Golgi localized β1-adrenergic receptors stimulate Golgi PI4P hydrolysis by PLCε to regulate cardiac hypertrophy
Figures
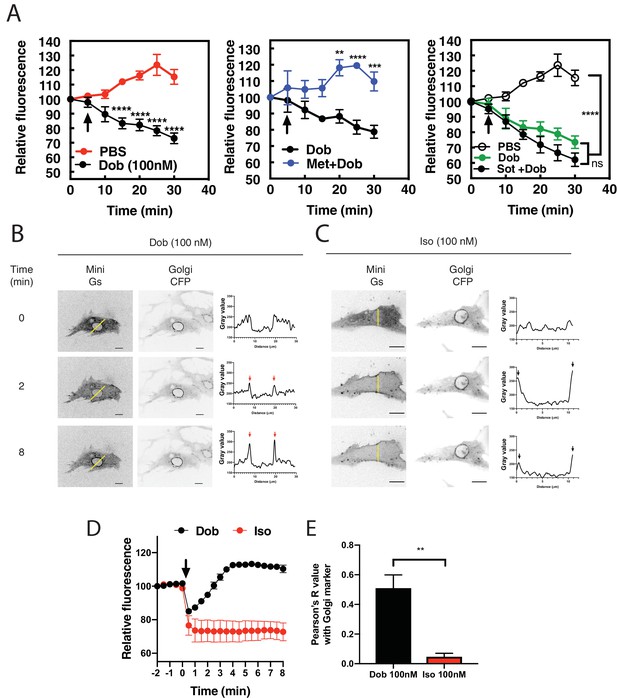
Dobutamine induces PI4P hydrolysis through the activation of internal βARs.
(A) NRVMs were transduced with FAPP-PH-GFP and stimulated as indicated. Time lapse live cell microscopy was used to quantitate Golgi associated FAPP-PH-GFP fluorescence was quantitated as previously described (Zhang et al., 2013; Malik et al., 2015). NRVMs were stimulated with dobutamine alone (100 nM, left) (N = 9), dobutamine in the presence or absence of metoprolol (100 μM, center) (N = 4), or dobutamine in the presence or absence of sotalol (5 mM, right) (N = 8). Data are not significantly different between dobutamine and dobutamine + sotalol. Data are from at least 4 cells for each N. (B) NRVMs were transfected with β1-ARs and NES-Venus-mini-Gs, followed by viral transduction with CFP-Giantin. Representative confocal fluorescence images of dobutamine-mediated NES-Venus-mini-Gs recruitment (100 nM, B left), CFP-Giantin Golgi marker (B, center) and histogram of representative NES-Venus-mini-Gs recruitment (B, right). Red arrow = Perinuclear region, Black arrow = sarcolemma. The yellow line indicates where histogram data was captured. Scale bars are 10 μm. (C) Representative confocal fluorescence images of Iso-mediated NES-Venus-mini-Gs recruitment (100 nM, left), CFP-Giantin Golgi marker (B, center) and histogram of representative NES-Venus-mini-Gs recruitment. Yellow line indicates where histogram data was captured. Scale bars = 10 μm (D) Mean data of fluorescence intensity of NES-Venus-mini-Gs at the perinuclear region corresponding to the Golgi ± SEM from at least 5 cells. (E) Pearson’s correlation coefficient for overlap of YFP-Mini-Gs and CFP-Giantin images. All symbols on time course graphs are presented as mean ± standard error from N = 4 or more independent preparations of myocytes. Agonists were added where indicated by the arrow.
-
Figure 1—source data 1
PI4P hydrolysis is stimulated by dobutamine and inhibited by a cell permeable antagonist.
- https://doi.org/10.7554/eLife.48167.005
-
Figure 1—source data 2
Dobutamine but not Iso stimulates Mini Gs translocation.
- https://doi.org/10.7554/eLife.48167.006
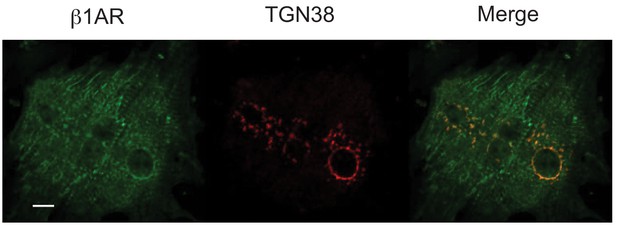
Staining of endogenous β1AR in cardiac myocytes.
Mouse neonatal cardiac myocytes were stained with anti-β1AR (abcam ab3442-1:100) and anti-TGN38 (Biorad AHP499G-1:1000) using the saponin permeabilization protocol described in the Materials and methods. Scale bar = 10 μm.
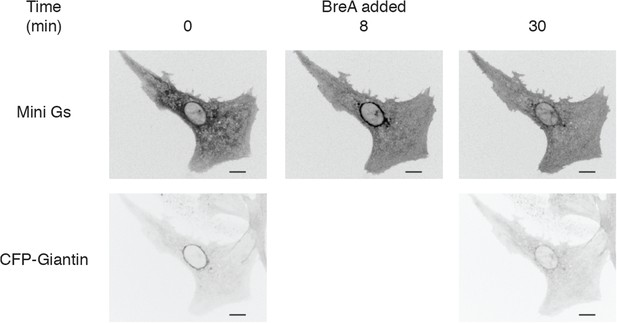
Disruption of the Golgi apparatus reverses mini-Gs protein recruitment to the perinuclear region by dobutamine.
NRVMs were transfected with β1-AR and NES-Venus-mini-Gs, followed by viral transduction with CFP-Giantin. Representative images of dobutamine-mediated NES-Venus-mini-Gs recruitment (100 nM, Upper panels) and CFP-Giantin Golgi marker (lower panels). Dobutamine was added at 2 min and Brefeldin A (5 μg/ml), was added at 8 min just after the 8 min image above was captured. NES-Venus-mini-Gs recruitment continually monitored. Scale bars are 10 μm.
Dobutamine activates β1ARs in the Golgi apparatus.
Confocal images of β1-AR-overexpressing cardiomyocytes with NES-Venus-mini-Gs, treated with 100 nM dobutamine. Total time represented by the movie is 10 min and pictures were taken every 15 s. Dobutamine was added at 2 min timepoint.
Isoproterenol does not activate β1ARs in the Golgi apparatus.
Confocal images of β1-ARs-overexpressing cardiomyocytes with NES-Venus-mini-Gs, treated with 100 nM isoproterenol. Total time represented by the movie is 10 min and pictures were taken every 15 seconds. Isoproterenol was added at 2 min timepoint.
Disruption of the Golgi apparatus inhibits activation of β1ARs in the Golgi apparatus.
Reversal of mini Gs recruitment to Golgi membrane after addition of Brefeldin A. Total time represented by the movie is 30 min and pictures were taken every 30 s. Dobutamine was added at 2 min timepoint for 8 min and then Brefeldin A was added for next 20 min.
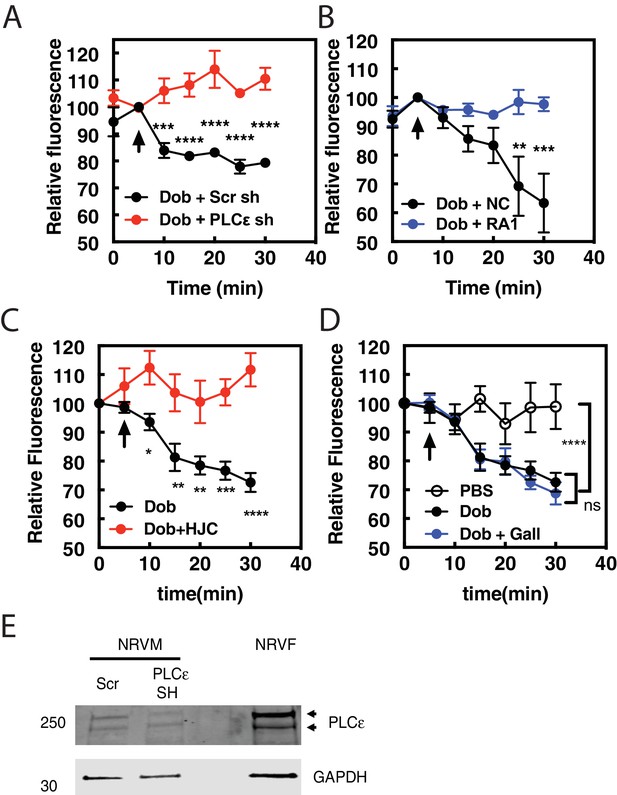
Dobutamine-mediated PI4P hydrolysis requires Epac and mAKAPβ bound PLCε.
NRVMs were transduced with FAPP-PH-GFP, stimulated with treatments as indicated and Golgi associate fluorescence was monitored with time. (A) PLCε knockdown prevents dobutamine-mediated PI4P hydrolysis. NRVMs were transduced for 48 hr with adenovirus expressing shRNA for PLCε or scrambled control shRNA before stimulation with 100 nM dobutamine (at arrow) (N = 3 independent experiments). (B) Disruption of PLCε-mAKAPβ interaction prevents PI4P hydrolysis stimulated by dobutamine. NRVMs were transduced with RA1 domain expressing adenovirus or control virus (NC-negative control) as previously described 24 hr before experimentation. Cells were stimulated with 100 nM dobutamine (at arrow) (N = 3 independent experiments). (C) Epac is required for dob-mediated PI4P hydrolysis. The Epac inhibitor HJC0726 (1 μM) was added to NRVMs 15 min before imaging and dobutamine (100 nM) was added at the arrow. (N = 3 independent experiments) (D) Gβγ is not required for dobutamine-mediated PI4P hydrolysis. The Gβγ inhibitor, Gallein (10 μM) was added 15 min prior to imaging and dobutamine added as indicated by the arrow (N = 3 independent experiments). Data are not significant between Dob and Dob + Gallein. Images for A, B,C and D PI4P hydrolysis were taken from at least 4 cells for each separate preparation of NRVMs. (E) Western blot of Adenoviral sh-RNA knockdown of PLCε. The two bands likely represent two splice variants of PLCε.
-
Figure 2—source data 1
Effects of inhibition of PLCε, Epac and Gβγ on dobutamine stimulated PI4P hydrolysis.
- https://doi.org/10.7554/eLife.48167.011
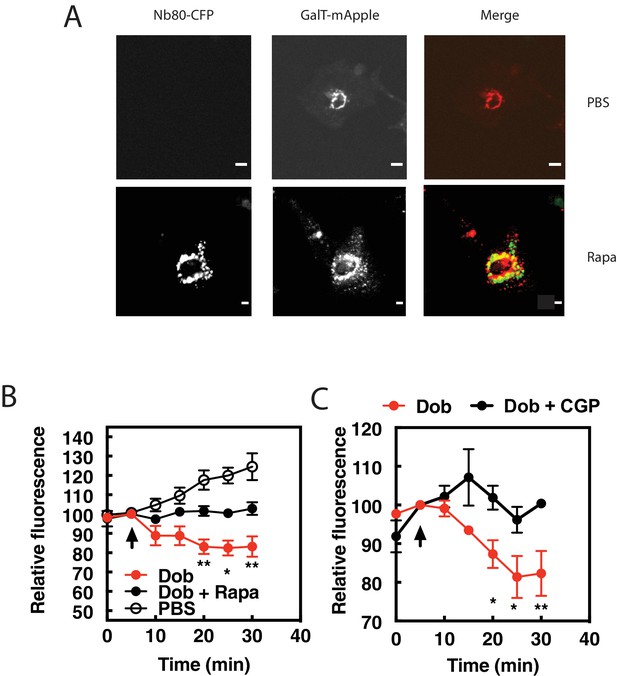
β1ARs in the Golgi are required for PI4P hydrolysis stimulated by dobutamine.
NRVMs were transduced with FRB-CFP-Nb80 and FKBP-GalT-mApple containing adenovirus, along with adenovirus containing FAPP-PH-GFP for 24 hr. (A) Confocal fluorescence images of NRVMs expressing FRB-CFP-Nb80 in the CFP channel and FKBP-GalT-mApple in the red channel before and after addition of rapamycin (see Materials and methods for details) Pearson’s correlation coefficient = 0.32 ± 0.03. Scale bars are 10 μm. (B) NRVMs were incubated with either rapamycin (1 μM) or DMSO control for 15 min prior to addition of dobutamine (100 nM) added at the arrow. Golgi associated FAPP-PH-GFP fluorescence was monitored by time lapse fluorescence video microscopy as in Figure 1A. (C) Cells were pretreated treated with the cell permeant β1AR selective antagonist, CGP-20712 (100 nM), or vehicle, followed by dobutamine addition and assessed as in B. Images were taken from at least n = 9 cells each from at least four separate preparations of NRVMs. Data were analyzed as means from N = 4 experiments. Agonists were added as indicated by the arrow.
-
Figure 3—source data 1
Effects of Golgi targeted NB80 and β1AR-specific antagonism on dobutamine stimulated PI4P hydrolysis.
- https://doi.org/10.7554/eLife.48167.013
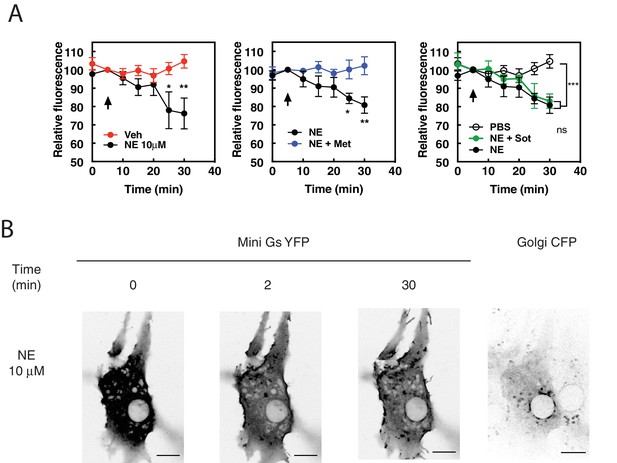
The physiological neurotransmitter, norepinephrine, can induce PI4P hydrolysis through internal receptors and can activate βARs at the Golgi.
(A) NRVMs were transduced with FAPP-PH-GFP and stimulated with norepinephrine (10 μM, left) in the presence of metoprolol (100 μM, center) or sotalol (5 mM, right) and analyzed as in Figure 1A. Data are not significant between norepinephrine and norepinephrine + sotalol. (B) NRVMs were transfected with β1AR and NES-Venus-mini-Gs, followed by viral transduction with CFP-Giantin. Representative images of norepinephrine-mediated NES-. Venus-mini-Gs recruitment (10 μM, left columns), CFP-Giantin Golgi marker (B, right). The Pearson’s Coefficient for colocalization of Venus-mini-Gs and CFP-Giantin is R = 0.455 ± 0.06. Scale bars are 10 μm. All experiments were performed in humidified environmental chamber at 37°C. Images for PI4P hydrolysis were collected as in Figure 1A, and were from at least n = 7 cells each from three separate preparations of NRVMs. Data were analyzed as means from N = 3 experiments. Agonists were added where indicated by the arrow.
-
Figure 4—source data 1
NE stimulates PI4P hydrolysis.
- https://doi.org/10.7554/eLife.48167.015
Norepinephrine activates β1ARs in the Golgi apparatus.
Representative confocal images of β1-ARs-overexpressing cardiomyocytes with NES-Venus-mini-Gs, treated with 10 µM norepinephrine. This confocal plane was chosen to emphasize both PM and Golgi Venus-mini-Gs recruitment. Total time represented by the movie is 30 min and pictures were taken every 30 s. Norepinephrine was added at 2 min timepoint.
Norepinephrine activates β1ARs in the Golgi apparatus.
Representative confocal images of β1-ARs-overexpressing cardiomyocytes with NES-Venus-mini-Gs, treated with 10 µM norepinephrine. This confocal plane was chosen to emphasize Golgi Venus-mini-Gs recruitment so PM recruitment is not clearly seen in this video. Total time represented by the movie is 30 min and pictures were taken every 30 s. Norepinephrine was added at 2 min timepoint.
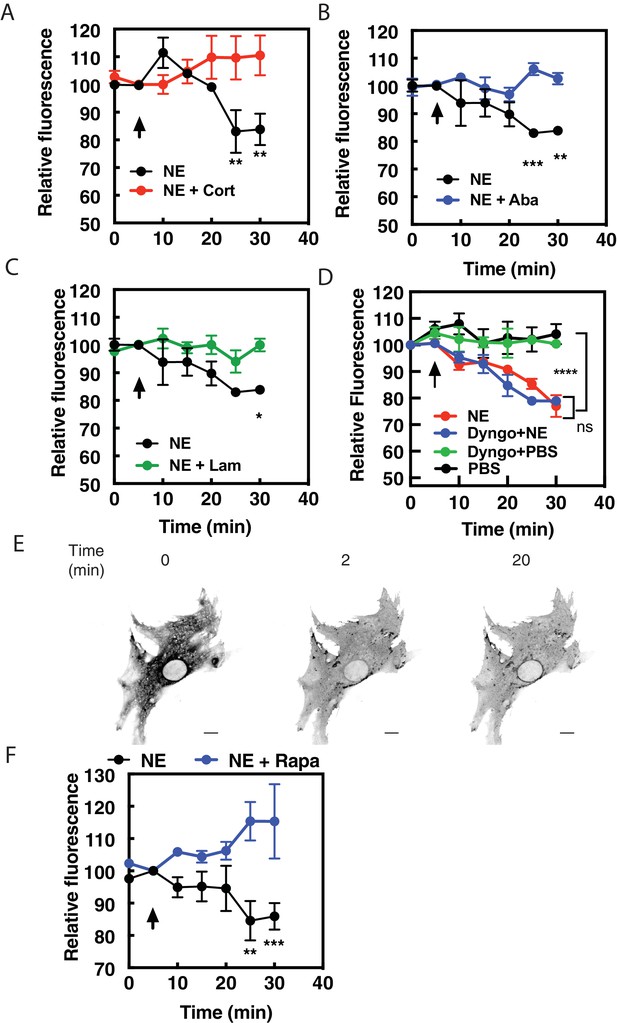
Norepinephrine requires OCT transporters but not receptor internalization to stimulated PI4P hydrolysis.
NRVMs were transduced with FAPP-PH-GFP and stimulated with norepinephrine (10 μM) in the presence of corticosterone (100 μM, (A), abacavir (10 μM, (B), lamotrigine (10 μM, (C) or Dyngo (40 μM, (D). Data are not significant between norepinephrine and norepinephrine + Dyngo. Images were collected as in Figure 1A for PI4P hydrolysis (N = 3) were from at least 4 cells each from separate preparations of NRVMs. Agonists were added where indicated by the arrow. (E) Dyngo has no effect on NES-Venus-mini-Gs Golgi recruitment by norepinephrine. Representative image of norepinephrine-mediated NES-Venus-mini-Gs recruitment in the presence of Dyngo (40 μM). (N = 3) Scale bars are 10 μm. (F) NRVMs were transduced with adenovirus containing FRB-CFP-Nb80 and FKBP-GalT-mApple along with adenovirus containing FAPP-PH-GFP for 24 hr prior to experimentation. NRVMs were incubated with either rapamycin (1 μM) or DMSO control for 15 min prior to addition of NE (10 μM) added at the arrow. Images were collected as in Figure 1A for PI4P hydrolysis and were from at least n = 10 cells each from three separate preparations of NRVMs. Data were analyzed as means from N = 3 experiments.
-
Figure 5—source data 1
NE requires membrane transport by OCT3 and Golgi resident βARs to stimulate PI4P hydrolysis.
- https://doi.org/10.7554/eLife.48167.020
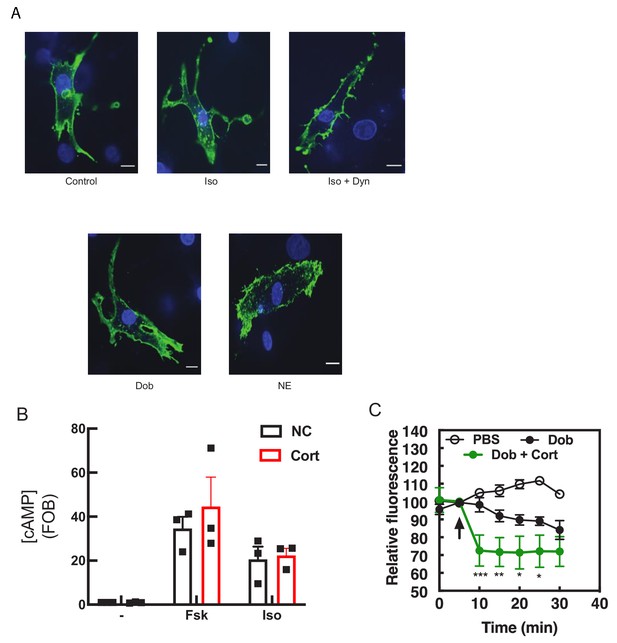
Receptor internalization does not contribute to Golgi β1AR localization.
(A) Dyngo blocks Isoproterenol induced β1-AR internalization. NRVMs were transfected with FLAG-β1-AR receptors and allowed to express for 48 hr. Cells were then stimulated with either vehicle control, Isoproterenol (10 μM), dobutamine (100 nM) or norepinephrine (10 μM) for 30 mins. Pretreatment with Dyngo (40 μM) was performed for 15 mins before agonist addition, where indicated. Cells are pseudocolored Green for M2-FLAG-Cy3 antibody, which is bound to FLAG-β1-AR and nuclei are stained with DAPI. Images are representative from at least three experiments. Scale bars are 10 μM. (B) Corticosterone has no effect on the ability of plasma membrane β1-AR to signal. NRVMs were stimulated in the presence of either Corticosterone (100 μM) or vehicle control with the indicated agonists for 10 mins. Cells were then lysed and cAMP measured according to the manufacturer’s instructions. Data is from three experiments. (C) Corticosterone does not inhibit Dobutamine-mediated PI4P hydrolysis. NRVMs were transduced with FAPP-PH-GFP and stimulated with dobutamine (100 mM) in the presence of Corticosterone (100 μM) or vehicle and analyzed as in Figure 1A. Images for PI4P hydrolysis collected as in Figure 1A, were from at least n = 7 cells each from three separate preparations of NRVMs. Agonists were added where indicated by the arrow.
Inhibition of receptor internalization does not alter activation of β1ARs in the Golgi apparatus.
Confocal images of β1-ARs-overexpressing cardiomyocytes with NES-Venus-mini-Gs, pretreated with 40 µM Dyngo. Total time represented by the movie is 30 min and pictures were taken every 30 s. Dyngo was added 15 min prior to experimentation and norepinephrine was added at 2 min timepoint.
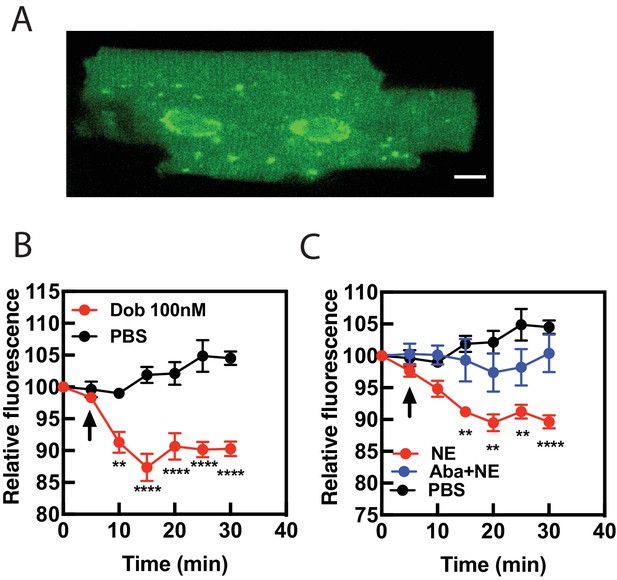
Dobutamine and NE stimulate PI4P hydrolysis in Adult Ventricular Myocytes (AVMs).
Freshly isolated mouse AVMs were infected with FAPP-PH-GFP (100 MOI) for 24 hr. (A) representative image of an AVM expressing FAPP-PH-GFP showing strong labeling surrounding the nucleus. (B) FAPP-PH-GFP expressing AVMs were treated with either dob or PBS control as indicated and were imaged as for NRVMs as in Figure 1A for PI4P hydrolysis. Fluorescence intensity in the region surrounding the nucleus was quantitated at 5 min intervals. C) AVMs were treated with PBS control, NE (10 μM) or NE plus abacavir and analyzed as in B. Data for B and C are from at least n = 3 cells each from three separate preparations of AVMs. Data were analyzed as means from N = 3 experiments.
-
Figure 6—source data 1
NE-Stimulated PI4P hydrolysis in adult cardiac myocytes requires OCT3.
- https://doi.org/10.7554/eLife.48167.023
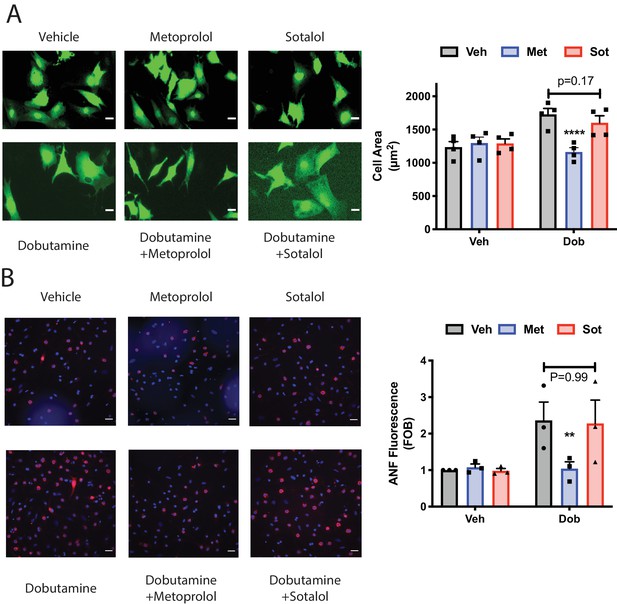
Dobutamine induced cardiomyocyte hypertrophy requires intracellular βARs.
(A) Dobutamine induces internal-receptor dependent increases in cell area. NRVMs were transduced with YFP virus prior to stimulation for 48 hr with dobutamine in the presence of the indicated antagonists or vehicle control. Following fixation, cell area was measured using image J. Representative images are on the left with mean data (fold over basal, FOB) on the right (N = 4 separate preparations of NRVMs), analyzed with a paired two way ANOVA. ****p<0.0001 vs. Dob and Dob+sotalol. Scale bars are 10 μm. B) Dobutamine induces an increase in ANF expression via an internal receptor-dependent mechanism. NRVMs were stimulated with dobutamine in the presence or absence of the indicated antagonists or vehicle control for 48 hr before fixation and staining for ANF. Fluorescence of ANF rings was then captured by confocal microscopy, followed by fluorescence intensity analysis with Image J. Representative images are on the left with mean data (fold over basal, FOB) on the right (N = 3 separate preparations of NRVMs), analyzed with a paired two way ANOVA. **p=0.007 vs. Dob and Dob+sotalol. The total number of cells analyzed was greater than 200 cells for each.
-
Figure 7—source data 1
Dobutamine-stimulated cardiomyocyte hypertrophy is blocked by a cell permeable βAR antagonist.
- https://doi.org/10.7554/eLife.48167.025
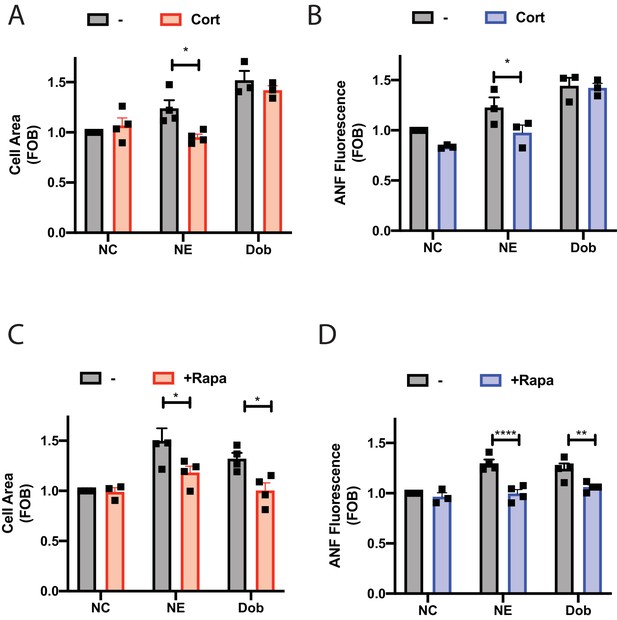
Dobutamine and norepinephrine induced cardiomyocyte hypertrophy requires Golgi-localized βARs.
Norepinephrine induced hypertrophy also requires agonist internalization. Norepinephrine induces internal-receptor dependent increases in cell area (A) and ANF expression (B). NRVMs were stimulated for 48 hr with norepinephrine (10 μM) or dobutamine (100 nM) in the presence of Corticosterone (100 μM) or vehicle control. Following fixation, cells were stained for ANF and using CellTracker Deep Red. Images were captured using a Thermo Fisher Cell Insight and analyzed by Cell Profiler. Both NE and Dobutamine significantly increase cell size (NE, p<0.05 and Dob p<0.001) and ANF expression (NE, p<0.01 and Dob p<0.001) in control conditions. Dobutamine and Norepinephrine require Golgi localized βAR to induce increase in cell area (C) and ANF expression (D). NRVMs were transduced with FRB-CFP-Nb80 and FKBP-mApple-GalT for 24 hr before stimulation. 15 min before agonist addition, Rapamycin or (1 μM) or vehicle control was added. Cells were then stimulated with either dobutamine (100 nM) or norepinephrine (10 μM) for 48 hr. Following fixation, cells were stained for ANF and using CellTracker Deep Red. Images were captured using a Thermo Fisher Cell Insight and analyzed by Cell Profiler. Both NE and dobutamine significantly increase cell size (NE, p<0.001 and Dob p<0.01) and ANF expression (NE, p<0.001 and Dob p<0.001) in control conditions. All data is from at least 1200 cells from three separate preparations of NRVMs. Data were analyzed as means from N = 3-4 experiments.
-
Figure 8—source data 1
NE-stimulated hypertrophy requires OCT3 and Golgi resident βARs.
- https://doi.org/10.7554/eLife.48167.027
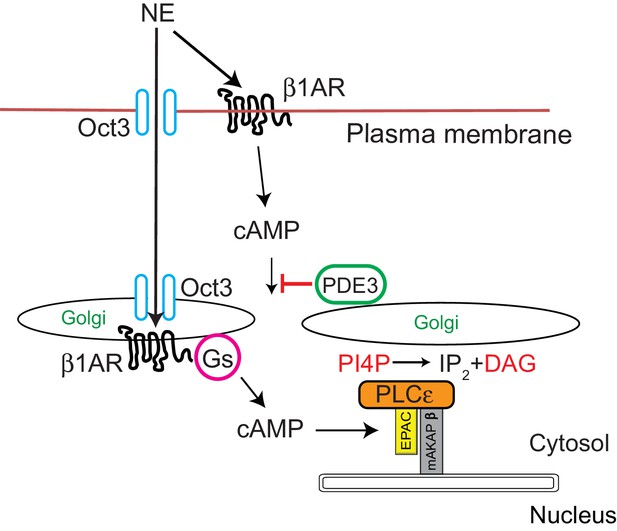
Signal transduction by cell surface and Golgi β1ARs.
β1ARs are located on both the plasma membrane and the Golgi apparatus in cardiac myocytes. Stimulation of cell surface β1ARs leads to production of cytosolic cAMP but this cAMP cannot access the Epac/PLCε/mAKAPβ due to PDE3 dependent hydrolysis of cAMP. To access Golgi β1AR, NE crosses the plasma membrane via the Oct3 transporter. We speculate that the Oct3 transporter is also present in the Golgi allowing access to the Golgi lumen and there is evidence for the presence of Oct3 on intracellular membranes as discussed in the text. Once activated in the Golgi, β1AR stimulates Gs and subsequent production of cAMP locally. Missing from this diagram is adenylyl cyclase which we presume is in the Golgi apparatus. There is evidence for adenylyl cyclase binding to mAKAPβ in cardiac myocytes. Local cAMP has privileged access to the Epac/PLCε/mAKAPβ complex which leads to PLCε-dependent production of local DAG from PI4P.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Rat) | NRVM | Freshly isolated myocytes from neonatal rats | ||
| Recombinant DNA reagent | NES-Venus-mini-Gs | Nevin Lambert, Augusta University, GA. Wan et al. (2018) | ||
| Recombinant DNA reagent (human) | FLAG-β1 adrenergic receptor | Addgene | RRID:Addgene_14698 | |
| Recombinant DNA reagent | CFP Giantin | Irannejad et al. (2017) | Golgi Marker Adenovirus expressing CFP Giantin MOI 50 | |
| Transfected construct (Rat) | PLCε-RA1 | Zhang et al. (2013) | Adenovirus expressing the RA1 domain fromPLCE1 MOI 50 | |
| Transfected construct (Rat) | PLCE1-shRNA | Zhang et al. (2013) | Adenovirus expressing PLCE1 shRNA MOI 50 | |
| Transfected construct | FAPP-PH-GFP | Zhang et al. (2013) | Adenovirus expressing FAPP-PH-GFP for PI4P detection MOI 50 | |
| Transfected construct | CFP-Nb80-FRB | This paper and Irannejad et al. (2017) | In this paper adenovirus was created for expressing CFP-Nb80-FRB previously created in Irannejad et al. (2017) MOI 50 | |
| Transfected construct | FKBP-mApple-GalT | This paper and Irannejad et al. (2017) | In this paper adenovirus was created for expressing FKBP-mApple-GalT previously created in Irannejad et al. (2017) MOI 50 | |
| Antibody | Anti-β1 adrenergic receptor (rabbit polyclonal) | Abcam | #ab3442 RRID: AB_10890808 | 1:100 dilution |
| Antibody | Anti-TGN38 (sheep anti Rat polyclonal) | Biorad | #AHP499G RRID:AB_2203272 | 1:1000 dilution |
| Antibody | M2-FLAG-Cy3 (mouse monoclonal) | Sigma | A9594 RRID:AB_439700 | 5 ug/mL |
| Chemical compound, drug | Butanedione-monoxime (BDM) | Sigma | 112135 | Myosin blocker |
| Chemical compound, drug | Isoproterenol (ISO) | Sigma | 1351005 | βAR agonist |
| Chemical compound, drug | Dyngo | Abcam | Ab120689 | Dynamin inhibitor |
| Chemical compound, drug | Sotalol | Sigma | S0278 | βAR antagonist |
| Chemical compound, drug | HJC0726 | Xiaodong Cheng, UT Houston Health Science Center. Zhu et al. (2015) | Epac inhibitor | |
| Chemical compound, drug | Brefeldin A | Biolegend | 420601 | Golgi disruptor |
| Chemical compound, drug | Gallein | Sigma | 371708 | G protein βγ subunit inhibitor |
| Chemical compound, drug | Corticosterone | Tocris | 3685 | Oct3 inhibitor |
| Chemical compound, drug | Metoprolol | Sigma | M5391 | βAR antagonist |
| Chemical compound, drug | Dobutamine | Tocris | 0515 | βAR agonist |
| Chemical compound, drug | Lamotrigine | Tocris | 2289 | OCT3 blocker |
| Chemical compound, drug | Abacavir | Tocris | 4148 | OCT3 blocker |
| Chemical compound, drug | Norepinephrine (NE) | Sigma | A0937 | AR agonist |
| Chemical compound, drug | Rapamycin (Rapa) | Tocris | 1292 | |
| Peptide, recombinant protein | Collagenase Type II | Worthington | CLS-2 | |
| Commercial assay or kit | cAMP Elisa | ENZO | ADI-900–066 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.48167.029





