In vivo imaging with a water immersion objective affects brain temperature, blood flow and oxygenation
Figures
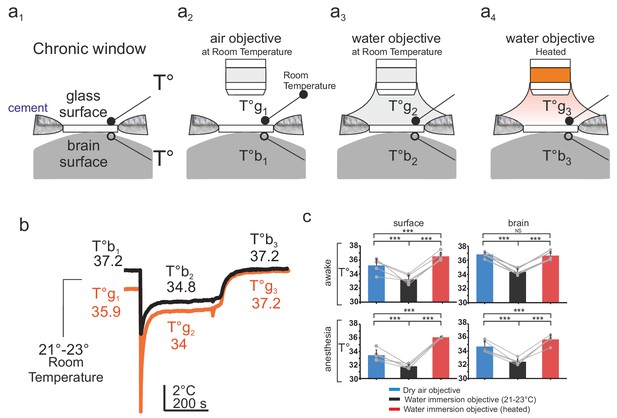
Brain temperature in different two-photon imaging conditions.
(a1–a4), Schematics of 4 imaging configurations. (a1) Two thermosensors were positioned above and below the glass of the chronic window. 2P imaging was performed with (a2) an air 60x air objective (NA = 0.7) at room temperature (21–23°C), (a3) a 60x water immersion objective (NA = 1.1) at 21–23°C, (a4) a 60X heated water immersion objective. (b) In the absence of water, resting temperature is higher at the brain surface (T°b1) than at the glass surface (T°g1), indicating constant energy loss. The water immersion objective used at room temperature causes a transient drop of temperature at both sites (T°b2, T°g2) which stabilize with 5 min. Heating the objective restores brain and glass surface temperature to ~37°C (T°b3, T°g3). (c) 1-D scatter plots showing average glass and brain temperature values measured in all conditions in the same five mice (Data presented as mean ± s.e.m; Wilcoxon signed rank test; *** for p<0.001, NS: non significant). Note that in awake animals, brain temperature is physiological at rest and can be restored upon heating the water immersion objective.
-
Figure 1—source data 1
Temperature with two objectives.
- https://doi.org/10.7554/eLife.47324.004
Objective heating system.
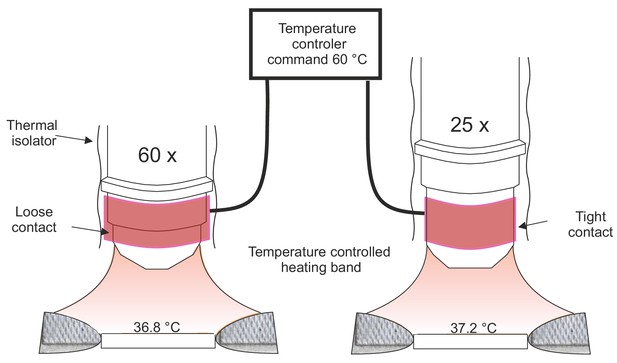
Left, the objective heater consists in a heating band with a temperature feedback connected to a temperature controller. In all experiments, the temperature command was set to the maximum value (60°C). In addition, a polyimide film (thermal isolator) was enwrapped around our 60X objective (NA = 1.1). Due to its shape, the objective was not in tight contact with the entire band width and the glass temperature could not reach ~37°C. Right, this was not the case with the large 25X objective (NA = 1.05), which straight shape ensured a tighter contact with the band and a higher glass temperature.
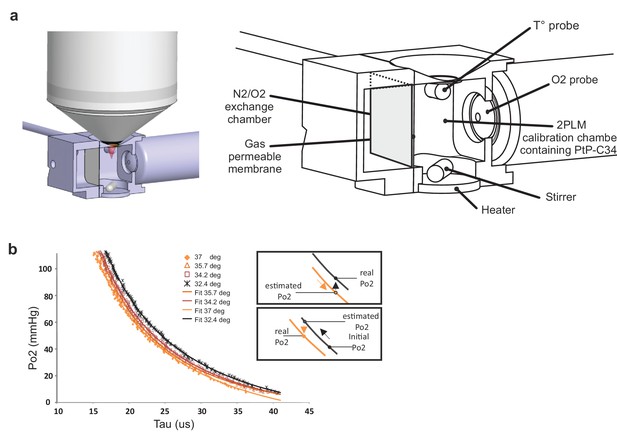
Calibration curves of PtP C343 at the temperatures measured below the glass window.
(a) 3D design of the calibration chamber (left) with an enlarged diagram (right). The gas exchange chamber communicated with the 2PLM calibration chamber through a gas permeable membrane. The temperature of the 2PLM calibration chamber was continuously controlled and recorded. The Po2 sensor (PtP-C343) solution was constantly homogenized by stirring. An amperometric oxygen probe was used to monitor Po2. Two-photon phosphorescence lifetime decays and Po2 were simultaneously acquired. The exchange chamber was initially filled with air and temperature was set at either 32.4°C, 34.2°C, 35.7°C or 37°C. Then, N2 was gradually substituted to air, and Po2 decreased slowly in the calibration chamber down to few mmHg. (b) The calibration curve shifted to the right with lowering the temperature. The two insets show how 2PM Po2 measurements must be corrected when lowering (Top) or increasing (Bottom) temperature.
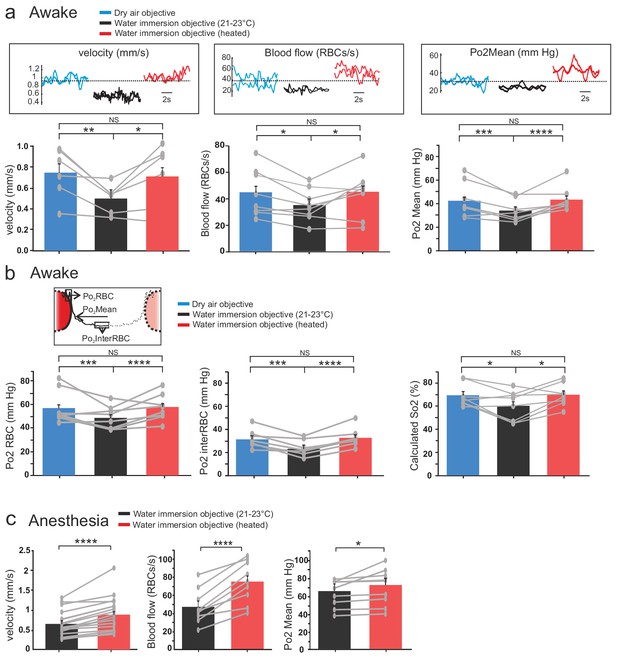
Effects of temperature on blood flow parameters and brain oxygenation in awake and anesthetized mice.
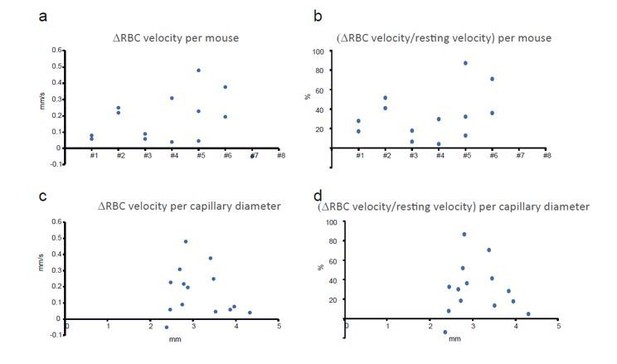
(a) In awake mice, imaging with a cool water immersion objective decreases capillary RBC velocity (left, n = 6 capillaries, three mice), flow (middle, n = 8 capillaries, four mice) and Po2 Mean (right, n = 8 capillaries, four mice). The effect is reversible upon heating the objective. Upper insets illustrate typical velocity, flow and Po2 measurements. (b) Inset, Schematic of EATs: Po2 at the RBC border (Po2 RBC), Po2 at distance from a RBC (Po2 InterRBC) which gives an estimate of pericapillary Po2, average Po2 in the capillary (Po2 Mean). 1-D scatter plots show that lowering temperature reversibly decreases Po2 RBC, ‘tissue’ Po2 and RBC saturation. Note that all Po2 values are calculated using calibrations curves acquired at the corresponding temperatures. (c) In mice anesthetized with ketamine/medetomidine and breathing air supplemented with oxygen (30%), resting Po2 is high but still increases upon heating the objective, brain temperature reaching ~35.7 C° and Po2 ~70 mmHg (velocity, n = 18 capillaries, eight mice; blood flow and Po2, n = 9 capillaries, three mice). See Materials and methods for the statistical tests. *, **, ***, **** for p<0.05, 0.01, 0.001 and 0.0001 respectively, NS: non significant.
-
Figure 3—source data 1
Blood flow parameters in awake mice.
- https://doi.org/10.7554/eLife.47324.007
-
Figure 3—source data 2
Blood flow parameters in anesthetized mice.
- https://doi.org/10.7554/eLife.47324.008
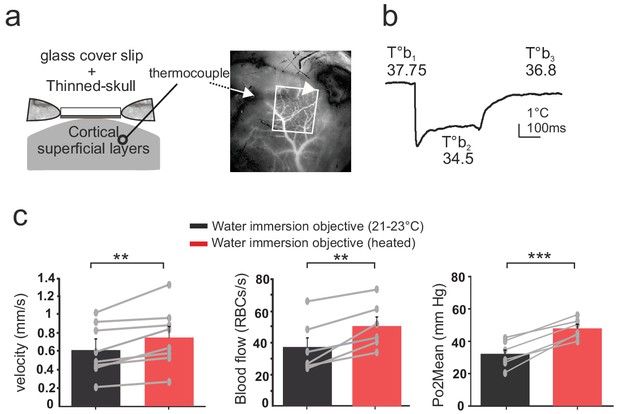
Effects of temperature in the barrel cortex of awake mice imaged through a reinforced thinned skull.
(a) Left, schematics of the preparation. The thermosensor was placed in the cortex superficial layers (II to IV). Right, the sensor core and tips (white arrow and arrow head, respectively) are observable as a dark shadow below the bone and the surface vessels, that were injected with texas red and imaged through the reinforced thinned-skull with a stereoscope. (b) Imaging with the cool water immersion objective causes a decrease of brain temperature (T°b2), which partially recovers upon heating the objective (T°b3). (c) Resting Po2, blood flow and velocity increase upon heating the objective (velocity, n = 8 capillaries, four mice; blood flow and Po2, n = 6 capillaries, three mice). See Materials and methods for the statistical tests. **, *** for p<0.01, 0.001, respectively.
-
Figure 4—source data 1
Thinned-skull.
- https://doi.org/10.7554/eLife.47324.010
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.47324.011