Dichotomous organization of amygdala/temporal-prefrontal bundles in both humans and monkeys
Figures
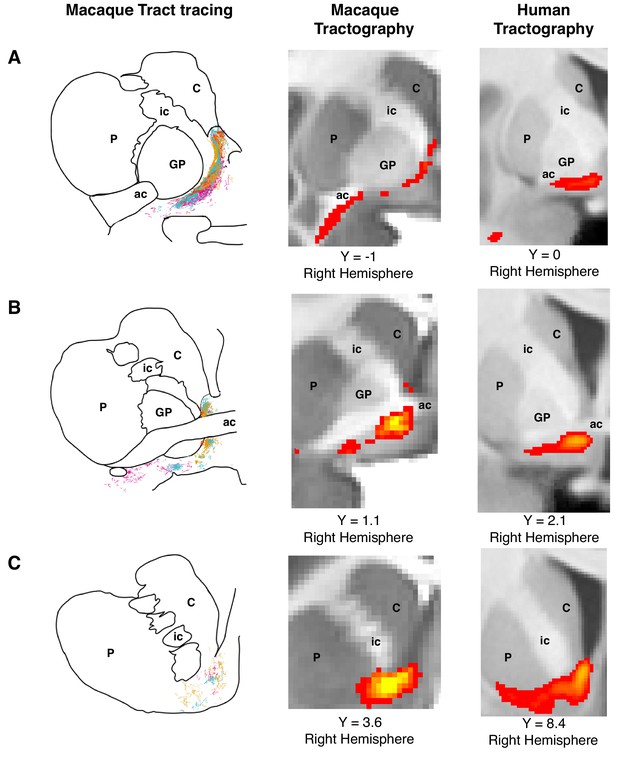
Histology-informed approach for the reconstruction of the frontal limb of the ventral amygdalofugal pathway.
(A-C, left and middle panels) Coronal sections depicting reconstructions of the frontal limb of the amygdalofugal pathway (AmF) in the macaque brain using probabilistic tractography based on diffusion MRI data (middle panels) and published reconstructions of the tract using radio-labeled fibers identified using tract-tracing injections in macaque (left panels; adapted with permission from Springer Nature (Oler et al., 2017); These panels are not available under CC-BY and are exempt from the CC-BY 4.0 license.) The AmF tractography reconstruction showed marked correspondence in anatomy and in the course of the fibers with the histological tracing data. AmF fibers leave the amygdala caudal to the anterior commissure (ac) and turn medially towards the medial wall of the hemisphere (A) and rostrally towards the frontal lobe by coursing ventrally to the ventromedial striatum (B–C). (A-C, right panels) The histology-informed reconstruction of the AmF was then used to guide the tractographic algorithm in the human brain, including when the seed, exclusion and waypoint masks were used in both species (see Materials and methods for details on the masks). The human AmF follows a course matching the results observed in the macaque brain (A-C, middle panels) with fibers projecting both medially (A) and rostrally (B–C). T1-weighted images are shown in radiological convention. ac, anterior commissure; C, caudate nucleus; GP, globus pallidus; ic, internal capsule; P, putamen.
© 2017 Springer Nature. All Rights Reserved. Figure 1A–C (left panels) is adapted with permission from Springer Nature (Oler et al., 2017).
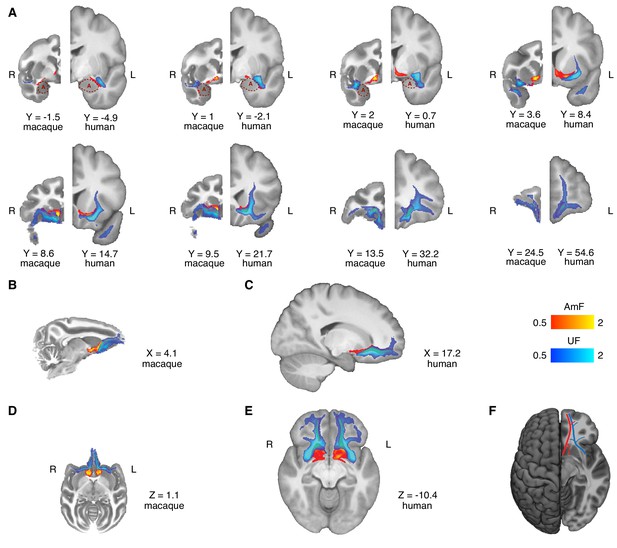
Anatomy and organization of the amygdalofugal pathway and uncinate fascicle in the basal ganglia and frontal lobe of the macaque monkey and human brain.
(A) The amygdalofugal pathway (AmF, red) and uncinate fascicle (UF, blue) are displayed on eight coronal brain slices. AmF and UF show the same medial-lateral organization in both macaque (left) and human (right) brain. (B–C) Sagittal view of the macaque (B) and human (C) brains displaying the preserved dorsal-ventral and medial-lateral organization of the two tracts across both species in caudal subcortical regions of the brain and their mergging in caudal orbitofrontal cortex white matter (WM). (D–E) The medial-lateral organization of the two tracts is visible on axial sections of macaque (D) and human (E) brains. The AmF follows a course medial to the UF with its fibers reaching the full extent of the ventral striatum and nucleus accumbens in both macaques (D) and humans (E). Here, the main body of the UF runs more laterally, primarily in the WM adjacent to the ventral-lateral striatum and the insular cortex. The tracts merge into an intermingled connectional system in the posterior OFC (area 13) WM and across the full extent of the ventromedial prefrontal cortex. Macaque coordinates are in F99 space and human coordinates are in MNI space. (F) Schematic template representing the organization of AmF (red) and UF (blue) in the ventral prefrontal cortex in the human brain. The dashed line (red) shows a bundle subcortical AmF fibers running underneath and through the ventral striatum.
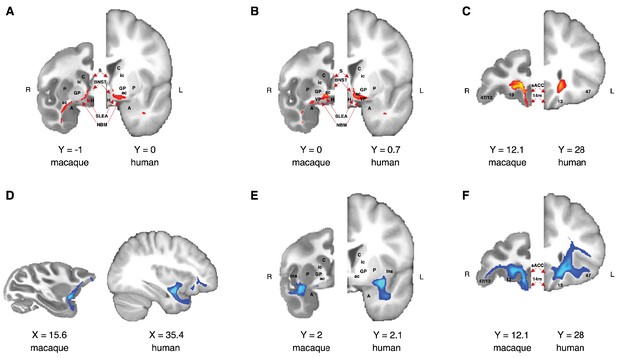
Comparison of the anatomical architecture of the ventral amygdalofugal pathway and uncinate fascicle in macaque monkey and human brains.
(A–C) Anatomy of macaque and human amygdalofugal pathway on three coronal slices showed from caudal (A) to rostral (C). (D–F) Anatomy of macaque and human uncinate fascicle in a sagittal slice and in two coronal slices showed from caudal (E) to rostral (F). Macaque coordinates are in F99 space and human coordinates are in MNI space. A, amygdala; BNST, bed nucleus of stria terminalis; H, hypothalamus; Ins, insular cortex; NBM, nucleus basalis of Meynert; sACC, subegnual anterior cingulate cortex (Brodmann area 25); S, septum; SLEA, sublenticular extended amygdala; VP, ventral pallidum; all other labels as in Figure 1 and Figure 4. Tractograms are thresholded with minimum and maximum values equal to 0.5 and 2, respectively.
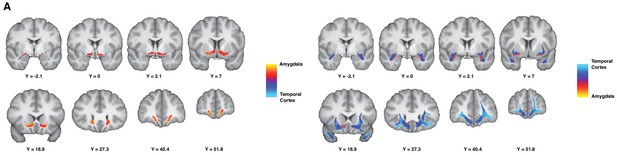
Gradient organization of ATR-prefrontal fibers.
White matter gradients showing the organization of fibers coursing between ATR and prefrontal areas. In the left panel, we represent the connectivity gradient ratio of the AmF bundle, indexing the relative probability of AmF streamlines originating in either the amygdala (red-yellow) or the temporal cortex (blue). The color scale represents the full range of the gradient ratio, from solely originating in the amygdala (yellow), to solely originating in the temporal cortex (light blue). In the right panel, we represent the same gradient ratio for the UF bundle.
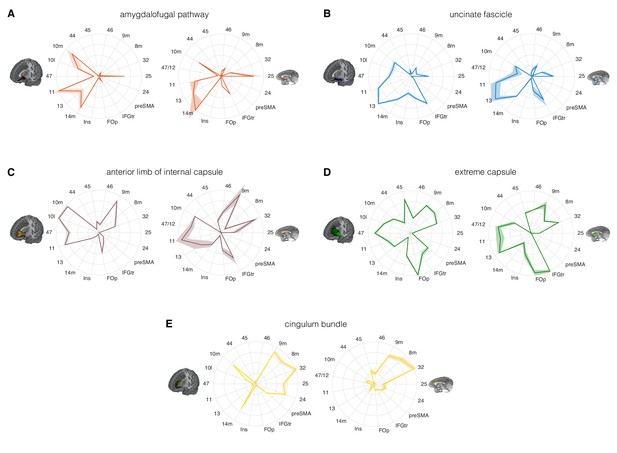
Comparison of the connectivity fingerprints of the amygdalofugal pathway, uncinate fascicle and adjacent white matter tracts in the human (left) and macaque (right) frontal lobe.
(A) The pattern of AmF fibers is preserved across the two species and predominantly targets medial areas in subgenual, orbital, ventral, and polar PFC. (B) Overall, UF axons show high similarity between the two species, but stronger innervation to the opercular and insular cortex is observable in the human UF. (C–E) Macaque–human comparison of the prefrontal fibers organization of three connectional systems located in the white matter dorsal to the AmF (internal capsule; C), dorsal to the UF (extreme capsule; D) or interacting with both tracts in the infralimbic and prelimbic cortex (cingulum bundle; E). 9 m, dorsomedial PFC; 8 m, caudal bank of the dorsomedial PFC; 32, anterior cingulate cortex; 25, subgenual cingulate cortex; 24, dorsal anterior cingulate cortex; preSMA, pre-supplementary motor area; IFGtr, inferior frontal gyrus, pars triangularis; FOp, frontal operculum; Ins, insular cortex; 14 m, ventromedial PFC; 13, caudal orbitofrontal cortex; 11, rostral orbitofrontal cortex; 47 (human) or 47/12 (macaque) lateral orbitofrontal cortex (also named lateral convexity in Murray and Rudebeck, 2018 and in Rudebeck and Murray, 2014); 10 l, lateral frontopolar cortex; 10 m, medial frontopolar cortex; 44, caudal bank of the ventrolateral PFC; 45, rostral bank of the ventrolateral PFC. Shades represent standard error of the mean (SEM).
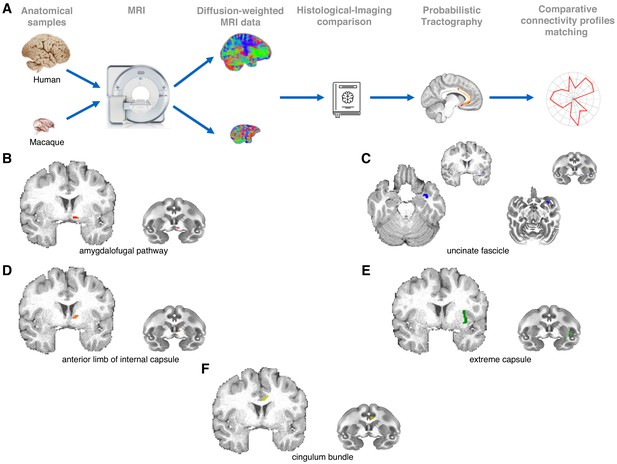
Methodological pipeline and white matter seed masks used to reconstruct each pathway.
(A) We combined high-quality, high-resolution diffusion imaging data from in-vivo human (n = 24) and post-mortem monkey (Macaca mulatta; n = 3) brains with probabilistic tractography analyses to reconstruct the course and connectivity pattern of two tracts connecting amygdala and anterior temporal cortex with the frontal lobe: the ventral amygdalofugal pathway and the uncinate fasciculus. For comparison, tractographic analyses were also run on three other limbic-PFC tracts: the anterior limb of the internal capsule, the extreme capsule, and the cingulum bundle. Anatomical reconstruction of the five tracts was made by reference to previously published atlases (Nieuwenhuys et al., 2008; Paxinos et al., 2000), tract-tracing studies (Fudge et al., 2002; Oler et al., 2017; Schmahmann and Pandya, 2006) and diffusion tractography studies (Catani et al., 2013; Croxson et al., 2005). We aimed to keep all seeding and tracking parameters constant across tracts and species. To constrain the tractography algorithm, a priori anatomical priors were used for the analyses, and homologous tract-specific exclusion masks were used in both species in order to obtain high fidelity results. (B–F) White matter seed masks were used to reconstruct the ventral amygdalofugal pathway (B), the uncinate fascicle (C), the internal capsule (D), the extreme capsule (E) and the cingulum bundle (F). All seeds are represented for the left hemisphere in both species.
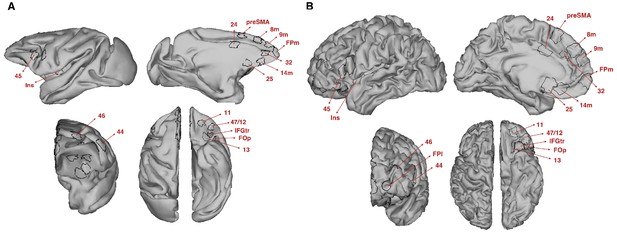
Target regions of interest used to estimate the prefrontal connectivity fingerprint for each tract.
(A–B) Regions of interest (ROI) were created on the cortical surface of macaque ((A); F99 space) and human ((B); MNI space) brains. Each ROI was then projected to the volumetric gray matter/white matter border and used for the connectivity fingerprint analyses. All ROI labels are as in Figure 4.
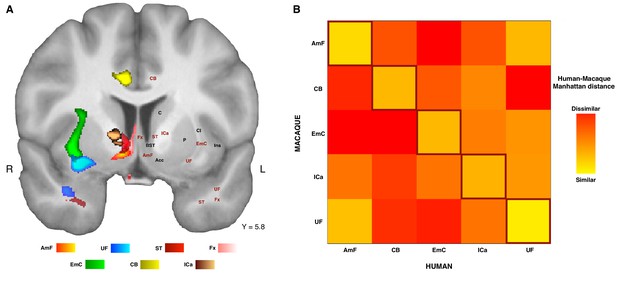
Organization of ATR-prefrontal tracts.
(A) Topographic organization of the AmF and UF with respect to other urrounding WM tracts. Despite the close proximity of these five bundles, they occupy different portions of white matter and can be dissociated from one another. (B) Dissimilarity matrix of the prefrontal course of the tracts of interest across species. The matrix shows a quantitative comparison of the connectivity fingerprints of the five frontal tracts that were reconstructed in both the human and the monkey brains. Human tracts are listed on the horizontal axis; macaque tracts are listed on the vertical axis. Pair-wise dissimilarity is defined as the ‘Manhattan distance’ between each pair of pathways across the two species. The greatest similarity was found when comparing each tract with its homolog in the other species (bright diagonal in the dissimilarity matrix). AmF, ventral amygdalofugal pathway; CB, cingulum bundle; ICa, anterior limb of the internal capsule; EmC, extreme capsule; UF, uncinate fascicle; ST, Stria Terminalis; Fx, Fornix.

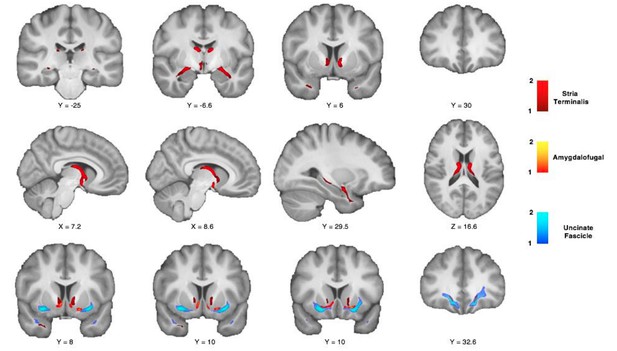
White matter seed masks for two limbic control tracts in the human brain.
(A–B) White matter seed masks used to reconstruct the stria terminalis (A) and fornix (B) in the human brain. As for the other tracts reconstructed in this article, seeds are represented for the left human hemisphere.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.47175.011





