Proinsulin misfolding is an early event in the progression to type 2 diabetes
Figures
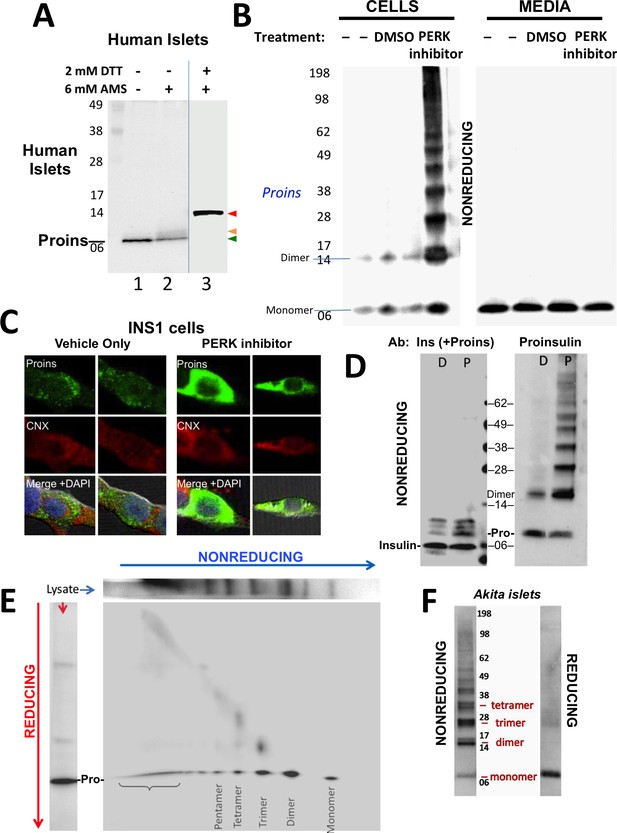
Detection of improperly folded proinsulin.
(A) Non-diabetic human pancreatic islets were lysed in RIPA buffer and divided into three equal parts, one of which was (partially) pre-reduced by boiling in the presence of 2 mM DTT (lane 3). This and a non-pre-reduced sample (lane 2) underwent alkylation with 6 mM AMS. A third sample was neither pre-reduced nor alkylated (lane 1). All samples were incubated for 1 hr at 37°C and finally resolved by SDS-PAGE under reducing conditions (200 mM DTT), electrotransfer to nitrocellulose, and immunoblotting for human proinsulin with mAb 20G11. The red arrowhead: proinsulin species with >1 alkylated Cys (upper band); beige arrowhead: at least one alkylated Cys (middle band); green arrowhead: no free Cys (bottom band). (B) Cell lysate (left) and overnight secretion (right) from untreated INS1E cells (–) or those treated with vehicle alone (DMSO) or PERK inhibitor (GSK2656157, 2 µM) were analyzed by nonreducing SDS-PAGE, electrotransfer to nitrocellulose, and immunoblotting for rodent proinsulin (mAb CCI-17). The positions of molecular mass markers are noted. (C). INS1E cells were treated overnight with vehicle (DMSO) or PERK inhibitor before formaldehyde fixation, permeabilization, and indirect immunofluorescence with mAb anti-proinsulin (GS-9A8, green) and rabbit anti-calnexin (red), with appropriate secondary antibodies. (D) INS1E cells were treated with DMSO (lane marked ‘D’) or PERK inhibitor (lane marked ‘P’). The cells were lysed and resolved in duplicate by nonreducing SDS-PAGE. The final gel was treated with 25 mM DTT for 10 min at 25°C, electrotransferred to nitrocellulose, and then immunoblotted with guinea pig anti-insulin that cross-reacts with proinsulin (‘Pro’) and conversion intermediates (left panel) or anti-proinsulin (CCI-17, right panel). The positions of molecular mass markers are noted. (E) INS1E cells treated with PERK inhibitor as in panel B) were lysed and resolved by a first dimensional nonreducing SDS-PAGE (shown horizontally, at top) and then in a second dimensional reducing SDS-PAGE (shown vertically, at left). The 2D gel was electrotransferred to nitrocellulose and immunoblotted for rodent proinsulin (mAb CCI-17). The bracket indicates high molecular weight proinsulin-containing complexes. (F) Islets from Akita male mouse were lysed in RIPA buffer and analyzed by anti-proinsulin immunoblotting (mAb CCI-17) under nonreducing (left) or reducing conditions (right). The positions of molecular mass markers are noted.
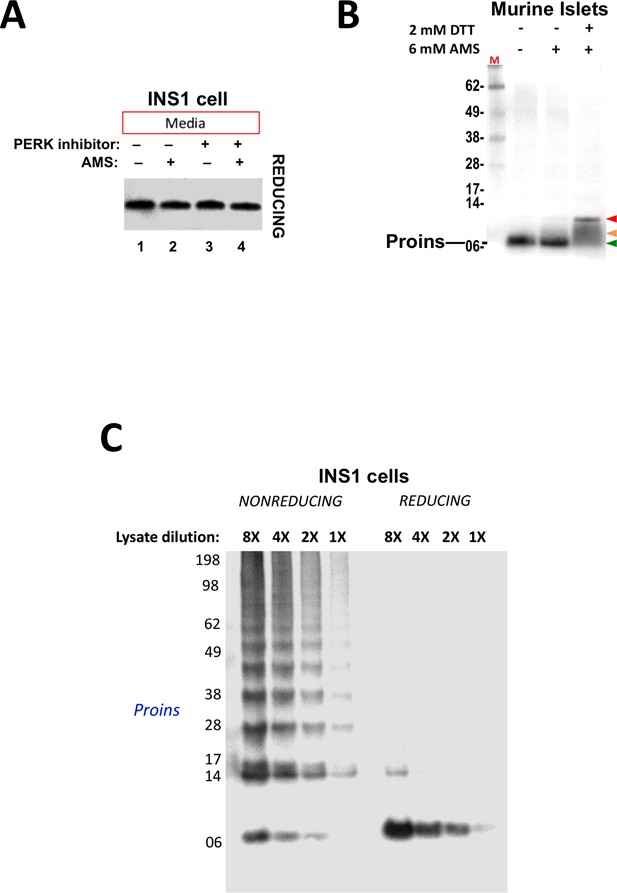
Proper and improper disulfide bond formation in proinsulin.
(A) Secreted proinsulin does not exhibit available free thiols. Media from INS1E cells incubated overnight without or with PERK inhibitor (‘+') were collected, divided in half, and either not alkylated or alkylated with 10 mM AMS. The reactions were quenched with 200 mM DTT and resolved by SDS-PAGE in the presence of 200 mM DTT, electrotransferred to nitrocellulose, and analyzed by immunoblotting for rodent proinsulin with mAb CCI-17. No alkylated proinsulin was detected in the secretion, indicating that these molecules are properly folded. (B) Presence of free thiols in a subpopulation of proinsulin molecules from mouse islet beta cells. Mouse islets were lysed in RIPA buffer and divided into three equal parts, one of which was (partially) pre-reduced by boiling in the presence of 2 mM DTT (third lane). This and a non-pre-reduced sample (middle lane) underwent alkylation with 6 mM AMS. The sample in the first lane was neither pre-reduced nor alkylated. All samples were incubated for 1 hr at 37°C and finally resolved by SDS-PAGE under reducing conditions (200 mM DTT), electrotransfer to nitrocellulose, and immunoblotting for rodent proinsulin (‘Proins’) with mAb CCI-17. The red arrowhead: proinsulin species with >1 alkylated Cys (upper band); beige arrowhead: at least one alkylated Cys (middle band); green arrowhead: no free Cys (bottom band). (C) Inhibition of PERK promotes formation formation of proinsulin disulfide-linked complexes in a rat beta cell line.. INS1E cells treated overnight with PERK inhibitor were lysed and analyzed by nonreducing or reducing SDS-PAGE in serial dilutions; the relative concentration of each sample is shown at the top. The final gel was treated with 25 mM DTT for 10 min at 25°C, electrotransferred to nitrocellulose, and immunoblotted for rodent proinsulin (‘Proins’) with mAb CCI-17. These data demonstrate linearity of immunoreactive signal by Western blotting.
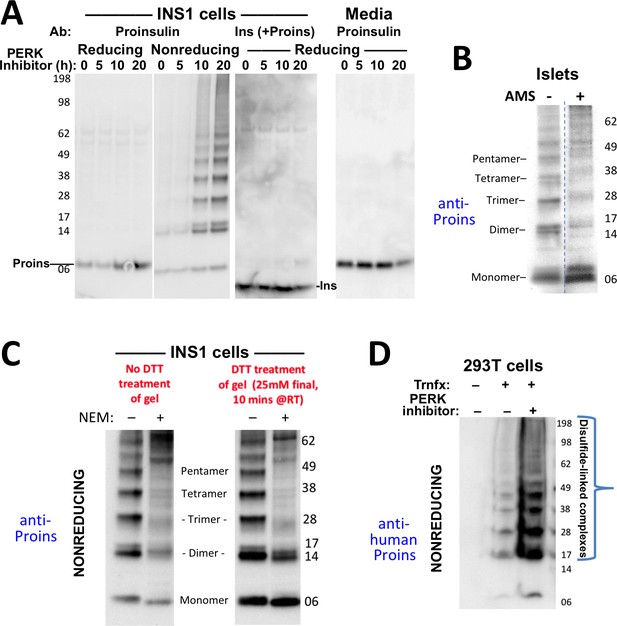
Formation of proinsulin disulfide-linked complexes.
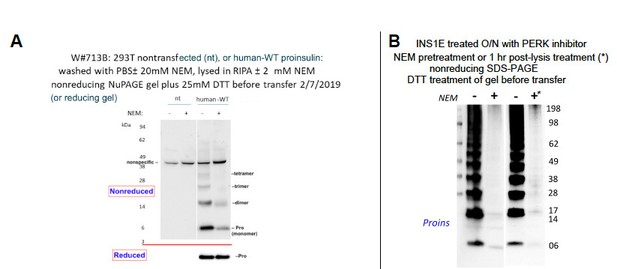
(A) INS1E cells were incubated for 20 hr in culture medium; the last 0, 5, 10, or 20 hr of this incubation included PERK inhibitor as indicated. At the end of the 20 hr, the media were collected and the cells were lysed; samples were resolved by nonreducing or reducing SDS-PAGE and electrotransferred to nitrocellulose. Panels 1, 2, and four were immunoblotted with mAb anti-proinsulin (CCI-17); panel three was immunoblotted with guinea pig anti-insulin. The positions of molecular mass markers are noted. (B) Murine pancreatic islets treated overnight with PERK inhibitor were lysed in SDS gel sample buffer under nonreducing conditions, and divided into two portions. One portion of lysate was incubated with 6 mM AMS for 1 hr, and then both portions were resolved by nonreducing SDS-PAGE, electrotransferred to nitrocellulose, and immunoblotted with mAb anti-proinsulin (CCI-17). The availability of free thiols results in proinsulin bands shifting in the AMS-treated lysate (right) compared to the untreated one (left). The positions of molecular mass markers are noted. (C). INS1E cells treated with PERK inhibitor overnight were washed with ice cold PBS either lacking or containing 20 mM NEM and lysed in the absence (lane 1) or presence of 2 mM NEM (lane 2). These samples were run on two halves of the same nonreducing SDS-PAGE; the right half-gel was incubated for 10 min with 25 mM DTT, while the left half-gel remained untreated. Both halves were then electrotransferred and immunoblotted with mAb anti-proinsulin. DTT treatment of the gel increased the signal strength of proinsulin monomers (less so for dimers). Conversely, alkylation greatly decreased the signal strength of disulfide-linked proinsulin oligomers but yielded a similar ratio of distinct proinsulin oligomeric species to that seen without alkylation. Molecular mass markers are indicated. (D) 293 T cells were either mock-transfected or transfected (‘Trnfx’) to express recombinant human proinsulin. Cells were treated overnight with vehicle (–) or PERK inhibitor before lysis, nonreducing SDS-PAGE, electrotransfer to nitrocellulose, and immunoblotting with mAb anti-human proinsulin (20G11). The positions of molecular mass markers are noted.

Proinsulin disulfide-linked complexes in human islets.
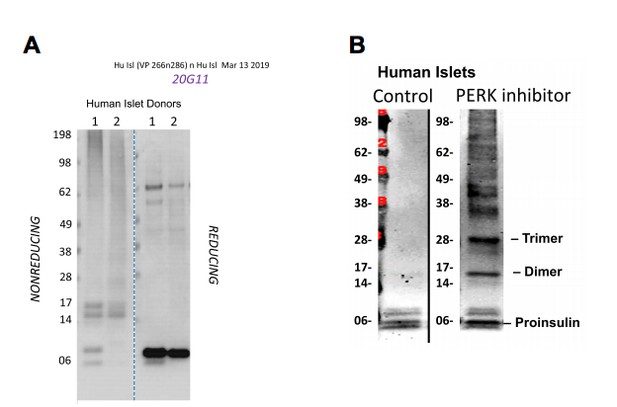
Lysates of human islets from two different donors were analyzed by nonreducing or reducing SDS-PAGE and processed as in panel C using mAb anti-human proinsulin (20G11).
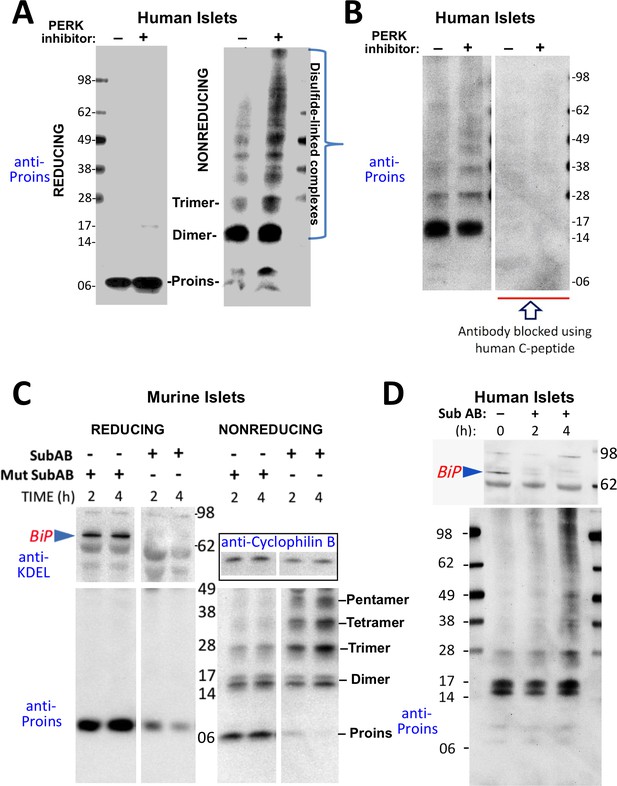
Misfolding of proinsulin in human (and rodent) pancreatic islets.
(A) Islets from humans not known to be diabetic (Prodo Labs) were treated overnight with vehicle or PERK inhibitor before lysis, reducing or nonreducing SDS-PAGE, electrotransfer to nitrocellulose, and immunoblotting for human proinsulin (mAb 20G11). The positions of molecular mass markers are noted. (B) Human islet lysates were immunoblotted with mAb anti-proinsulin (20G11)±purified human C-peptide as a blocking/competitor peptide (molecular mass markers indicated). (C) Mouse pancreatic islets were isolated and maintained in an overnight recovery medium including 11.1 mM glucose. The islets were then incubated with active SubAB (1 µg/mL final) or the same concentration of inactive mutant SubAA272B for 2 hr or 4 hr, as indicated. At each time point the islets were lysed and analyzed by reducing or nonreducing SDS-PAGE and immunoblotting with anti-KDEL (recognizing multiple ER resident proteins including BiP, as indicated), mAb anti-proinsulin (CCI-17, lower panels), and anti-cyclophilin B (loading control, boxed). Molecular mass markers are noted. (D) Islets from humans not known to be diabetic (obtained from the IIDP) were incubated with active SubAB (1.5 µg/mL final) for 0 hr, 2 hr or 4 hr, as indicated. At each time point the islets were lysed and analyzed by nonreducing SDS-PAGE and immunoblotting with anti-KDEL (upper panel) and mAb anti-proinsulin (20G11, lower panel). The positions of molecular mass markers are noted.
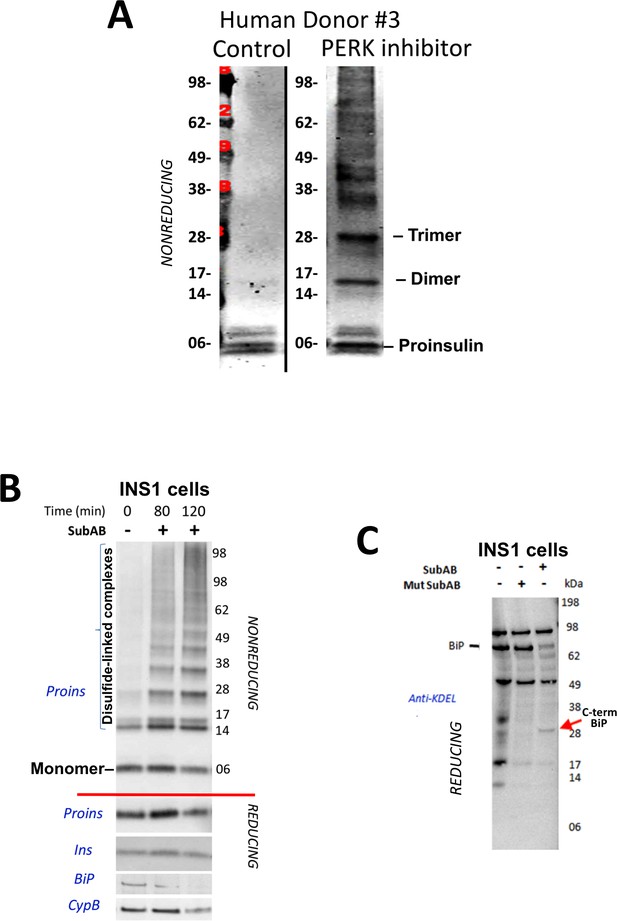
Proinsulin misfoding induced by perturbation of the ER folding environment.
(A) Inhibition of PERK promotes formation of proinsulin disulfide-linked complexes in human islets. Live islets from a human donor were incubated overnight in the absence of presence of PERK inhibitor (GSK2656157, 2 µM) and analyzed by nonreducing SDS-PAGE, electrotransfer to nitrocellulose, and immunoblotting with mAb anti-human proinsulin (20G11). (B) Loss of intact BiP promotes rapid formation of proinsulin disulfide-linked complexes. INS1E cells were either control or incubated for the indicated short periods with SubAB toxin before cell lysis and immunoblotting for the antigens shown. Proins = proinsulin (below the red line show samples analyzed under reducing conditions in which all proinsulin migrates as a monomer). Ins = insulin; BiP = GRP78; CypB = Cyclophilin B (loading control). (C) Cleavage of BiP by SubAB. INS1E cells incubated without or with SubAB toxin were immunoblotted with anti-KDEL. Loss of intensity of the intact BiP band (~78 kD) coincided with the appearance of a ~ 28 kD BiP fragment (‘C-term BiP’) as has been noted previously (Paton et al., 2006).
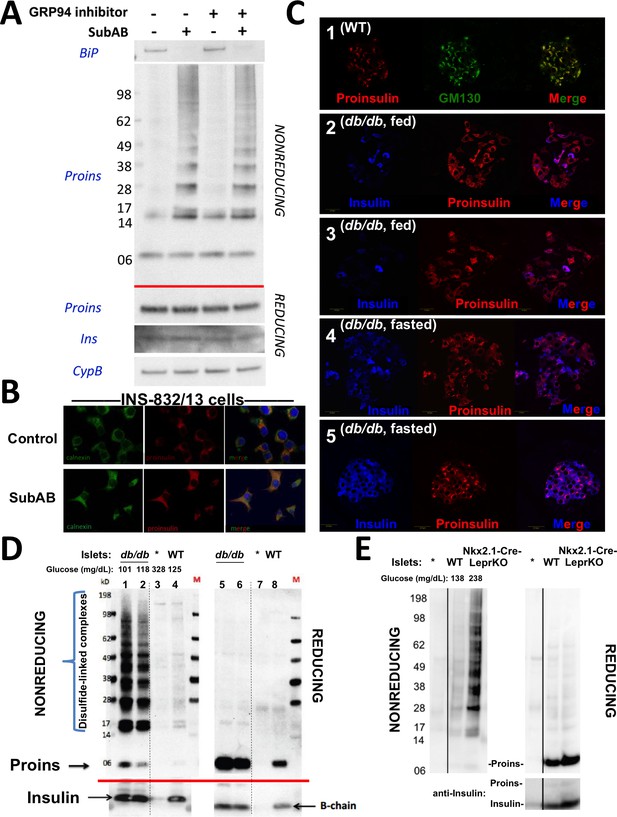
Improper proinsulin folding from pharmacological or physiological alteration of the ß-cell ER folding environment.
(A) INS1E cells were incubated for 24 hr ± 20 µM GRP94 inhibitor (PU-WS13). During the last 3 hr, SubAB was added where indicated. Cell lysates were analyzed by immunoblotting for BiP (top panel), and mAb anti-proinsulin (CCI-17) under nonreducing conditions (above red line) or with anti-proinsulin (Proins), anti-insulin (Ins), and anti-cyclophilin B (CypB, loading control) under reducing conditions (below red line). The positions of molecular mass markers are noted. (B) INS832/13 incubated ±active SubAB (1.5 μg/mL, 4 hr) were processed for immunofluorescence with rabbit anti-calnexin (green) or mAb anti-proinsulin (red). (C) Sections of wild-type or LepRdb/db mouse pancreas (C57BL/6) were de-paraffinized and prepared for indirect immunofluorescence. 1) Wild-type islets immunostained with mAb anti-proinsulin (CCI-17, in red), or the Golgi complex labeled with mab anti-GM130 (in green). 2–5) LepRdb/db mice with random blood glucose >500 mg/dL, as follows. 2 and 3) Mice fed ad lib; immunostained for insulin (blue) and mAb anti-proinsulin (CCI-17, red). 4 and 5) Mice fasted overnight, and immunostained as above. The third panel in each case is a merged image of the single-channel fluorescence. (D) Isolated islets from young male LepRdb/db mice (lanes 1, 2, 5 and 6; random blood glucose values shown above) or wild-type C56BL/6 (‘WT’, lanes 4, 8) or a high-fat fed Ins1+/-,Ins2-/- male (lanes 3 and 7, marked with asterisk) were lysed in RIPA buffer and analyzed by nonreducing or reducing SDS-PAGE and immunoblotting with mAb anti-proinsulin (CCI-17, above red line) or guinea pig anti-insulin (below red line). The positions of molecular mass markers are noted. (E) Isolated islets from WT and Nkx2.1-Cre-mediated LepR-KO mice (random blood glucose values shown above) were lysed in RIPA buffer and analyzed under nonreducing or reducing conditions as in panel D. Molecular mass markers are noted. Asterisk denotes lysate from Ins1+/-,Ins2-/- islets as in panel D. The positions of molecular mass markers are noted. Islet lysates from WT and Nkx2.1-Cre-mediated LepR KO mice were also immunoblotted with guinea pig anti-insulin (bottom right) that weakly cross reacts with proinsulin (Proins).
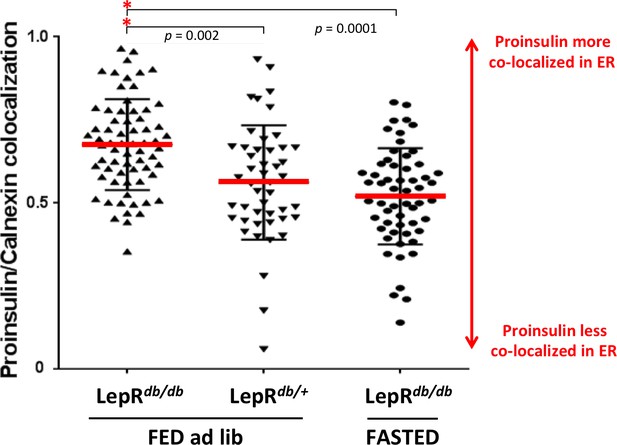
Intracellular proinsulin distribution in the LepRdb/db mouse.
Sections of homozygous diabetic LepRdb/db or heterozygous LepRdb/+ (control) mouse pancreas from conditions of Figure 4C (fed ad lib or fasted overnight) were immunostained with mAb anti-proinsulin (CCI-17 as in Figure 4C) and rabbit polyclonal anti-calnexin (ER marker) using two-color immunofluorescence. Proinsulin-positive cells were examined for extent of proinsulin co-localization with the ER using by Manders’ method using ImageJ software analyzed with ‘Coloc2’ algorithm. Statistical analyses were performed with GraphPad Prism (version 7.00) using one-way ANOVA (Kruskal-Wallis) with Dunn’s multiple comparison test to compare the sum of ranks. Data are individual pancreatic ß-cells with mean plus standard deviation (SD) with p values shown.
-
Figure 4—figure supplement 1—source data 1
- https://doi.org/10.7554/eLife.44532.011
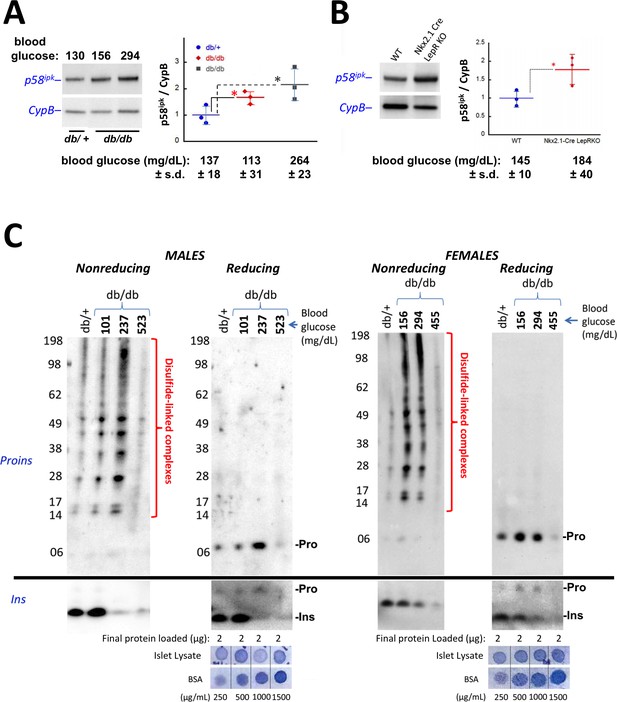
Accumulation of improperly folded proinsulin and detection of ER stress response are early events in the development of diabetes in leptin receptor-deficient mice.
(A) Islet lysates from LepRdb/+ heterozygote (control) or LepRdb/db mice at different stages of diabetes progression (random blood glucose values shown above) were immunblotted for p58ipk and cyclophilin B (CypB, loading control); the graph at right shows the quantitation of the p58ipk / CypB ratio from three independent experiments (mean ± s.d., each point a different animal; asterisk, p=0.05 by Mann Whitney U test and p<0.05 by t-test). (B) Islet lysates from WT and Nkx2.1-Cre-mediated LepR-KO mice were immunblotted for p58ipk and CypB as in panel A; the graph at right shows quantitation of the p58ipk / CypB ratio from three independent experiments (mean ± s.d., each point a different animal; p=0.05 by Mann Whitney U test and p<0.05 by t-test). (C) Islet lysates from LepRdb/+ heterozygote (control) or LepRdb/db mice at different stages of diabetes progression (random blood glucose values shown above; blood glucose in LepRdb/+male was 138 mg/dL and in female was 117 mg/dL) were analyzed by nonreducing or reducing SDS-PAGE and immunoblotting with mAb anti-proinsulin (CCI-17, above black line; molecular mass markers are noted) or guinea pig anti-insulin (below black line). Islet protein content from each mouse was measured (Bramhall et al., 1969) relative to known BSA standards shown at bottom; 2 µg islet protein was analyzed for each sample. Left sets of gels are from males; right sets of gels are from females, as indicated. In LepRdb/db mice, intra-islet abundance of misfolded disulfide-linked proinsulin complexes reached maximum with random blood glucoses ranging from 150 to 300 mg/dL but this was accompanied by a decline of intra-islet mature insulin levels. Overtly diabetic animals (random blood glucose >450) exhibited low insulin levels and ultimately exhibited low proinsulin levels as well.
-
Figure 5—source data 1
- https://doi.org/10.7554/eLife.44532.014
-
Figure 5—source data 2
- https://doi.org/10.7554/eLife.44532.015
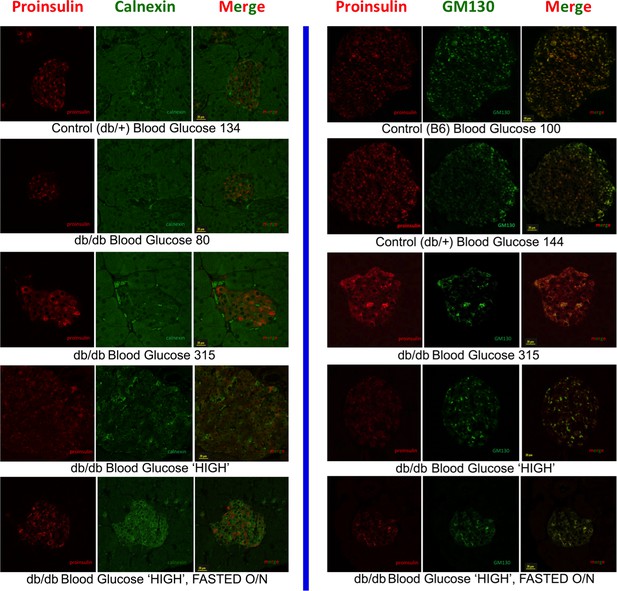
Intracellular proinsulin distribution in the LepRdb/db mouse.
Left side: Double immunofluorescence with mAb anti-proinsulin (red) and rabbit anti-calnexin (green) in islets of heterozygous LepRdb/+ (control) and homozygous LepRdb/db mice with various levels of blood sugar indicative of worsening diabetes. The cytoplasmic proinsulin distribution tended to broaden towards the ER marker as a function of worsening glycemic control. The bottom panel involves fasting overnight as in Figure 4C. Right side: Double immunofluorescence with mAb anti-proinsulin (red) and mouse anti-GM130 (green, using sequential blocking protocol) in islets of C57BL/6 (B6, control), heterozygous LepRdb/+ (control), and homozygous LepRdb/db mice with various levels of blood sugar indicative of worsening diabetes. Under normoglycemic conditions, proinsulin tended to distribute more strongly in the Golgi region, with some apparent recovery of this distribution also seen in the islet ß-cells of frankly diabetic animals after overnight fast (bottom panel).
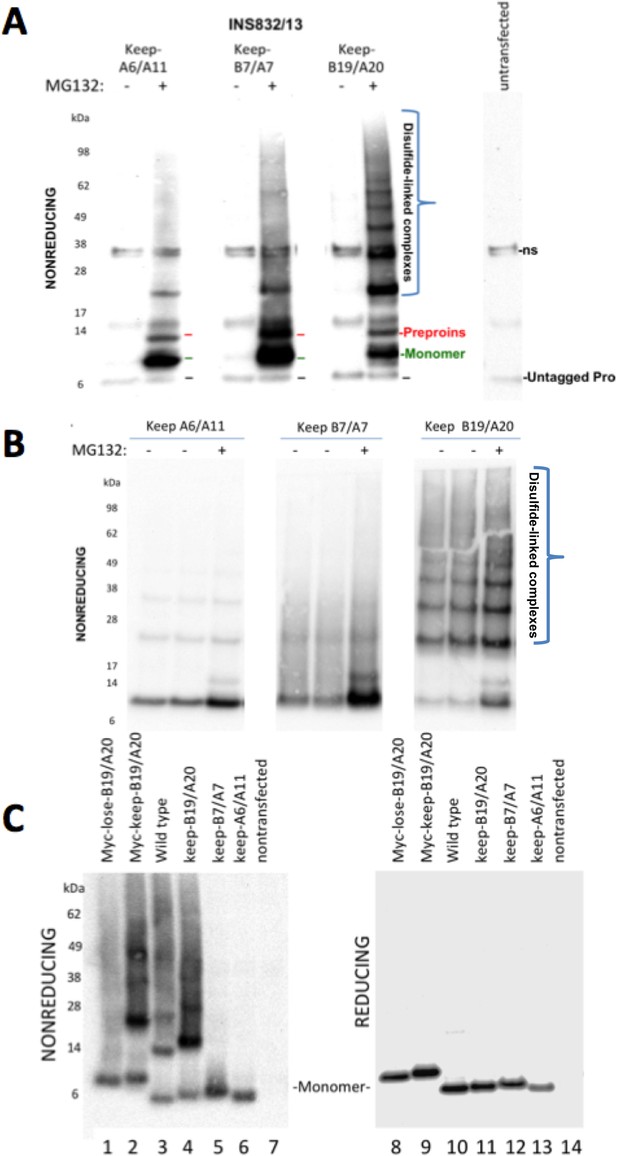
Proinsulin Cys residues that contribute to covalent complex formation.
(A) INS832/13 were transfected to express myc-tagged recombinant human proinsulin ‘keep-A6/A11’, ‘keep-B7/A7’, or ‘keep-B19/A20’ constructs. At 48 hr post-transfection, cells were treated with vehicle alone (–) or MG132 (10 µg/mL) for 7 hr before cell lysis and analysis by nonreducing SDS-PAGE and immunoblotting with mAb directed against a sequence (PLALEGSLQKRGIV) spanning the junction of the proinsulin C-peptide and insulin A-chain. A control of mock-transfected cells (far right lane) is shown for comparison; ‘ns’=nonspecific band. The positions of molecular mass markers are noted. (B) 293 T cells were transfected to express the same constructs and treated and analzyed as in panel A. The positions of molecular mass markers are noted. (C) 293 T cells were transfected to express the same constructs as in panel A, or myc-tagged ‘lose-B19/A20’, myc-tagged ‘keep-B19/A20’, and untagged wild-type human proinsulin. Cell lysates were resolved by SDS-PAGE under nonreducing and reducing conditions and analyzed as in panel A; molecular mass markers are noted.
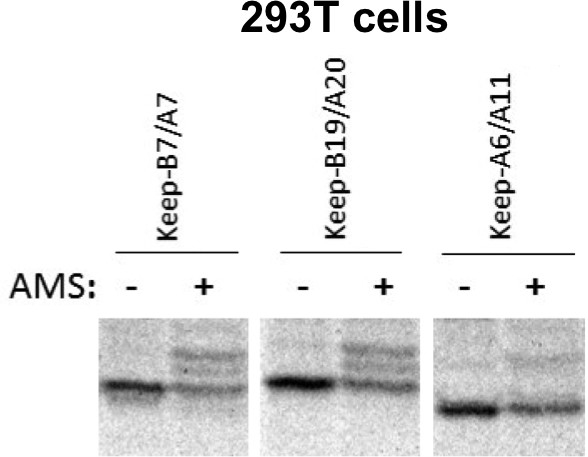
Free thiols in recombinant proinsulin mutants.
293 T cells transiently transfected to express recombinant myc-tagged proinsulin mutants known as keep-B7/A7, keep-B19/A20, or keep-A6/A11 were metabolically labeled with 35S-amino acids for 30 min, lysed in RIPA buffer, immunoprecipitated with anti-myc, alkylated with 40 mM AMS for 1 hr at room temperature, and then boiled in SDS-gel sample buffer containing 200 mM DTT. The gels, analyzed by autoradiography, demonstrate that each of the 2-Cys proinsulin mutants has available free thiols that can react with the alkylating agent, causing upward band shift.
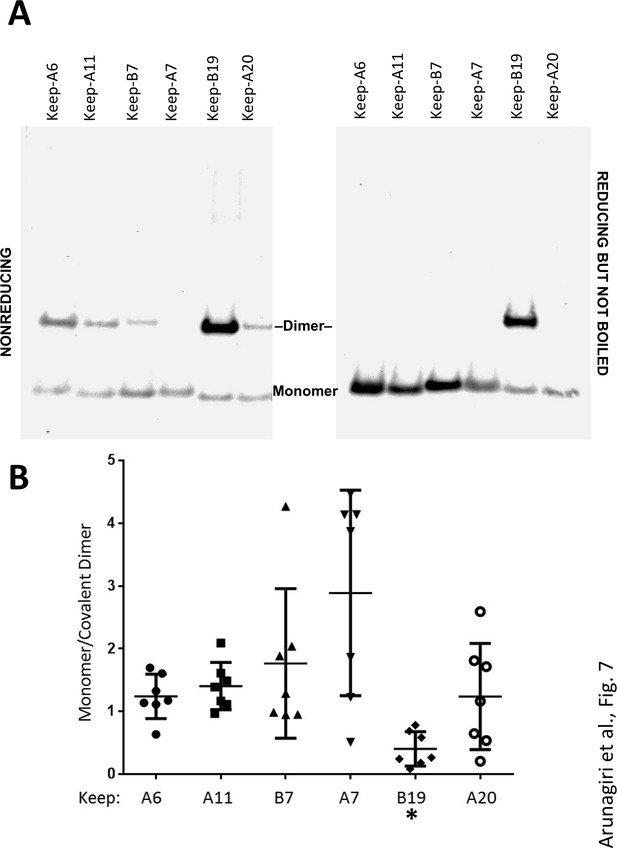
Proinsulin intermolecular disulfide crosslinking is promoted by Cys(B19).
(A) 293 T cells were transfected to individually express six distinct human proinsulin mutants bearing only one cysteine. At 28 hr post-transfection, cell lysates were analyzed either by nonreducing SDS-PAGE (left) or incubated in SDS-gel sample buffer plus 200 mM DTT at room temperature for 10 min prior to SDS-PAGE. The gels were identically electrotransferred to nitrocellulose, and immunoblotted for proinsulin as in Figure 6. (B) Seven independent experiments like that shown in the left panel of Figure 7A were quantified for monomer to covalent dimer ratio: keep-A7 was mostly monomeric whereas keep-B19 predominantly formed covalent dimers (asterisk signifies p<0.05 when compared to each keep mutant except keep-A20).
-
Figure 7—source data 1
- https://doi.org/10.7554/eLife.44532.020
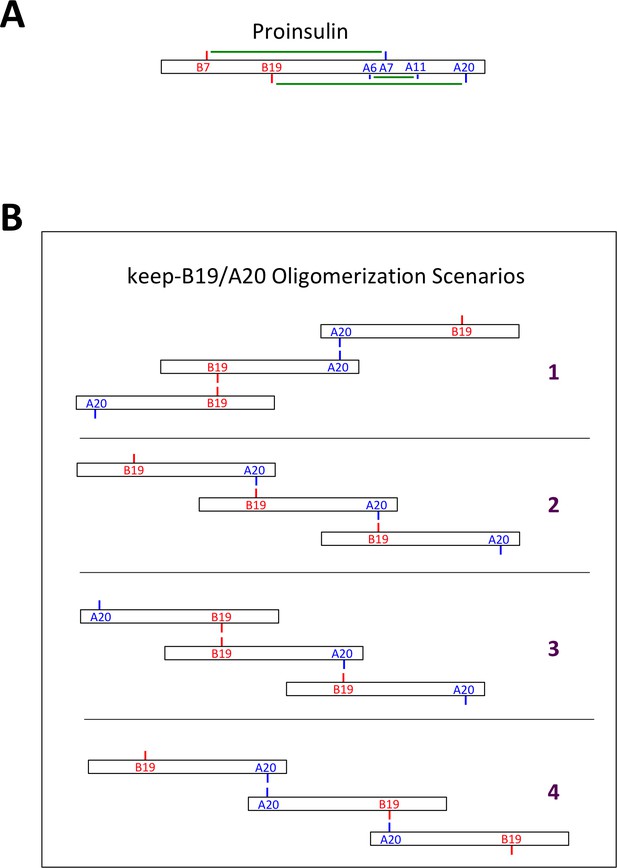
Native and non-native proinsulin disulfide pairing.
(A) Native intramolecular disulfide bonding of proinsulin. Native disulfide bonds of proinsulin are indicated schematically as green lines connecting cysteine residues of the insulin B-chain (in red) or A-chain (in blue). (B) Some possible scenarios of intermolecular disulfide bonding of proinsulin mutant keep-B19/A20. Possible intermolecular disulfide-linked oligomerization involving homotypic B19-B19 and A20-A20 interactions (scenario 1), or heterotypic B19-A20 interactions (scenario 2), or combinations of homotypic and heterotypic interactions (scenarios 3 + 4). Terminal Cys residues of disulfide-linked proinsulin oligomers exist as free thiols that can propagate into larger disulfide linked complexes, or could form mixed disulfides with ER oxidoreductases.
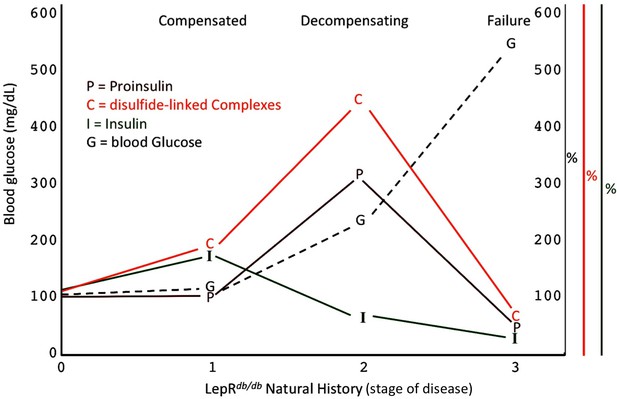
Islet dysfunction during the natural history of diabetes in the LepRdb/db mouse, as a model.
A schematic is shown, indicating progression of early islet dysfunction during the natural history of diabetes in the LepRdb/db mouse. In the first stage of postnatal life, random blood glucose is in the normal range and insulin content in islets is actually slightly greater than that seen in the control condition. Total proinsulin levels are not diminished, but there is a slight increase in proinsulin disulfide-linked complexes. At the second stage, random hyperglycemia (150–300 mg/dL) is observed, accompanied by islet insulin levels that are less than that seen in the control condition. However, total proinsulin levels are notably increased, accompanied by an increase in proinsulin disulfide-linked complexes. At a third stage is a worsening of random hyperglycemia (>450 mg/dL), accompanied by low islet insulin and low islet proinsulin, which has been attributed to ß-cell dedifferentiation, or ß-cell death, or could potentially represent a combination of both.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.44532.022





