Accelerated redevelopment of vocal skills is preceded by lasting reorganization of the song motor circuitry
Figures
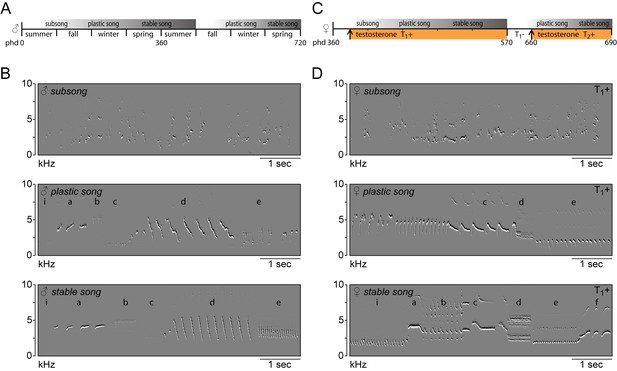
Song in juvenile male and adult female canaries progresses through the same stages of development.
(A) Schematic of natural song development in juvenile males. (B) Example spectral derivative spectrograms illustrating the different song developmental stages of a male canary. Subsong was recorded at 45 days of age, plastic song at 120 days of age and stable song at 1 year old. (C) Schematic of song development in adult female canaries during a first testosterone treatment (T1+), after removal of testosterone (T1-), and during a second testosterone treatment (T2+). (D) Example spectral derivative spectrograms from an adult female canary illustrating subsong after 3 days of testosterone treatment, plastic song after 30 days of treatment, and stable song after 200 days of treatment. Lowercase letters indicate different phrases of repeated syllables.
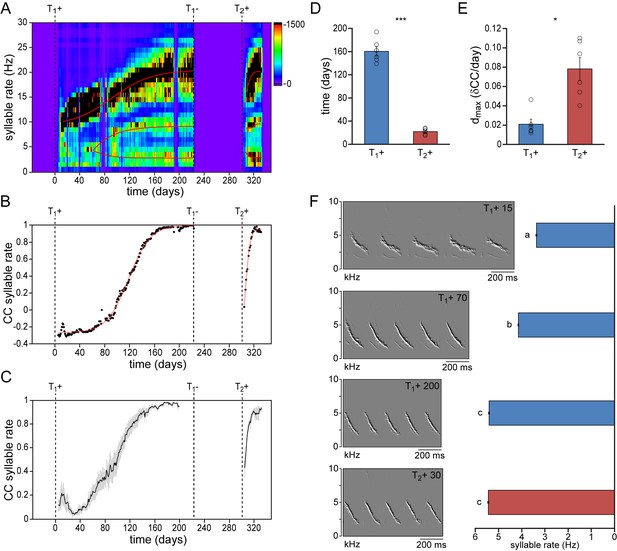
Syllable repetition rates develop faster in birds with prior singing experience.
(A) Syllable rate histogram from a female canary during two subsequent testosterone treatments. Color scales indicate the number of daily syllables produced for each discrete syllable rate. Curves were fitted through the most occurring syllable rates and are shown in red. (B) The correlation coefficient (CC) between the stabilized distribution of syllable rates at the end of the 1st testosterone treatment and each other day during the development and re-development of song in the same bird as shown in A. (C) The mean syllable rate correlation plot for all animals. Grey bars indicate the SEM. (D) Group statistics of all studied birds demonstrating that stable syllable rates were achieved more quickly during a 2nd testosterone treatment (red bars) than during the 1st treatment (blue bars). (E) The peak day-to-day increase in the syllable rate CC (dmax) was higher during a 2nd testosterone treatment than during the 1st treatment. (F) Example spectral derivative spectrograms from one bird and corresponding bar graphs illustrating syllable rates at 15, 70, and 200 days after a 1st testosterone treatment and 30 days after a 2nd treatment. Columns in D-E represent the mean ± SEM and open circles indicate individual data points (*p<0.05, ***p≤0.001, paired t-test, n = 6 animals). Columns in F represent the mean ± SEM (a,b,c: p≤0.001, ANOVA; n = 100 syllables). Source data for temporal song features are available in the Figure 2—source data 1.
-
Figure 2—source data 1
Source file for quantitative comparisons of temporal song features.
- https://doi.org/10.7554/eLife.43194.007
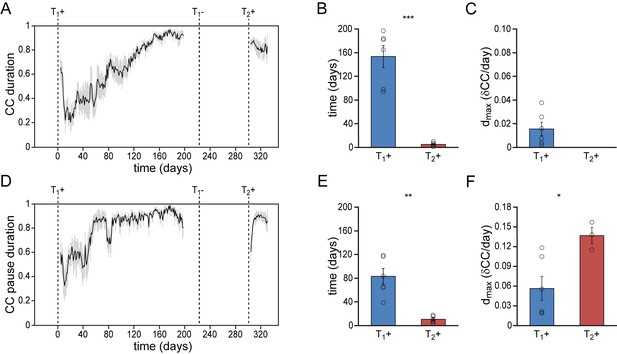
Differential re-development of temporal song features.
(A) Mean syllable duration correlation plot for all animals showing a gradual development during a 1st testosterone treatment (T1+), followed by an immediate recovery of syllable duration during a 2nd treatment (T2+). (B) Group statistics demonstrating that stable syllable durations were achieved more quickly during a 2nd testosterone treatment (red bars) than during the 1st treatment (blue bars). (C) The peak day-to-day increase in the syllable duration CC (dmax) for T1+. Dmax could not be calculated for T2+, as we observed no developmental increase of this song feature during the 2nd testosterone treatment. (D) Mean correlation plot for the pause duration between subsequent song syllables. Pause durations stabilized relatively fast during a 1st testosterone treatment (T1+), and required a phase of re-development during a 2nd treatment (T2+). (E) Group statistics demonstrating that stable pause durations were achieved more quickly during a 2nd testosterone treatment (red bars) than during the 1st treatment (blue bars). (F) The peak day-to-day increase in the pause duration CC (dmax) was significantly higher for T2+ than for T1+. Grey bars in A and D indicate the SEM. Columns in B,C,E,F represent the mean ± SEM and open circles indicate individual data points (*p<0.05, **p<0.01, ***p≤0.001, paired t-test, n = 6 animals).
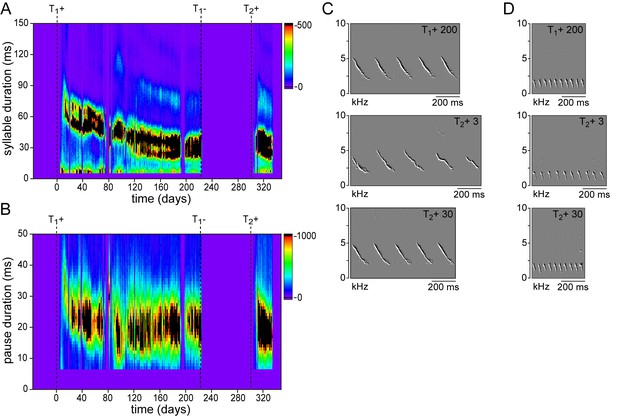
Development and re-development of temporal song features.
Example song feature histograms from one individual illustrating the gradual acquisition during a 1st testosterone treatment (T1+) and the rapid recurrence during a 2nd treatment (T2+) for (A) syllable duration, and (B) pause duration. (C,D) Example spectral derivative spectrograms from two different birds illustrating a slower syllable rate with longer pause durations at the onset of song production during the 2nd (T2+ 3) testosterone treatment compared to stable songs during the 1st (T1+ 200) and 2nd (T2+ 30) treatment.
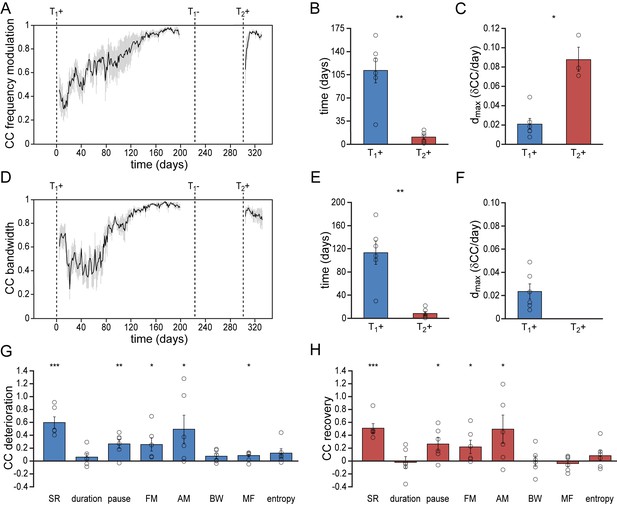
Differential re-development of song features.
(A) Mean correlation plot for all animals illustrating the development of the frequency modulation (FM) distribution in the song during the development (T1+) and re-development (T2+) of song. The correlation plot illustrates a gradual FM development during T1+, followed by a short phase of FM re-development during T2+. (B) Group statistics demonstrating that the stabilization of the FM distribution in the song took less time during a 2nd testosterone treatment (red bars) than during the 1st treatment (blue bars). (C) The peak day-to-day increase in the FM CC (dmax) was significantly higher during a 2nd testosterone treatment than during the 1st treatment. (D) Mean correlation plot for the syllable bandwidth showing a gradual development during a 1st testosterone treatment (T1+), followed by an immediate recovery of syllable bandwidth during a 2nd treatment (T2+). (E) Group statistics demonstrating that stable syllable bandwidths were achieved more quickly during a 2nd testosterone treatment (red bars) than during the 1st treatment (blue bars). (F) The peak day-to-day increase in the syllable bandwidth CC (dmax) for T1+. Dmax could not be calculated for T2+, as we observed no developmental increase of this song feature during the 2nd testosterone treatment. (G) Deterioration in the distribution patterns of all analyzed song features during absence of song production (T1-), and (H) subsequent recovery of song features during testosterone-induced re-development of song (T2+). Grey bars in A and D indicate the SEM. Columns in B,C and E-H represent the mean ± SEM and open circles indicate individual data points (*p<0.05, **p<0.01, ***p≤0.001, paired t-test (B,C,E,F) and one-sample t-test (G,H), n = 6 animals). Source data for acoustic features are available in the Figure 3—source data 1.
-
Figure 3—source data 1
Source file for quantitative comparisons of spectral song features.
- https://doi.org/10.7554/eLife.43194.011
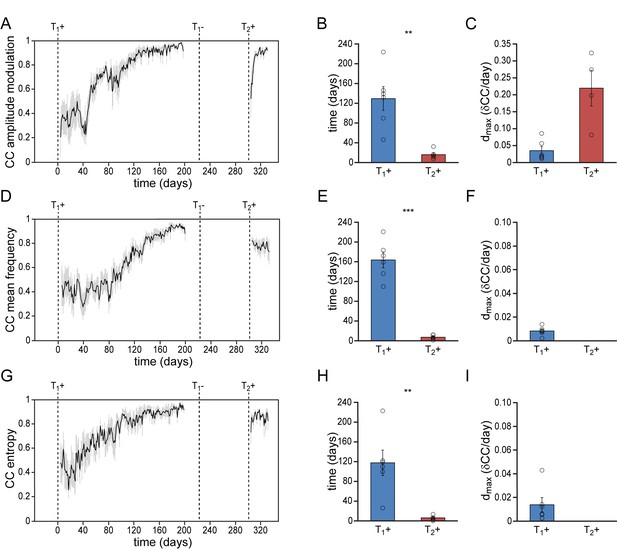
Differential re-development of spectral song features.
(A) Mean amplitude modulation (AM) correlation plot for all animals. The AM distribution demonstrated a gradual consolidation during the 1st testosterone treatment (T1+), and displayed a short phase of re-development during a 2nd testosterone treatment (T2+) (B,C). Both mean frequency (D–F) and wiener entropy (G–I) demonstrated a gradual development during the 1st testosterone treatment (T1+) followed by an immediate recovery during a 2nd treatment (T2+). Dmax could not be calculated for T2+ in F,I, as we observed no developmental increase of these song features during the 2nd testosterone treatment. Grey bars in A, D and G indicate the SEM. Columns in B,C, E,F, and H,I represent the mean ± SEM and open circles indicate individual data points (**p<0.01, ***p≤0.001, paired t-test, n = 6 animal).
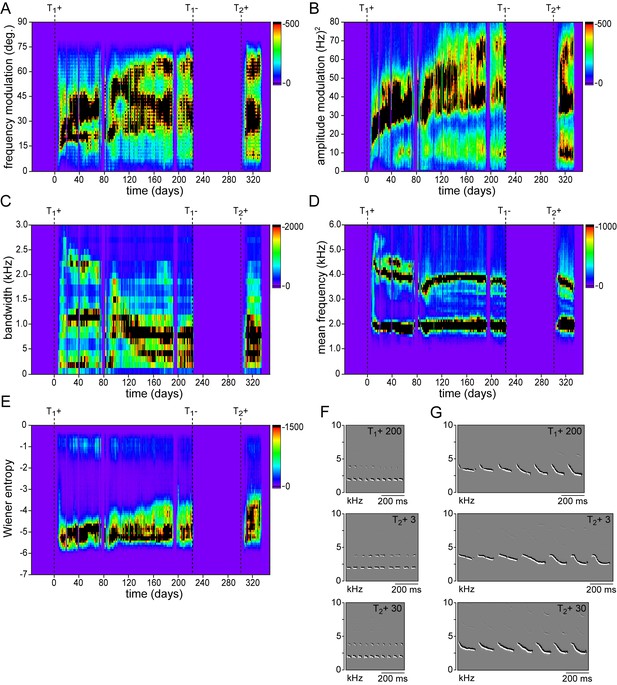
Development and re-development of spectral song features.
Example song feature histograms illustrating the gradual acquisition during a 1st testosterone treatment (T1+) and the rapid recurrence during a 2nd treatment (T2+) for (A) frequency modulation, (B) amplitude modulation, (C) bandwidth, (D) mean frequency, and (E) Wiener entropy. (F,G) Example spectral derivative spectrograms from stable songs at 200 days after a 1st testosterone treatment (T1+ 200), plastic songs at 3 days after a 2nd testosterone treatment (T2+ 3), and stable songs 30 days after a 2nd treatment (T2+ 30).
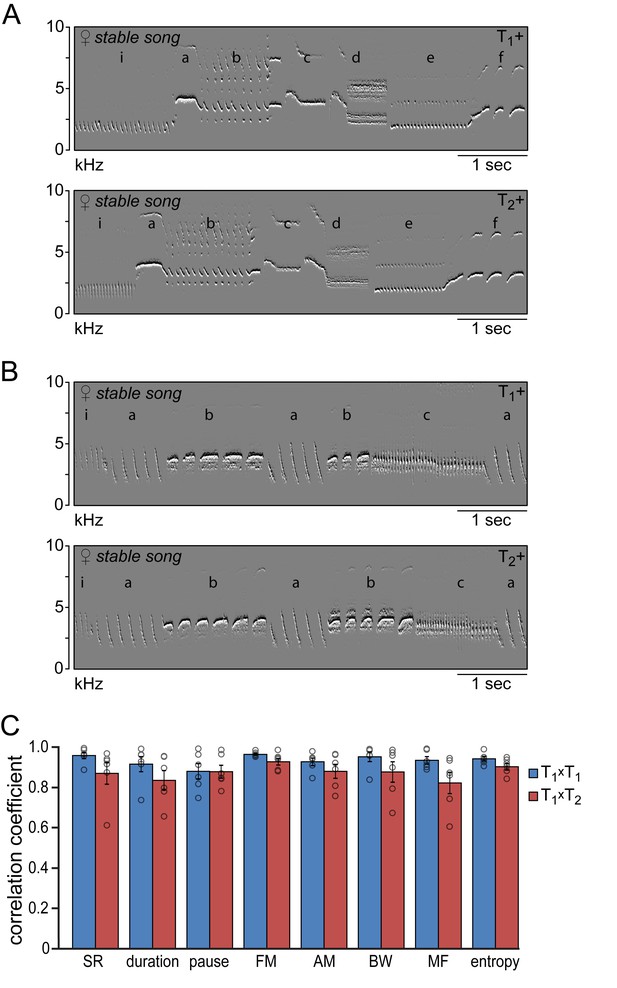
Similarity of time and frequency parameters after subsequent testosterone treatments.
(A,B) Example spectral derivative spectrograms of stable song from two animals during the 1st (T1+) and 2nd (T2+) testosterone treatment illustrating a strong similarity in song structure. (C) Similarity analyses between stable song patterns from the 1st and 2nd testosterone treatment periods (T1xT2, red bars) demonstrated a high level of correlation of more than 80% for all analyzed song features. Correlation coefficients between songs from T1+ and T2+ were not significantly different from the CCs obtained when cross-correlating song patterns within the T1+ period (T1xT1, blue bars). Columns in C represent the mean ± SEM and open circles indicate individual data points (NS, paired t-test, n = 6 animals). Source data for similarity calculations are available in the Figure 4—source data 1.
-
Figure 4—source data 1
Source file for song similarity calculations between subsequent testosterone treatments.
- https://doi.org/10.7554/eLife.43194.015
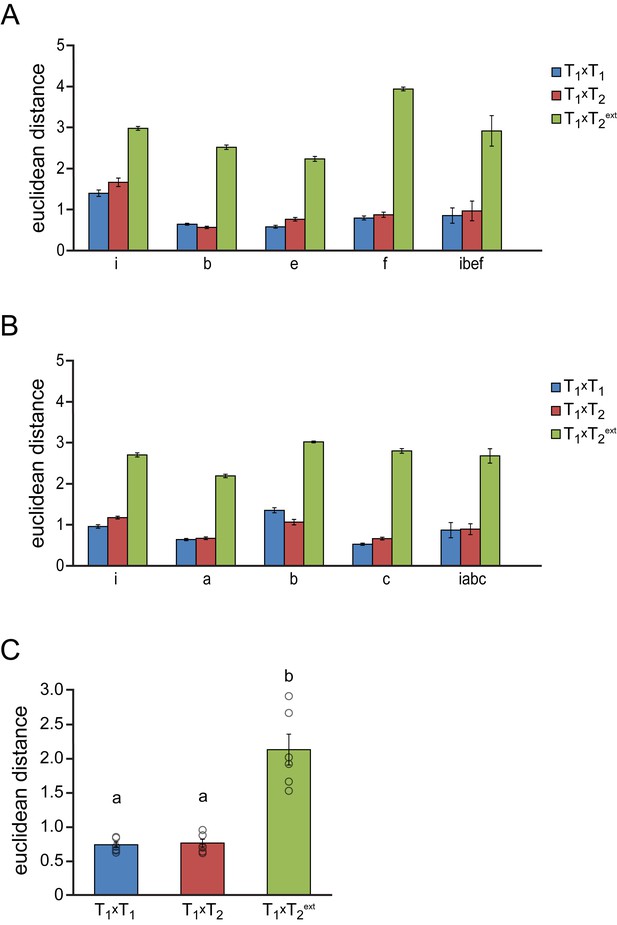
Syllable similarity during subsequent testosterone treatments.
Euclidean distances are shown between syllables of the same type during a 1st testosterone treatment (T1xT1, blue bars), between syllables of the same type between the 1st and 2nd testosterone treatment (T1xT2, red bars), and between syllables of different types between the 1st and 2nd testosterone treatment (T1xT2ext, green bars). (A,B) Euclidean distances between individual song syllables and their means within and between testosterone treatments for the two birds shown in Figure 4. Lowercase letters correspond to the song phrases indicated in Figure 4A and B. (C) Group statistics demonstrating a strong similarity between syllables of the same type, but not between syllables of different types when comparing song syllables between the two testosterone treatments. Columns in A-C represent the mean ± SEM and open circles in C indicate individual data points (a,b: p<0.001, ANOVA, n = 6 animals).
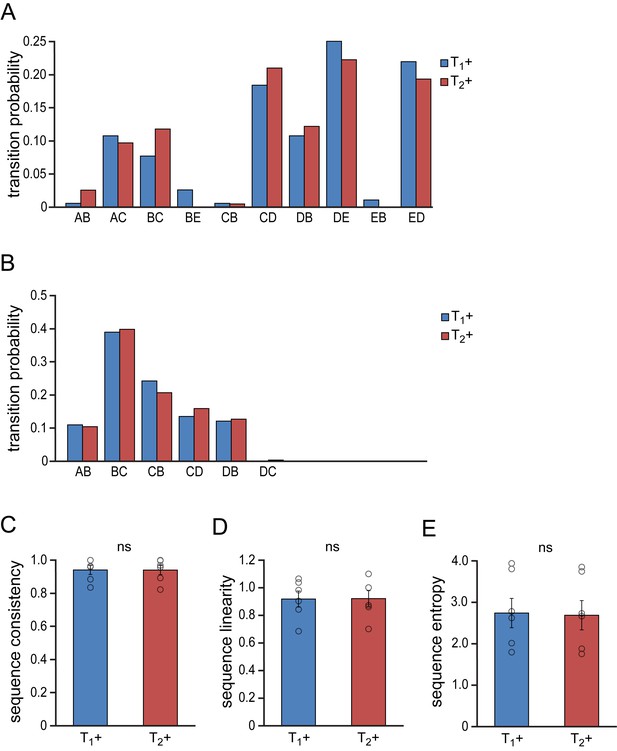
Similarity of song syntax during subsequent testosterone treatments.
(A,B) Example probability distributions of song phrase transitions from two animals during the 1st (T1+, blue bars) and 2nd (T2+, red bars) testosterone treatment illustrating the strong similarity in phrase transitions between the two treatment periods. (C–E) Group statistics demonstrating stable consistency (C), linearity (D) and entropy (E) of phrase transitions during the two developmental phases. Columns in C-E represent the mean ± SEM and open circles indicate individual data points (NS, paired t-test, n = 6 animals).
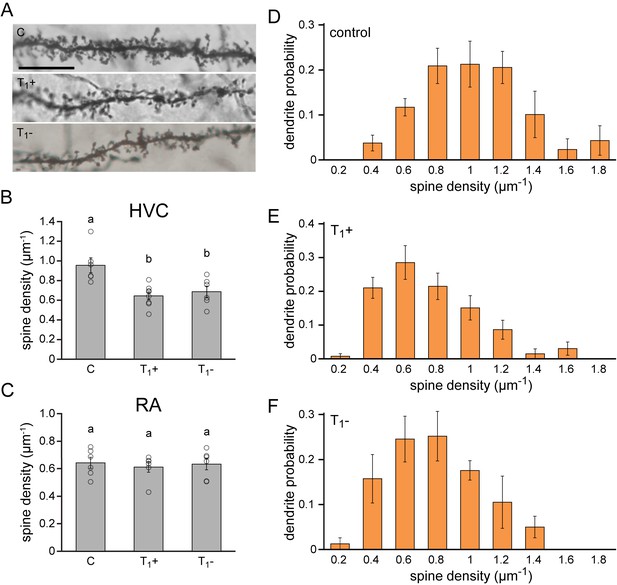
Lasting synaptic pruning in the forebrain motor nucleus HVC.
(A) Photomicrographs of dendrite segments from non-singing control animals (C), singing female canaries sacrificed after five months of testosterone treatment (T1+), and non-singing individuals sacrificed 2.5 months after testosterone withdrawal (T1-). (B) Compared to naive control birds (C), spine densities were significantly reduced in testosterone-treated, singing birds (T1+), and remained significantly reduced up to 2.5 months after birds stopped singing by withdrawing testosterone (T1-). (C) No significant differences in spine densities were observed in RA between the experimental periods. (D–F) The probability distribution of dendrites with different spine densities in HVC demonstrated a shift towards more dendrites with fewer spines in testosterone treated (T1+), singing birds and testosterone removed (T1-), non-singing birds compared to non-singing control birds. Columns in B-F represent the mean ± SEM and open circles indicate individual data points (a,b: p<0.01, ANOVA, n = 6 animals). Scale bar = 100 µm. Source data for spine quantifications are available in the Figure 5—source data 1.
-
Figure 5—source data 1
Source file for spine quantification data.
- https://doi.org/10.7554/eLife.43194.017
Tables
Plasma levels of testosterone (T) during different hormone treatments.
https://doi.org/10.7554/eLife.43194.018| Treatment | T levels (pg/ml) | Significance* |
|---|---|---|
| control | 238 ± 65 | |
| T1+ | 6486 ± 488 | p<0.001 |
| T1- | 138 ± 30 | NS |
| T2+ | 7279 ± 1249 | p<0.01 |
-
* P-values of comparisons between different hormone treatments against control values (ANOVA with Dunnett’s t correction for multiple comparisons against one control).
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.43194.019





