Molecular basis of signaling specificity between GIRK channels and GPCRs
Figures
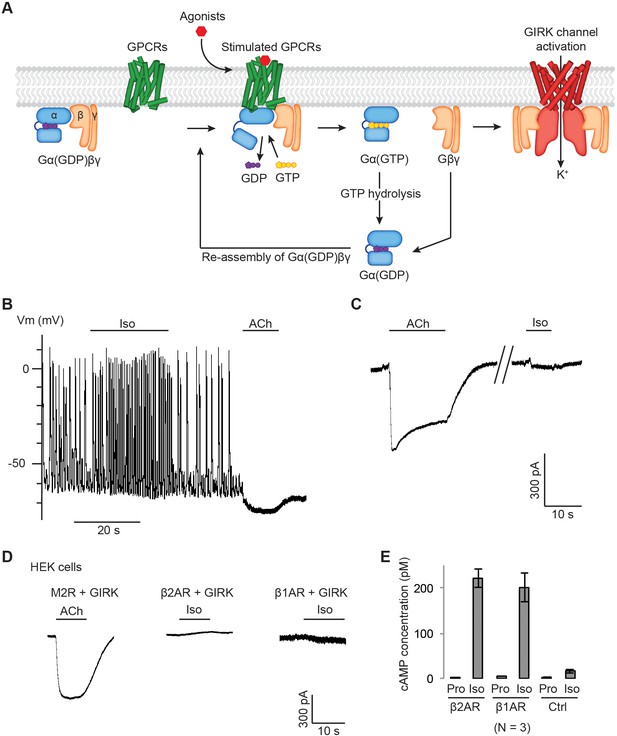
Gβγ specificity between GPCRs and GIRK channels.
(A) A schematic representation of GPCR signal transduction and GIRK channel activation. Agonist binding promotes the formation of a GPCR-Gα(GDP)βγ complex. The activated GPCR then triggers the exchange of GDP to GTP on the Gα subunit. Gα(GTP) and Gβγ subunits subsequently dissociate from the GPCR. Dissociated Gβγ directly binds to and activates GIRK channels. Dissociated Gα(GTP) hydrolyzes GTP to GDP, which then reassociates with Gβγ to form Gα(GDP)βγ. (B) A representative current-clamp recording of spontaneous action potentials from an acutely isolated murine sinoatrial node (SAN) cell. 1 µM isoproterenol (Iso) or acetylcholine (ACh) was applied as indicated. (C) A representative voltage-clamp recording from the same SAN cell in (B). The membrane potential was held at −80 mV, and 1 µM Iso or ACh was applied as indicated. (D) Representative voltage-clamp recordings of HEK-293T cells transiently co-transfected with GIRK channels, and either M2Rs, β2ARs or β1ARs. The membrane potential was held at −80 mV. 10 µM ACh or Iso was applied as indicated. (E) Validation of the function of βARs. HEK-293T cells expressing βARs or untransfected HEK-293T cells (Ctrl) were treated with 10 µM propranolol (Pro) or isoprennaline (Iso), and intracellular cAMP levels were quantified (N = 3, ±SD). See also Figure 1—figure supplement 1.
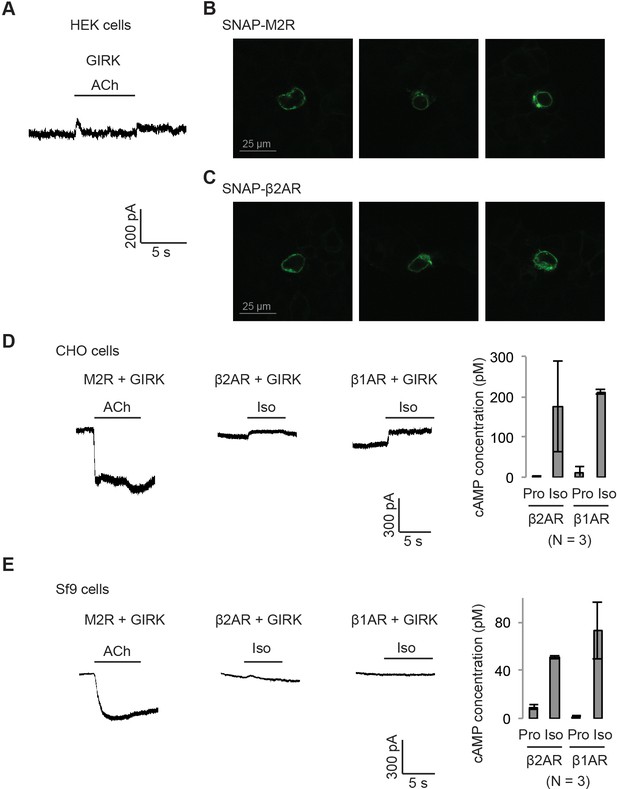
Gβγ specificity in heterologously expressed GIRK channels.
(A) Endogenous M2Rs do not activate GIRK. HEK-293T cells were transiently transfected with GIRK channels. The membrane potential was held at −80 mV. 10 µM ACh was applied as indicated. (B) (C) Confocal images of HEK-293T cells expressing M2Rs or β2ARs. HEK2-93T cells were transiently transfected with (B) SNAP-M2Rs or (C) SNAP-β2ARs. Receptors were stained using Alexa Fluor 488 conjugate to a SNAP-ligand. Three representative images are shown for each receptor. (D) Representative voltage-clamp recordings of CHO cells transiently co-transfected with GIRK channels, and either M2Rs, β2ARs or β1ARs. The membrane potential was held at −80 mV. 10 µM ACh or Iso was applied as indicated. The function of βARs was validated by quantifying intracellular cAMP (N = 3, ±SD). (E) Representative voltage-clamp recordings of Sf9 cells co-infected with GIRK channels, and either M2Rs, β2ARs or β1ARs. The membrane potential was held at −80 mV. 10 µM ACh or Iso was applied as indicated. The function of βARs was validated by quantifying intracellular cAMP (N = 3, ±SD).
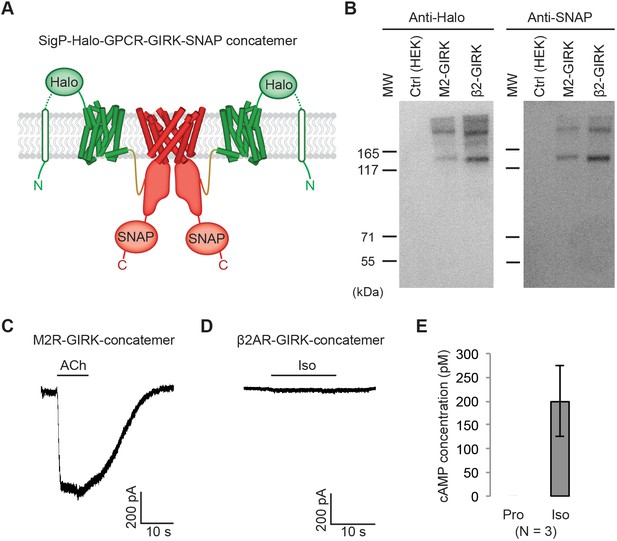
Effect of artificially enforced GPCR-GIRK co-localization.
(A) A schematic representation of GPCR-GIRK concatemer constructs. GIRK was directly fused to the C-terminus of GPCRs. A cleavable signal peptide and a Halo tag were added to the N-terminus of each concatemer. Additionally, a SNAP tag was added to the C-terminus of each concatemer. (B) Western-Blot analysis of GPCR-GIRK concatemer constructs. HEK-293T cells were transiently transfected with either M2R-GIRK or β2AR-GIRK concatemers. The expected size of these concatemers is ~150 kDa. (C) (D) Representative voltage-clamp recordings of HEK-293T cells transiently transfected with M2R-GIRK concatemers or β2AR-GIRK concatemers. Membrane potential was held at −80 mV. 10 µM ACh or Iso was applied as indicated. (E) Validation of the function of β2AR-GIRK concatemers. HEK-293T cells expressing β2AR-GIRK concatemers were treated with 10 µM propranolol (Pro) or isoproterenol (Iso), and intracellular cAMP levels were quantified (N = 3, ±SD).
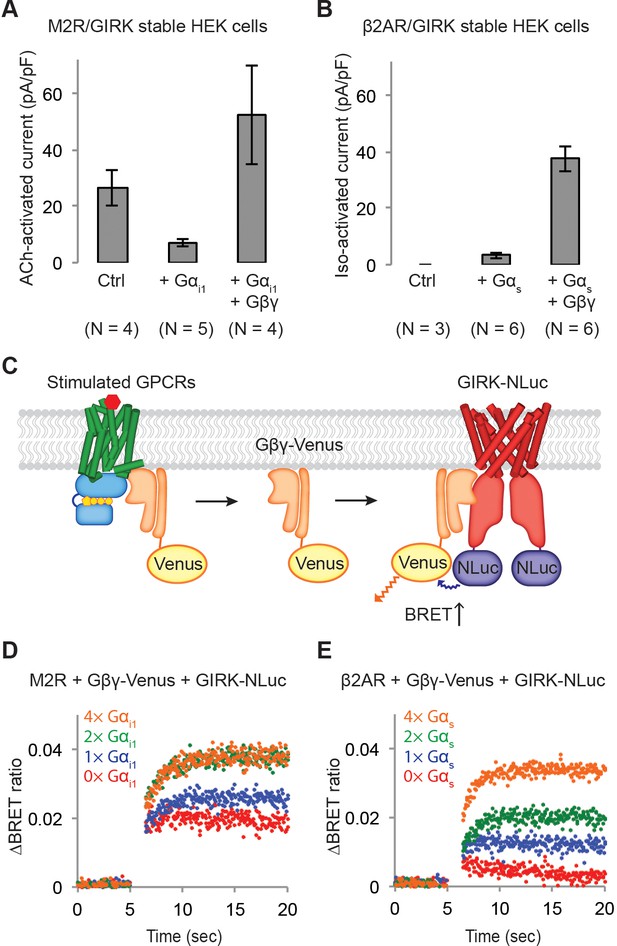
Influence of G protein levels on specificity.
(A) GIRK currents induced by M2R agonist ACh. Cells from a stable HEK-293T cell line expressing M2Rs and GIRK channels were transiently transfected with a vector expressing either GFP (Ctrl), Gαi1, or Gαi1 and Gβγ. 10 µM ACh was applied, and the evoked inward current was normalized to the capacitance of the cell (±SEM). (B) GIRK currents induced by β2AR agonist Iso. Cells from a stable HEK-293T cell line expressing β2ARs and GIRK channels were transiently transfected with a control vector expressing either GFP, Gαs, or Gαs and Gβγ. 10 µM Iso was applied, and the evoked inward current was normalized to the capacitance of the cell (±SEM). (C) A schematic representation of the BRET assay. Upon agonist stimulation of a GPCR, Gβγ-Venus is released. Gβγ-Venus then binds to GIRK-NLuc, which increases the BRET signal. (D) (E) Representative changes in BRET signal upon stimulation of GPCRs. In (D), HEK-293T cells were transfected with M2Rs, Gβγ-Venus, GIRK-NLuc, and increasing amounts of Gαi1. In (E), HEK-293T cells were transfected with β2ARs, Gβγ-Venus, GIRK-NLuc, and increasing amounts of Gαs. Agonists were applied at t = 5 s. See also Figure 3—figure supplements 1 and 2.
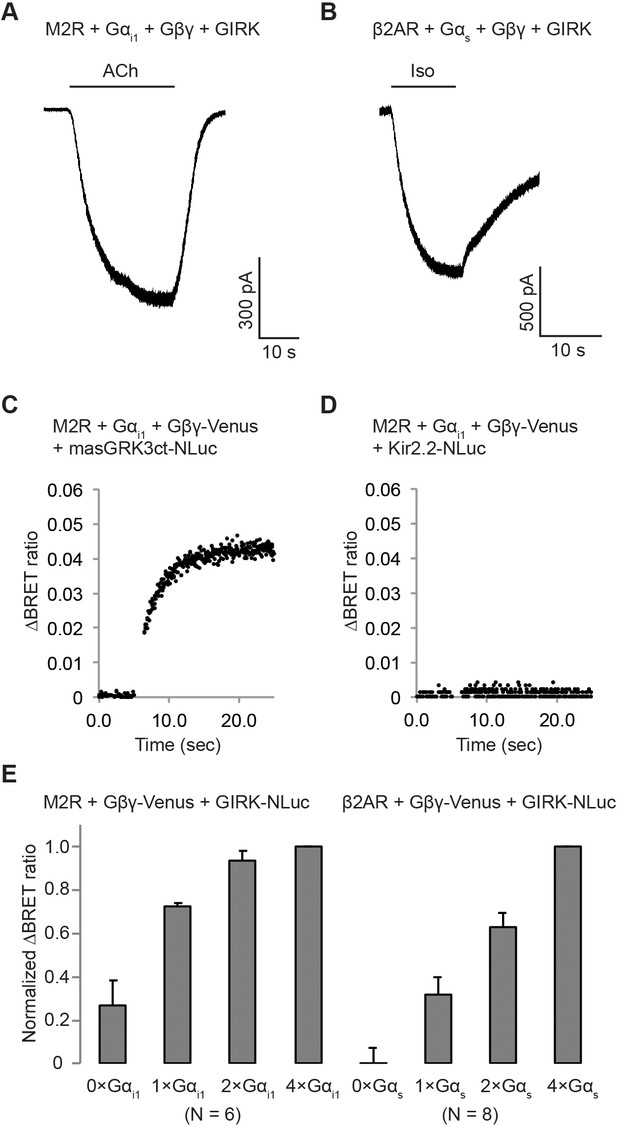
β2ARs activate GIRK channels in the presence of over-expressed G protein trimers.
(A) A representative voltage-clamp recording of HEK-293T cells stably expressing M2Rs and GIRK. The cells were transiently transfected with Gαi1 and Gβγ. (B) A representative voltage-clamp recording of HEK-293T cells stably expressing β2ARs and GIRK. The cells were transiently transfected with Gαs and Gβγ. (C) A representative time-resolved BRET ratio curve from HEK-293T cells expressing M2Rs, Gαi1, Gβγ-Venus, and masGRK3ct-NLuc, which is known to interact with Gβγ. (D) A representative time-resolved BRET ratio curve from HEK-293T cells expressing M2Rs, Gαi1, Gβγ-Venus, and Kir2.2-NLuc, another inward-rectifier K+ channel that is structurally similar to GIRK but does not bind to Gβγ. (E) Normalized changes in BRET signal upon stimulation of GPCRs. ΔBRET ratio was measured in the presence of different Gα expression levels and was normalized to that in the presence of 4 × Gα (N = 6–8, ±SEM).
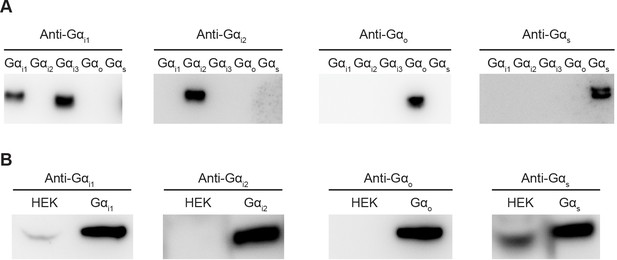
Comparison of endogenous Gα levels in HEK-293T cells.
(A) Evaluation of antibody specificity. Different Gα proteins were heterologously expressed, purified and analyzed by Western Blot. Anti-Gαi1 antibody recognizes both Gαi1 and Gαi3. Anti-Gαi2, Gαo, and Gαs antibodies specifically recognize their target Gα. (B) Comparison of endogenous Gα levels in HEK-293T cells. HEK-293T cells were lysed and analyzed by Western Blot. 5 ng of purified Gα was loaded as a reference.
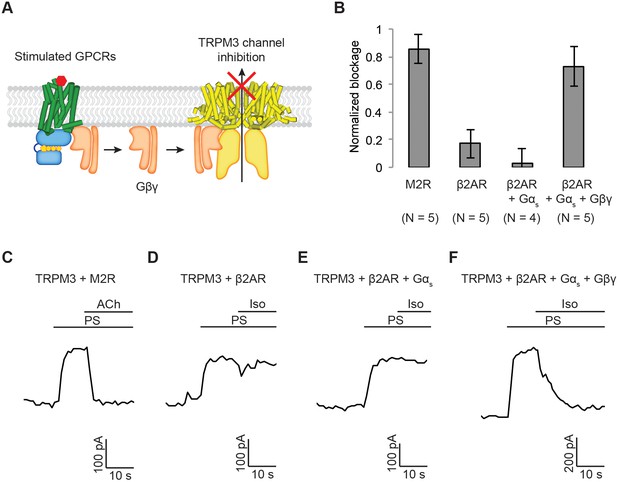
Generalization of Gαi-coupled GPCR target specificity.
(A) A schematic representation of TRPM3 channel inhibition by Gβγ. Upon agonist stimulation, released Gβγ directly binds to and inhibits TRPM3 channels. (B) The amount of current blocked upon GPCR stimulation was normalized to the first peak current (±SEM). (C)-(F) Representative voltage-clamp recordings of HEK-293T cells transiently transfected with (C) TRPM3 and M2Rs (D) TRPM3 and β2ARs (E) TRPM3, β2ARs, and Gαs, or (F) TRPM3, β2ARs, Gαs, and Gβγ. A ramp protocol from −100 mV to +100 mV was applied to the cells every second. The currents at +100 mV were plotted. TRPM3 currents were evoked by 10 µM pregnenolone sulfate (PS). M2Rs and β2ARs were stimulated by 10 µM ACh and Iso, respectively.
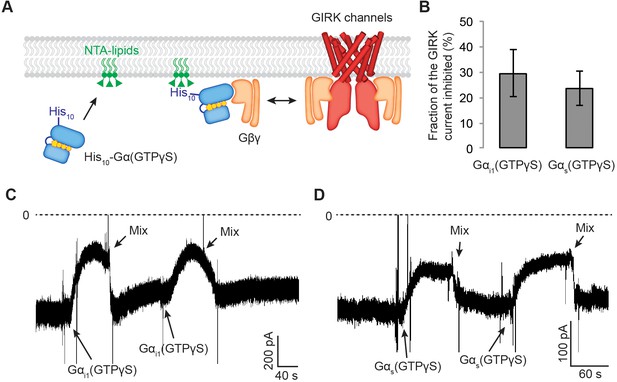
Gαs(GTP-γS) and Gαi1(GTP-γS) do not differentially compete with GIRK channels for Gβγ.
(A) A schematic representation of the competition assay between His10-Gα(GTP-γS) and GIRK for Gβγ in a reconstituted planar lipid bilayer system. In these experiments, we controlled the amount of lipid-associated Gα(GTP-γS) to evaluate the competition quantitatively. We first incorporated a fixed amount of Ni-NTA-lipids into the lipid bilayer and applied enough His10-Gα(GTP-γS) to saturate all the available Ni-NTA binding sites (Figure 5—figure supplement 1B–1D). Tethered His10-Gα(GTP-γS) competes with GIRK for Gβγ and therefore inhibits GIRK. (B) Current inhibition by His10-Gα(GTP-γS) was normalized to the initial current levels (N = 3, ±SD). (C) (D) Representative inward GIRK currents from lipid bilayers. GIRK was partially activated by PIP2, Na+, and a low concentration of Gβγ. Dashed lines represent the baseline current (0 pA). (C) His10-Gαi1(GTP-γS) or (D) His10-Gαs(GTP-γS) was directly perfused to the bilayer membrane several times followed by mixing the solutions in the bilayer chamber. The transient decrease in the current upon Gα(GTP-γS) application is an artifact due to the absence of Na+ in His10-Gα(GTP-γS) solution. See also Figure 5—figure supplement 1.
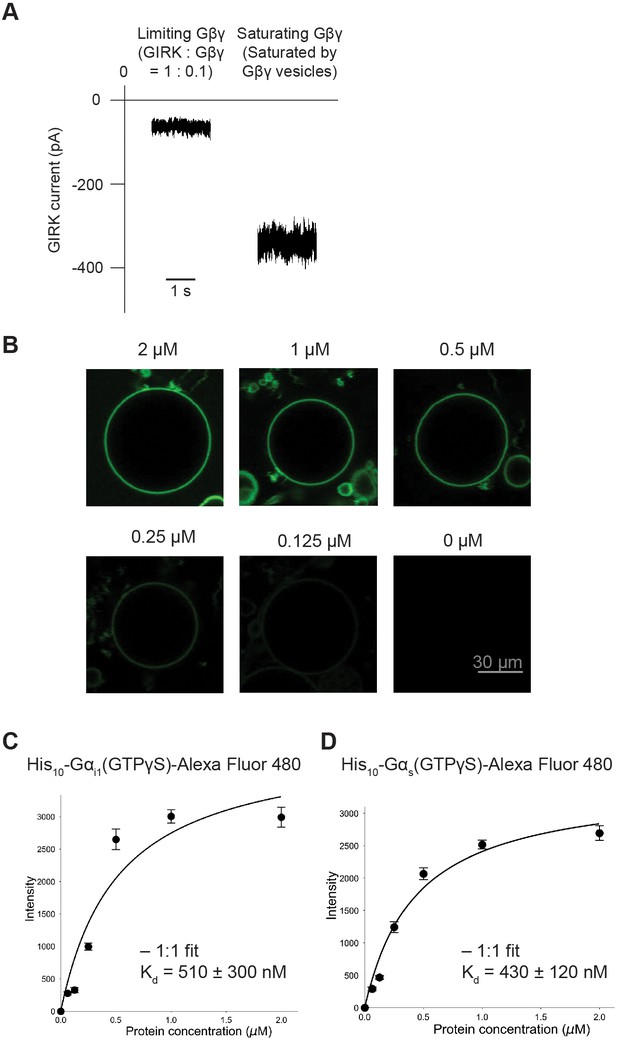
Purified His10-Gα(GTP-γS) binds to the GUV membrane containing Ni-NTA lipids.
(A) GIRK channels were partially activated by low amounts of Gβγ. Proteoliposomes containing GIRK and Gβγ at a ratio of 1:0.1 (wt:wt) were fused to the planar lipid bilayer membrane. GIRK was then activated by adding 32 µM C8-PIP2 and 32 mM Na+ to the bilayer chamber (left trace). Subsequently GIRK was fully activated by fusing Gβγ vesicles (right trace). The ~5 fold increase in the inward GIRK current suggests the partial activation of GIRK channels due to the presence of limiting amounts of Gβγ in the left trace. (B) Typical confocal images of the giant unilamellar vesicle (GUV) equator planes. The corresponding concentration of Alexa Fluor 488-labeled His10-Gαi1(GTP-γS) is indicated. (C) (D) Gα(GTP-γS) binding curves to GUVs. The fluorescence intensities measured at different protein concentrations were fitted using a 1:1 binding model.
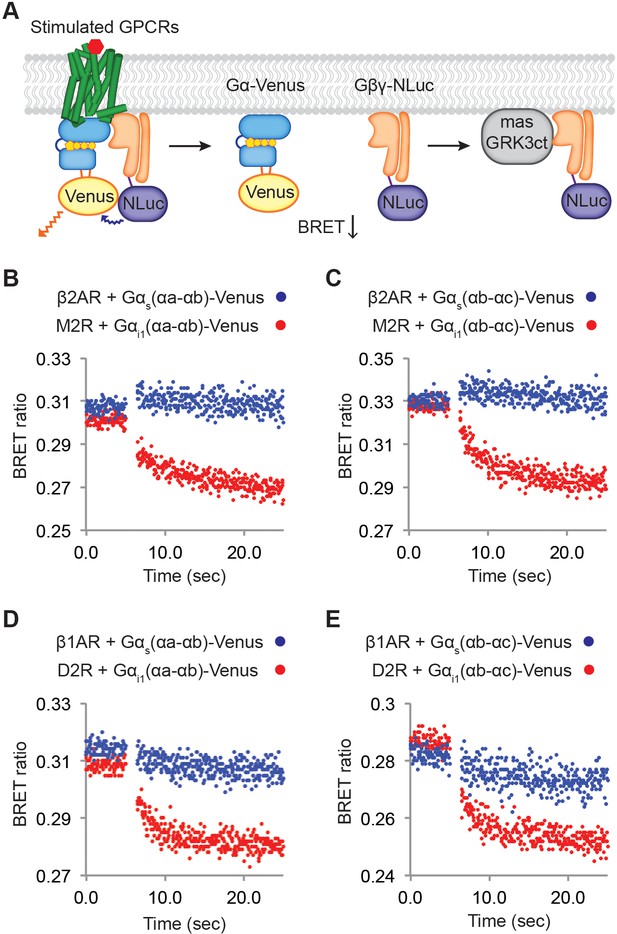
Relative rates of Gβγ release by Gαi versus Gαs-coupled receptors.
(A) A schematic representation of the experiment that monitors Gβγ release by BRET. Upon agonist stimulation, GPCRs release Gα and Gβγ. The dissociation of Gα-Venus and Gβγ-NLuc results in a decrease of the BRET signal. Released Gβγ-NLuc was chelated by masGRK3ct, a fusion of the C-terminal PH domain of GRK3 and a myristic acid attachment peptide. (B)-(E) Representative time-resolved BRET ratio curves. In (B) and (C), M2Rs released more Gβγ than β2ARs did within the same time period, independent of which Gα-Venus construct was used. In (D) and (E), D2Rs released more Gβγ than β1ARs did within the same time period, independent of which Gα-Venus construct was used. See also Figure 6—figure supplements 1 and 2, and Table 1.
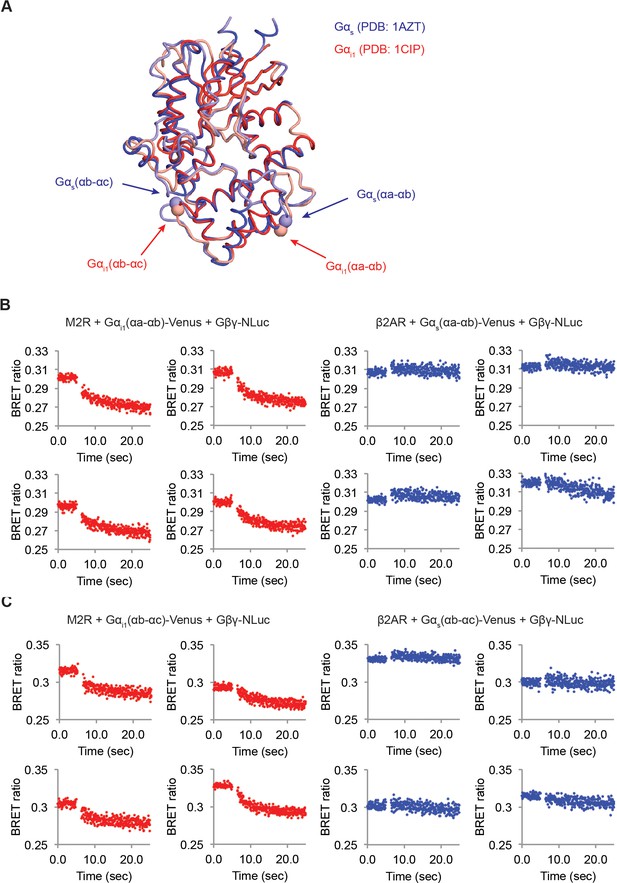
M2Rs catalyze release of Gβγ at higher rate compared to β2ARs.
(A) Structural comparison between Gαs and Gαi1. Crystal structures of Gαs (Blue, PDB: 1AZT) and Gαi1 (Red, PDB: 1GG2) were superimposed. The Venus insertion sites are indicated as arrows. The Cα atoms of residues Gαs-113 and 144, and Gαi1-91 and 121 are represented as spheres. (B) (C) Time-resolved BRET ratio curves from HEK-293T cells transiently transfected with different GPCRs and Gα-Venus constructs.
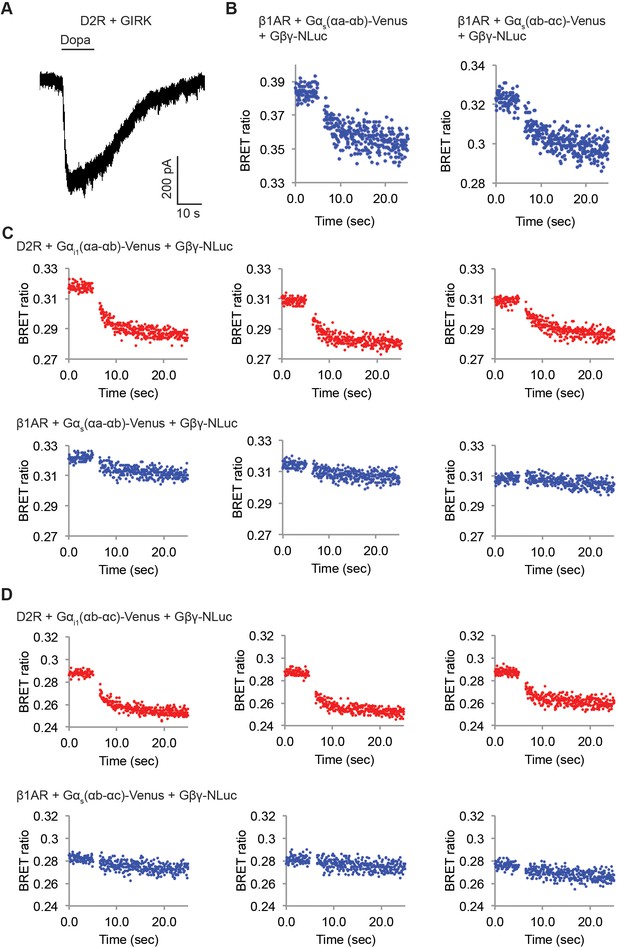
D2Rs catalyze release of Gβγ at higher rate compared to β1ARs.
(A) A representative voltage-clamp recording of a HEK-293T cell transiently transfected with GIRK channels and D2Rs. The membrane potential was held at −80 mV. 10 µM Dopamine (Dopa) was applied as indicated. (B) Validation of the function of Venus-inserted Gαs. Time-resolved BRET ratio curves from HEK-293T cells transiently transfected with β1ARs and different Gα-Venus constructs were shown. (C) (D) Time-resolved BRET ratio curves from HEK-293T cells transiently transfected with different GPCRs and Gα-Venus constructs.
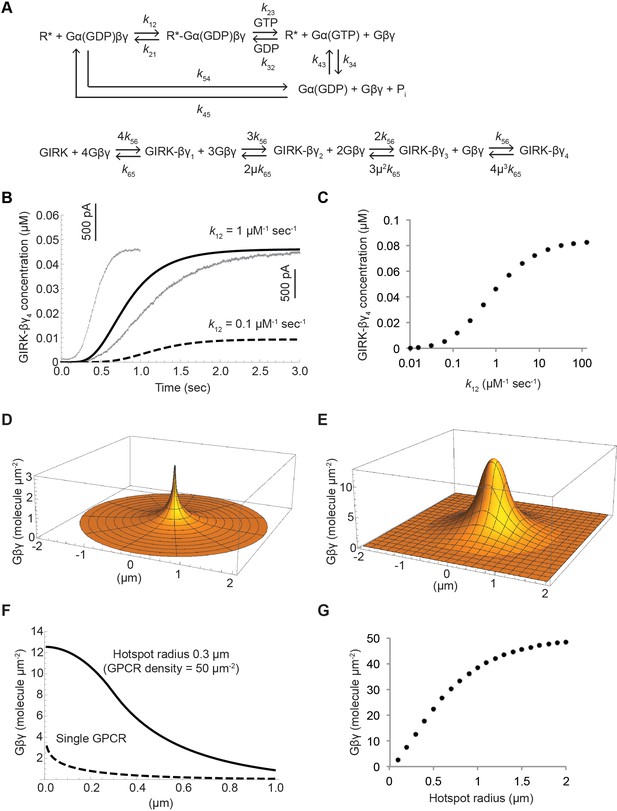
Kinetic model of Gβγ specificity.
(A) Reaction scheme used to model GPCR activation of GIRK. kxy are the rate constants of the reactions between two G protein states. Rate, equilibrium and cooperativity constants are summarized in Table 2. (B) ACh-stimulated GIRK currents from two different SAN cells are shown in grey. Calculated GIRK-βγ4 concentration as a function of time for two different k12 magnitudes are shown in black solid and dashed curves. (C) Calculated steady state GIRK-βγ4 concentration as a function of k12 magnitude. (D) Steady state two-dimensional Gβγ concentration profile (molecules µm−2; one molecule µm−2 = 0.2 µM in a layer 80 Å thick below the membrane surface) surrounding a single GPCR generating 1 Gβγ sec−1 with mean Gβγ lifetime 1 s and diffusion coefficient 0.2 µm2 sec−1. (E) Steady state two-dimensional concentration profile of Gβγ in and surrounding a hotspot of radius 0.3 µm with a density of 50 GPCR µm−2. Gβγ lifetime and diffusion coefficient are the same as in (D). (F) Two dimensional cross sections of concentration profiles in (D) and (E). (G) Steady state Gβγ concentration at the center of hotspot as a function of hotspot radius. See also Figure 7—figure supplement 1, and Table 2.
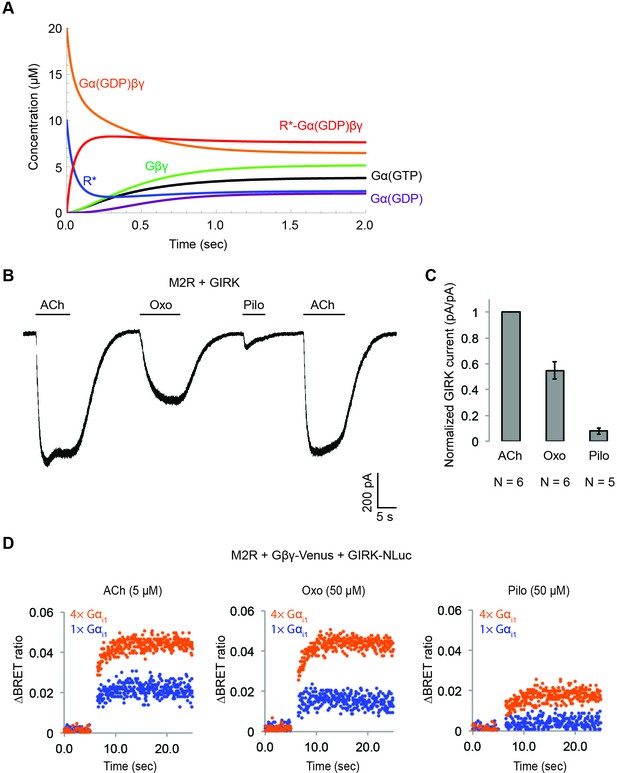
Simulation of GPCR-activation of GIRK.
(A) Calculated receptor and G protein concentrations as a function of time (k12 = 1 µM−1 sec−1). (B) Muscarinic partial agonists activate GIRK channels to a limited extent. Whole-cell voltage-clamp recordings were performed on stable HEK-293T cells expressing M2Rs and GIRK channels. The membrane potential was held at −80 mV. 10 µM ACh, 100 µM Oxotremorine (Oxo), or 100 µM Pilocarpine (Pilo) was applied as indicated. (C) Partial agonist-activated GIRK currents were normalized to ACh-activated GIRK currents (N = 5–6, ±SEM). (D) Representative changes in BRET signal upon stimulation of M2Rs with different agonists. HEK-293T cells were transfected with M2Rs, Gβγ-Venus, GIRK-NLuc, and increasing amounts of Gαi1. 5 µM acetylcholine (ACh), 50 µM oxotremorine (Oxo), or 50 µM pilocarpine (Pilo) was applied at t = 5 s.
Tables
Quantitative-BRET measurements of Gβγ release from different Gα constructs.
Averaged Nano-Luc intensity, basal BRET ratio, and ΔBRET ratio were summarized (N = 3–4, ±SD).
| NLuc intensity | Basal BRET ratio | ΔBRET ratio | |
|---|---|---|---|
| M2R-Gαi1(αa-αb) | (13.85 ± 0.36) x105 | 0.301 ± 0.003 | 0.031 ± 0.002 |
| β2AR- Gαs(αa-αb) | (11.91 ± 2.80) x105 | 0.310 ± 0.007 | 0.002 ± 0.006 |
| M2R-Gαi1(αb-αc) | (8.08 ± 1.77) x105 | 0.310 ± 0.014 | 0.029 ± 0.006 |
| β2AR- Gαs(αb-αc) | (8.64 ± 2.62) x105 | 0.311 ± 0.014 | 0.005 ± 0.005 |
| D2R-Gαi1(αa-αb) | (23.29 ± 1.46) x105 | 0.312 ± 0.005 | 0.028 ± 0.006 |
| β1AR- Gαs(αa-αb) | (24.51 ± 1.09) x105 | 0.315 ± 0.007 | 0.007 ± 0.003 |
| D2R-Gαi1(αb-αc) | (5.58 ± 4.31) x105 | 0.287 ± 0.001 | 0.032 ± 0.004 |
| β1AR- Gαs(αb-αc) | (7.77 ± 0.34) x105 | 0.279 ± 0.004 | 0.008 ± 0.003 |
Parameters used for simulation of GPCR-activation of GIRK.
k12: The rate of formation of the productive GPCR-G protein complex (Sungkaworn et al., 2017). k21: The rate of dissociation of the productive GPCR-G protein complex (Sungkaworn et al., 2017). k23: The rate of nucleotide exchange and subsequent dissociation of GPCRs, Gα(GTP), and Gβγ (Sungkaworn et al., 2017). k32: The rate of the reverse reaction of nucleotide exchange and dissociation of GPCRs and G proteins. k34: The rate of GTP hydrolysis, based on Breitwieser and Szabo, 1988. k43: The rate of the reverse reaction of GTP hydrolysis. k45: The on-rate between Gα(GDP) and Gβγ, adapted from Sarvazyan et al., 1998. k54: The off-rate between Gα(GDP) and Gβγ, calculated based on k45 and Kd = 3 nM (Sarvazyan et al., 1998). k56: The on-rate between the GIRK and Gβγ is diffusion limited (Wang et al., 2016). k65: The off-rate between the GIRK and Gβγ were calculated based on k56 and our previous Kd measurement (Shea et al., 1997).
| Reaction | Forward-rate | Backward-rate | Note |
|---|---|---|---|
| R* + Gα(GDP)βγ ⇌ R*-Gα(GDP)βγ | 1 µM−1 sec−1 (k12) | 1 sec−1 (k21) | Sungkaworn et al., 2017 |
| R*-Gα(GDP)βγ ⇌ R* + Gα(GTP) + Gβγ | 1 sec−1 (k23) | 0 M−2 sec−1 (k32) | Sungkaworn et al., 2017 |
| Gα(GTP) ⇌ Gα(GDP) + Pi | 2 sec−1 (k34) | 0 M−1 sec−1 (k43) | Breitwieser and Szabo, 1988 |
| Gα(GDP) + Gβγ ⇌ Gα(GDP)βγ | 0.7 × 106 M−1 sec−1 (k45) | 0.002 sec−1 (k54) | Sarvazyan et al., 1998 |
| GIRK-βγn-1 + (5 - n)Gβγ ⇌ GIRK-βγn + (4 - n)Gβγ | (5 - n) × 1 × 107 M−1 sec−1 ((5 - n) × k56) | n × µn-1 × 600 sec−1 (n × µn-1 × k65) | Shea et al., 1997; Wang et al., 2016 |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Rabbit monoclonal anti Gαi1 | Abcam | Cat. #: ab140125 | 1:1000 |
| Antibody | Rabbit monoclonal Anti Gαi2 | Abcam | Cat. #: ab157204 | 1:1000 |
| Antibody | Mouse monoclonal anti Gαo | Santa Cruz Biotechnology | Cat. #: sc-13532 | 1:1000 |
| Antibody | Rabbit polyclonal anti Gαs/olf | Santa Cruz Biotechnology | Cat. #: sc-383 | 1:1000 |
| Antibody | Rabbit polyclonal anti SNAP tag | NEB | Cat. #: P9310S | 1:1000 |
| Antibody | Rabbit polyclonal anti Halo tag | Promega | Cat. #: G9281 | 1:1000 |
| Commercial assay or kit | cAMP ELISA Detection Kit | GenScript | Cat. #: L00460 | |
| Commercial assay or kit | NLuc substrate | Promega | Cat. #: N1110 | 1:50 |
| Cell line (Homo Sapiens) | Flp-In-T-REx-293 | Thermo Fisher | RRID:CVCL_U427 | |
| Cell line (Homo Sapiens) | HEK-293 tsA201 | Sigma | RRID: CVCL_2737 | |
| Cell line (Cricetulus griseus) | Chinese Hamster Ovary Cells | Sigma | RRID: CVCL_0213 | |
| Cell line (Spodoptera frugiperda) | Sf9 cells | Sigma | RRID: CVCL_0549 | |
| Software | Mathematica | Wolfram | SCR_014448 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.42908.018





