An autoregulatory cell cycle timer integrates growth and specification in chick wing digit development
Figures
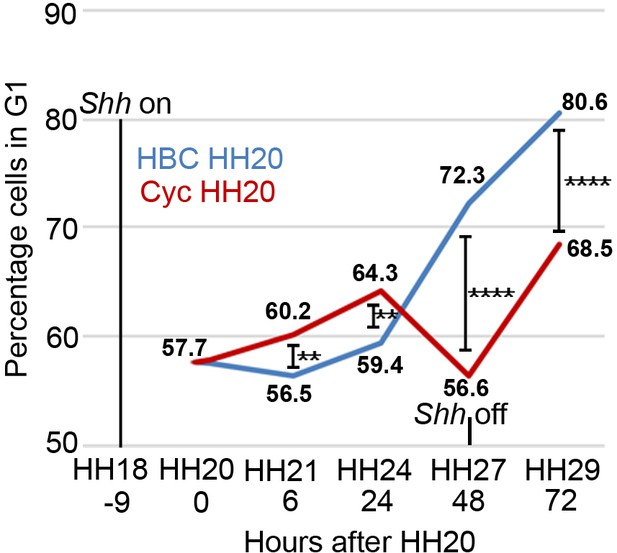
Shh signalling influences polarising region proliferation.
In normal development (blue line - HBC carrier control) the percentage of polarising region cells in G1-phase of the cell cycle slightly decreases over 6 hr between HH20 and HH21 and then increases throughout wing outgrowth (Note, Shh expression terminates at HH27/28 and data and statistics taken from Chinnaiya et al., 2014; Pickering and Towers, 2016. In all cases, between 10 and 14 polarising regions were dissected and data were obtained from 5 to 10,000 cells). The inhibition of Shh signalling using cyclopamine/HBC at HH20 (red line) increases the percentage of G1-phase cells over 24 hr until HH24, then there is a sharp decrease over the next 24 hr until HH27 when Shh expression normally terminates. Over the next 24 hr until HH29, G1-phase cell numbers recover in posterior mesenchyme after Shh expression has terminated (unpaired Pearson’s χ2 test **- p =< 0.01, and ****- p =< 0.0001; see Chinnaiya et al., 2014; Pickering and Towers, 2016).
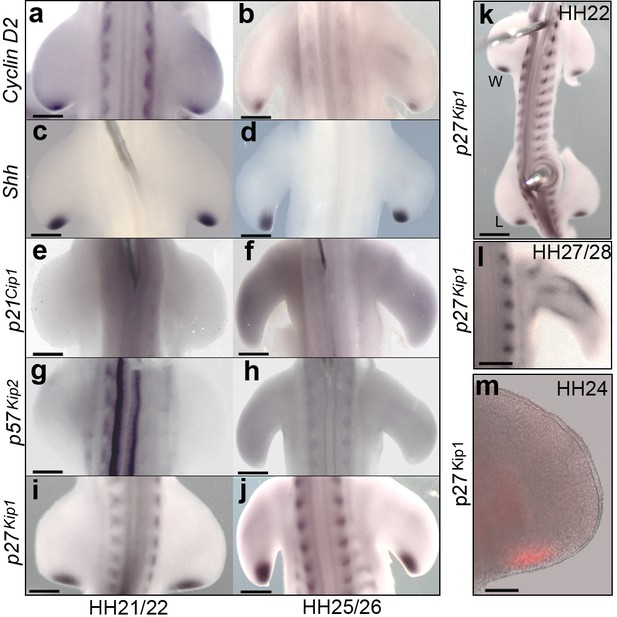
The polarising region expresses G1-S phase regulators.
Polarising region cells express Cyclin D2 and Shh at HH21/22 (a, c) and at HH25/26 (b, d); but not p21cip1 and p57kip2 (e-h). Polarising region cells express p27kip1 at HH21/22 (i) and HH25/26 (j). p27kip1 is also expressed in the leg (L) polarising region at HH22, as well as in the wing (W) (k), and in differentiating myogenic cells in the wing at HH27/28 (l). p27kip1 protein is present in the polarising region at HH24 (m, n = 3/3). Note, gene expression patterns were observed in all embryos analysed (n => 12). Scale bars: a, c, e, g, i, l – 150 μm; b, d, f, h, j – 300 μm; k – 250 μm; - m – 75 μm.
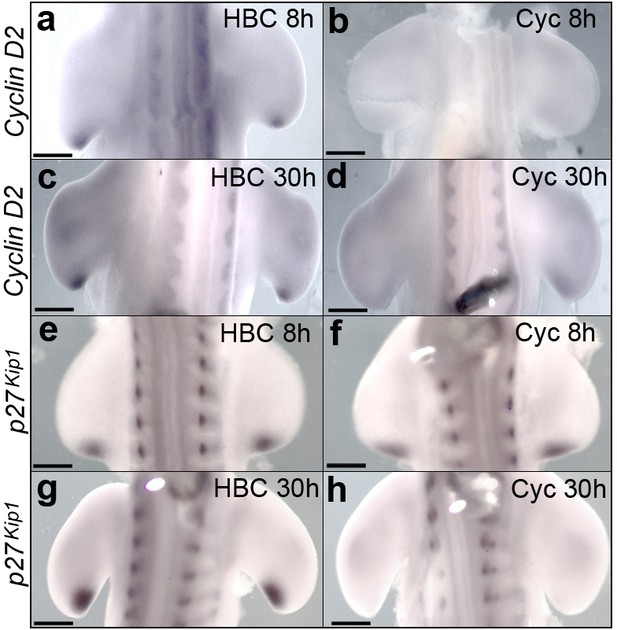
Shh signalling regulates Cyclin D2 and p27kip1 expression.
Application of cyclopamine at HH20 causes loss of Cyclin D2 expression in the polarising region at 8 hr (a, b, n = 4/4 in each experiment) and 30 hr (c, d n = 4/4 in each experiment). Although p27kip1 expression is unaffected at 8 hr (e, f, n = 6/6 in each experiment) it is undetectable at 30 hr (g, h, n = 5/5 in each experiment). Scale bars: a, b, e, f – 150 μm; c, d, g, h – 300 μm.
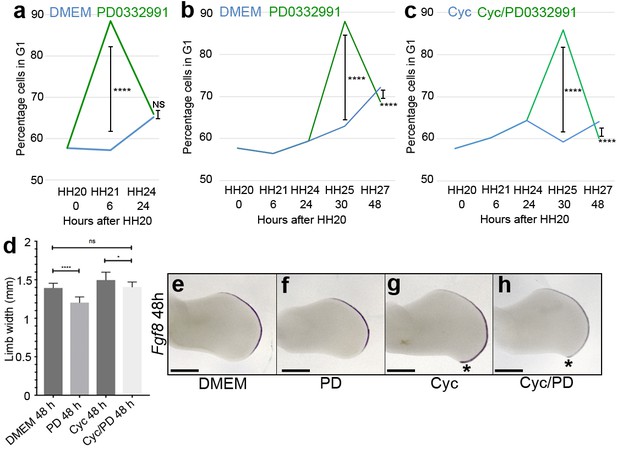
D cyclin inhibition prevents polarising region over-proliferation.
Application of PD0332991 at HH20 (green line) increases the percentage of polarising region cells in G1-phase to 88.4% after 6 hr compared with 57.2% in DMEM-treated controls (a, blue line - Pearson’s χ2 test - p < 0.0001, n = 12 polarising regions in both experiments; 11,745 and 11,310 cells analysed, respectively – note HH20 G1-phase data taken from Chinnaiya et al., 2014; Pickering and Towers, 2016). After 24 hr, G1-phase cell numbers are similar: 65.1% in DMEM-treated polarising regions and 65.9% in PD0332991-treated polarising regions (a - Pearson’s χ2 test – p > 0.05 and are not significantly different, n = 12 polarising regions in both experiments; 13,062 and 11,833 cells analysed, respectively). Application of PD0332991 at HH24 (green line) to control HBC-treated wing buds (blue line) significantly increases the percentage of polarising region cells in G1-phase to 87.8% (11,932 cells analysed) after 6 hr compared with 62.9% in DMEM/HBC-treated controls (b, blue line - Pearson’s χ2 test - p < 0.0001; 5,235 cells analysed n = 12 polarising regions in both experiments – note HH20, HH21 and HH24 G1-phase data taken from Chinnaiya et al., 2014; Pickering and Towers, 2016). After 24 hr, G1-phase cell numbers are still significantly different: 72.2% in DMEM/HBC-treated polarising regions and 68.7% in PD0332991-treated polarising regions (b - Pearson’s χ2 test – p < 0.0001, n = 12 polarising regions in both experiments; 11,472 and 10,669 cells analysed, respectively). Application of PD0332991 at HH24 to cyclopamine-treated wing buds (green line – note cyclopamine added at HH20) significantly increases the percentage of polarising region cells in G1-phase to 85.9% after 6 hr compared with 59.3% in cyclopamine-treated wing buds (c, blue line - Pearson’s χ2 test - p < 0.0001, n = 12 polarising regions in both experiments; 10,940 and 11,637 cells analysed, respectively – note HH20, HH21 and HH24 G1-phase data taken from Chinnaiya et al., 2014; Pickering and Towers, 2016). After 24 hr, G1-phase cell numbers are still significantly different: 64.1% in cyclopamine-treated polarising regions and 60.1% in cyclopamine/PD0332991-treated polarising regions (c - Pearson’s χ2 test – p < 0.0001, n = 12 polarising regions in both experiments; 11,118 and 10,001 cells analysed, respectively). PD0332991 reduces expansion of the wing bud by 7% compared with DMEM-treated wings, and by 17% compared with cyclopamine-treated wings at 48 hr (d unpaired t test *- p = <0.05, and ****- p =< 0.0001, n => 7 in all cases). Note loss of posterior overgrowth in PD0332991-treated wing buds (asterisks in g and h). Examples of wing buds from which measurements are taken are shown hybridised for Fgf8 (e-h). Scale bars: 300 μm.
-
Figure 4—source data 1
Flow cytometry graphs for cell cycle analysis.
(A) DMEM 6 h. (B) PD0332991 6 h. (C) DMEM 24 h. (D) PD0332991 24 h. (E) DMEM 6 h. (F) Cyc 6 h. (G) PD0332991 6 h. (H) Cyc/PD0332991 6 h. (I) DMEM 24 h. (J) 10 Cyc 24 h. (K) PD0332991 24 h. (L) Cyc/PD0332991 24 h.
- https://doi.org/10.7554/eLife.47625.006
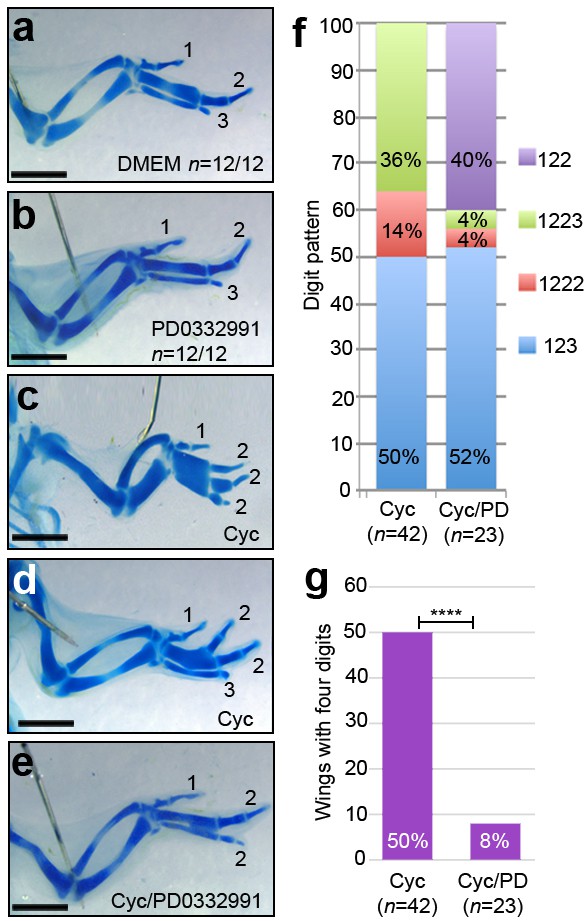
D cyclin inhibition prevents digit development caused by loss of Shh signalling.
Application of DMEM (a) and PD0332991 at HH24 (b) does not affect digit patterning (n = 12/12 in both cases). Application of cyclopamine at HH20 results in a 1-2-2-2 digit pattern (c, f - n = 6/42, 14%), a 1-2-2-3 pattern (d, f - n = 15/42, 36%) and a 1-2-3 pattern (f, - n = 21/42, 50%) in day 10 wings. Application of cyclopamine at HH20 and PD0332991 at HH24 results in a 1-2-2 digit pattern (e, f, n = 9/23–40%), a 1-2-3 pattern (f - n = 12/23, 52%) a 1-2-2-2 pattern and 1-2-2-3 pattern (f – both n = 1/23, 4%) in day 10 wings. 50% of cyclopamine-treated wings have four digits, 8% of cyclopamine/PD033299-treated wings have four digits (g, χ2 test p =< 0.0001). Scale bars: 1 mm.
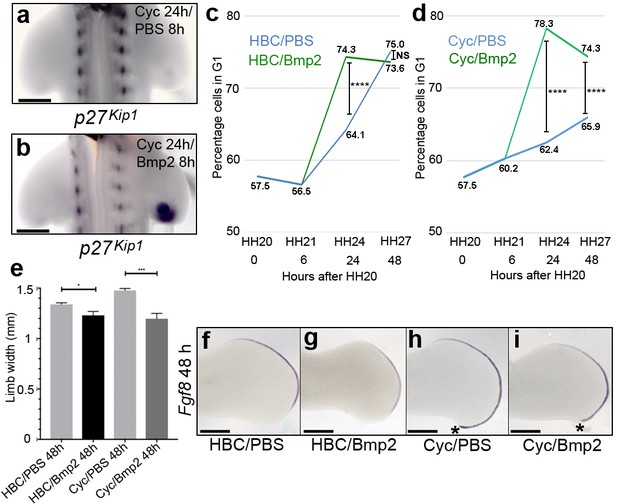
Bmp2 regulates p27kip1 expression and inhibits proliferation.
Application of cyclopamine at HH20 and a PBS-soaked bead to the right-hand wing bud 16 hr later causes loss of p27kip1 expression in the polarising region at 24 hr (a, n = 3/3). However, a Bmp2-soaked bead induces p27kip1 in the polarising region (b, n = 6/6). Application of Bmp2-soaked beads to HH21 wing buds significantly increases the percentage of cells in G1-phase to 74.3% (green line) compared with 64.1% in PBS-soaked bead controls (blue line) at 24 hr (c, Pearson’s χ2 test - p < 0.0001 – note HBC carrier added also, n = 14 polarising regions in both experiments; 7,309 and 9,339 cells analysed, respectively– note HH20, HH21 G1-phase data taken from Chinnaiya et al., 2014; Pickering and Towers, 2016). After 48 hr, the percentages of cells are very similar (75% and 73.6%, respectively) and not significantly different (c, Pearson’s χ2 test – p > 0.05; 8,402 and 6,576 cells analysed, respectively). Application of Bmp2-soaked beads to cyclopamine-treated HH20 wing buds (green line) significantly increases the percentage of G1-phase cells to 78.3% compared with 62.4% in PBS-bead/cyclopamine-treated controls (blue line) at 24 hr, and to 74.3% compared with 65.9% at 48 hr. (d, n = 14 polarising regions in all experiments; 6.924, 9,363, 10,869 and 9,790 cells analysed, respectively - Pearson’s χ2 test - p < 0.0001, – note HH20, HH21 G1-phase data taken from Chinnaiya et al., 2014; Pickering and Towers, 2016). Bmp2-soaked beads reduce expansion of the wing bud by 8.3% compared to PBS bead-treated wings at 24 hr and by 18.9% compared with PBS-soaked bead-cyclopamine-treated wings at 48 hr (e-i, unpaired t test *- p =< 0.05, and ***- p =< 0.0005, n = 7 in all cases). Note reduced posterior/distal growth in Bmp2-treated wing buds (asterisks in h and i). Examples of wing buds from which measurements are taken are shown hybridised for Fgf8 (f-i). Scale bars: a, b – 150 μm, e-h - 300 μm.
-
Figure 6—source data 1
Flow cytometry graphs for cell cycle analysis.
(A) HBC/PBS 24 h. (B) HBC/Bmp2 24 h. (C) Cyc//PBS 24 h. (D) Cyc//Bmp2 24 h. (E) HBC/PBS 48 h. (F) HBC/Bmp2 48 h. (G) Cyc/PBS 48 h. (H) Cyc/Bmp2 48 h.
- https://doi.org/10.7554/eLife.47625.009
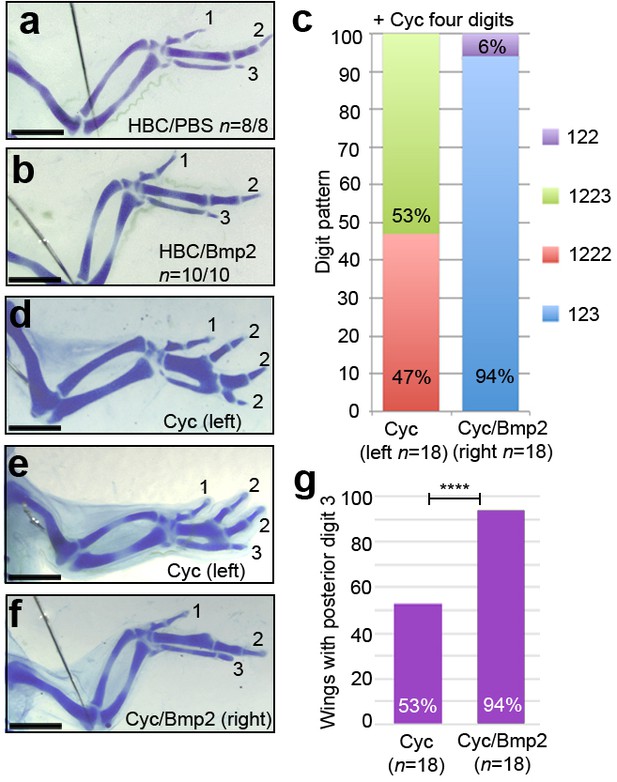
Bmp2 inhibits digit development and specifies digit 3.
Application of HBC/PBS-soaked beads (a) or HBC/Bmp2-soaked beads at HH21 (b) do not affect digit patterning (n = 8/8 and 10/10, respectively). Wings with four digits following cyclopamine-treatment at HH20 have a 1-2-2-2 digit pattern (c, d - 47%, n = 8/18) or a 1-2-2-3 pattern (c, e - 53%, n = 10/18). Application of Bmp2-soaked beads 2 hr later into the right-hand wings buds of the same cyclopamine-treated embryos results in a 1-2-3 digit pattern (c, f - 94%, n = 17/18) or a 1-2-2 pattern (c, 6%, n = 1/18). 53% of cyclopamine-treated wings have a posterior digit 3, but 94% of cyclopamine/Bmp2-treated wings have a posterior digit 3 (g, χ2 test p =< 0.0001). Scale bars: 1 mm.
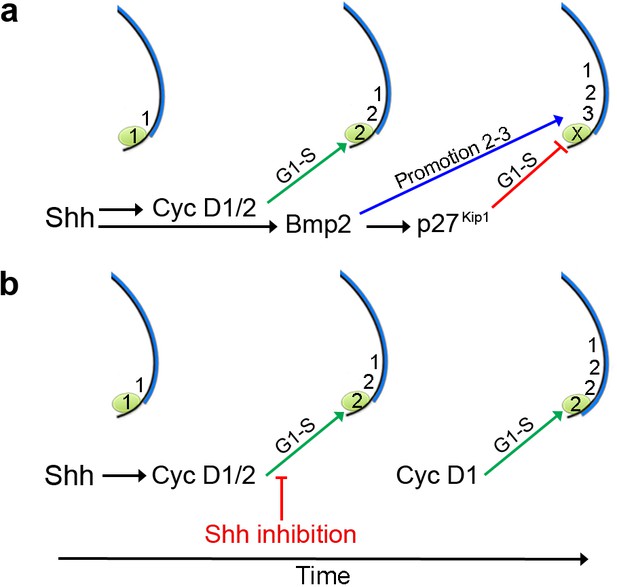
An autoregulatory polarising region cell cycle timer.
(a) Shh signalling is required for Cyclin D2 expression in the polarising region (green). Bmp2 signalling acts downstream of Shh to control p27kip1 that inhibits the D-cyclin-dependent formation of a digit from the polarising region (X), and that also promotes the digit 2 to digit 3 positional value in adjacent cells. Shh signalling also stimulates Cyclin D1-dependent proliferation in the digit-forming field, which is required for the progressive specification of antero-posterior digit positional values - 1, 2 and then 3, over 12 hr. (b) Inhibition of Shh signalling during digit specification prevents Bmp2 signalling from promoting the digit 2 to digit 3 positional value in the digit-forming field adjacent to the polarising region. In addition, loss of Bmp2 signalling prevents p27kip1 from inhibiting polarising region proliferation and digit development. Since blocking Shh signalling attenuates Cyclin D2 function, Cyclin D1 is likely to be responsible for the over-proliferation of polarising region cells.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.47625.012





