Atypical memory B-cells are associated with Plasmodium falciparum anemia through anti-phosphatidylserine antibodies
Figures
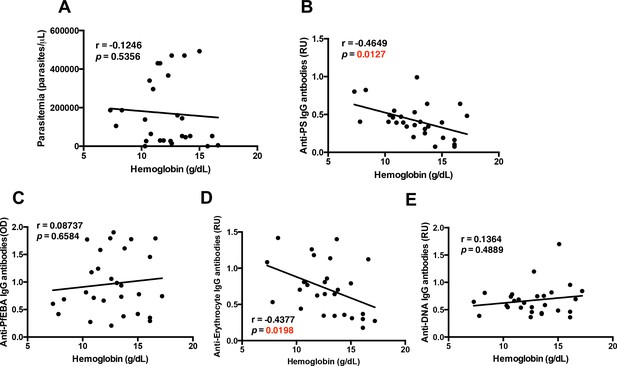
Specific autoantibodies correlate with malarial anemia in P.-falciparum-infected returned travelers.
Non-parametric Spearman correlation analysis comparing hemoglobin with (A) parasitemia, (B) anti-PS IgG antibodies, (C) anti-PfEBA IgG antibodies, (D) anti-erythrocyte IgG antibodies and (E) anti-DNA IgG antibodies.
-
Figure 1—source data 1
Source data for Figure 1 .
- https://doi.org/10.7554/eLife.48309.004
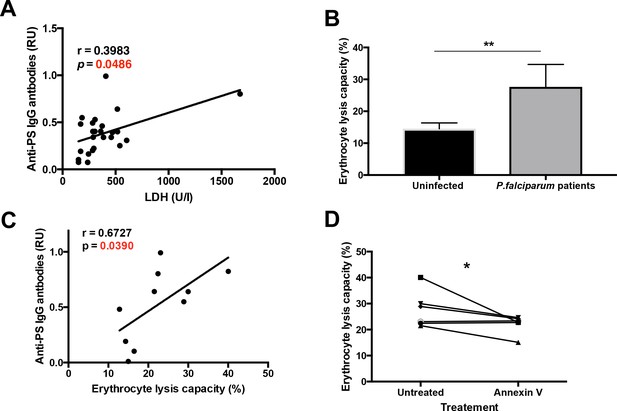
Plasma from P. falciparum patients mediates erythrocyte lysis, which can be partially inhibited by Annexin V.
(A,B) Correlation of plasma anti-PS IgG antibodies with the LDH levels (A) or with the erythrocyte lysis capacity (B) of the plasma of P. falciparum patients. (C) Complement-mediated lysis of erythrocytes exposing PS by P. falciparum patient’s plasma compared to plasma from uninfected controls, expressed as percentage of maximal lysis. (D) Complement-mediated lysis of erythrocytes exposing PS, pre-incubated or not with Annexin V, before incubation with the plasma of P. falciparum patients (n = 6). Results show the means and standard deviations of triplicated determinations. Significance was assessed by nonparametric Spearman correlation analysis (A,B) or unpaired Student's t-test (C,D). *p≤0.05, **p≤0.01.
-
Figure 2—source data 1
Source data for Figure 2.
- https://doi.org/10.7554/eLife.48309.006
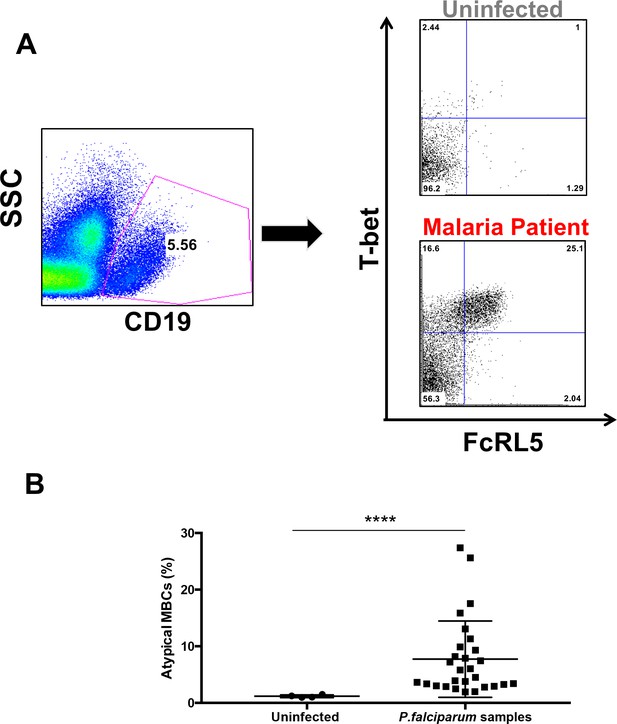
Atypical MBCs expand in P.-falciparum-infected patients and decline after treatment.
(A) Gating strategy for the characterization of FcRL5+ T-bet+ B-cells (CD19+) with representative plots of one uninfected control and one P. falciparum patient. (B) Percentage of CD19+ FcRL5+ T-bet+ B-cells in samples from uninfected controls and P. falciparum patients. Significance assessed by unpaired Student's t test. ****p≤0.0001.
-
Figure 3—source data 1
Source data for Figure 3.
- https://doi.org/10.7554/eLife.48309.014
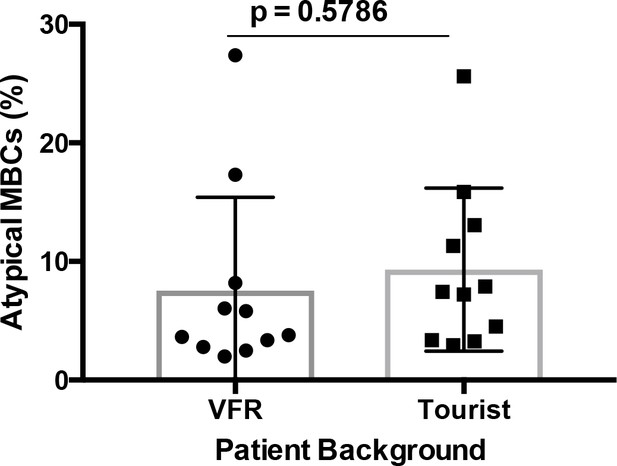
Atypical MBCs do not correlate significantly with patient background.
Comparison of the percentage of atypical MBCs in the circulation of P. falciparum patients by background (visiting friends or relatives (VFR) and tourists). Significant assessed by unpaired Student's t test.
-
Figure 3—figure supplement 1—source data 1
Source data for Figure 3—figure supplement 1.
- https://doi.org/10.7554/eLife.48309.009
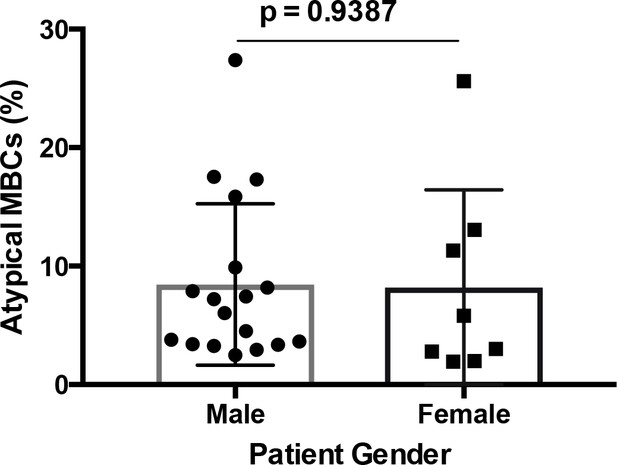
Atypical MBCs do not correlate significantly with patient gender.
Comparison of the percentage of atypical MBCs in the circulation of P. falciparum patients by gender. Significant assessed by unpaired Student's t test.
-
Figure 3—figure supplement 2—source data 1
Source data for Figure 3—figure supplement 2.
- https://doi.org/10.7554/eLife.48309.011
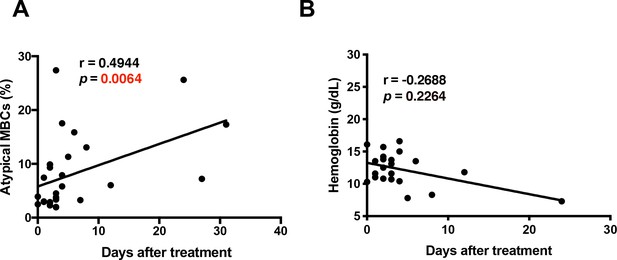
The time after treatment at which samples were collected correlates significantly with atypical MBCs but not with hemoglobin levels.
Non-parametric Spearman Correlation analysis comparing the days after treatment when samples were collected and levels of (A) atypical MBCs and (B) hemoglobin.
-
Figure 3—figure supplement 3—source data 1
Source data for Figure 3—figure supplement 3.
- https://doi.org/10.7554/eLife.48309.013
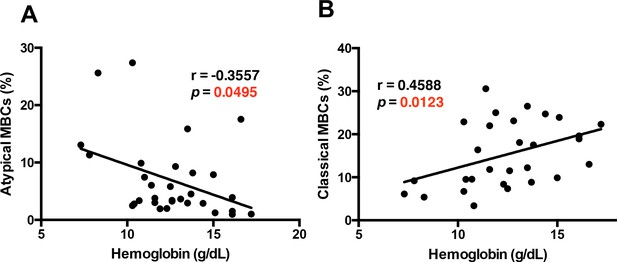
The atypical MBC subset correlates with the development of anemia in P. falciparum patients.
Correlation analysis of atypical (A) and classical (B) MBC subsets from the PBMC of P. falciparum patients compared with hemoglobin levels. Significance was assessed by non-parametric Spearman correlation analysis.
-
Figure 4—source data 1
Source data for Figure 4.
- https://doi.org/10.7554/eLife.48309.027
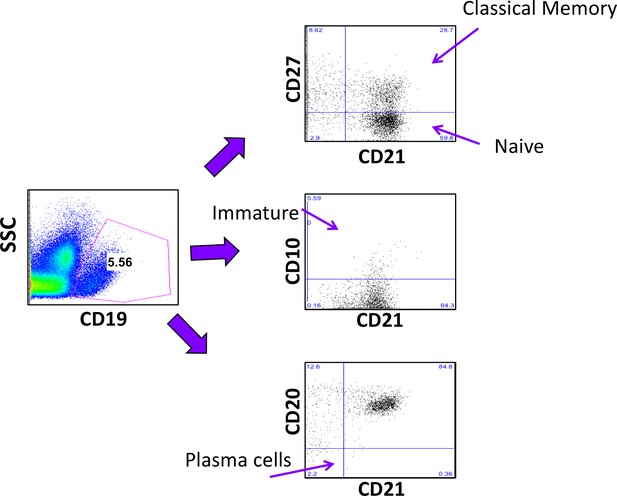
Gating strategy for relevant B-cell sub-populations.
B-cell subpopulations of human PBMC (Weiss et al., 2009).
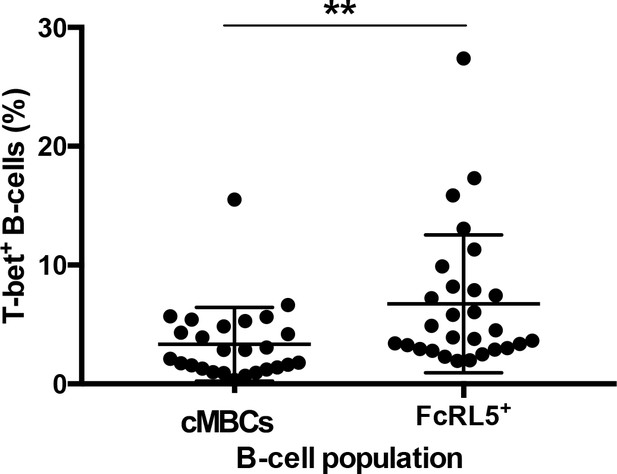
Expression of T-bet in FcRL5+ cells compared to classical MBCs.
Significance assessed by unpaired Student's t test. **p≤0.01.
-
Figure 4—figure supplement 2—source data 1
Source data for Figure 4—figure supplement 2.
- https://doi.org/10.7554/eLife.48309.018
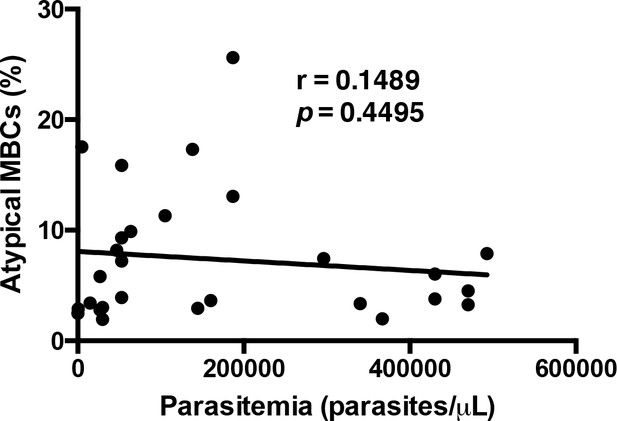
Atypical MBCs do not correlate significantly with parasitemia.
Non-parametric Spearman correlation analysis comparing the percentage of atypical MBCs and parasite levels.
-
Figure 4—figure supplement 3—source data 1
Source data for Figure 4—figure supplement 3.
- https://doi.org/10.7554/eLife.48309.020
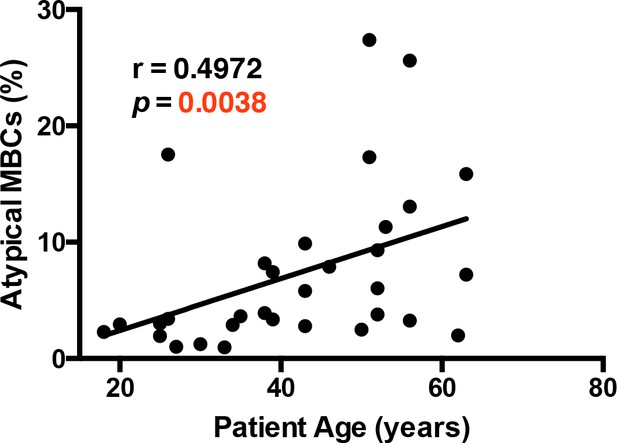
Atypical MBCs correlate significantly with patient's age.
Non-parametric Spearman correlation analysis comparing the percentage of atypical MBCs and patient's age.
-
Figure 4—figure supplement 4—source data 1
Source data for Figure 4—figure supplement 4.
- https://doi.org/10.7554/eLife.48309.022
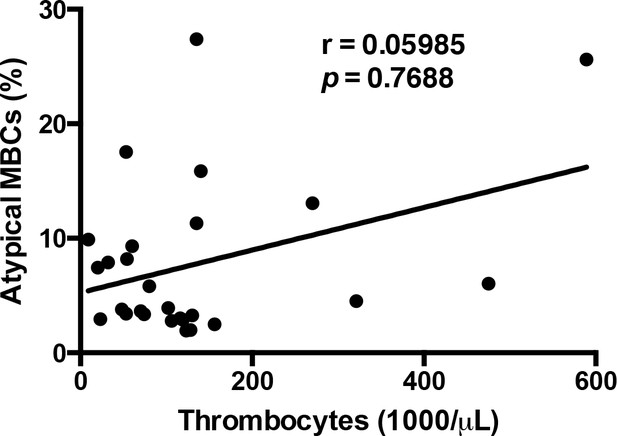
Atypical MBCs do not correlate significantly with thrombocyte levels.
Non-parametric Spearman correlation analysis comparing the percentage of atypical MBCs and thrombocyte levels in the circulation.
-
Figure 4—figure supplement 5—source data 1
Source data for Figure 4—figure supplement 5.
- https://doi.org/10.7554/eLife.48309.024
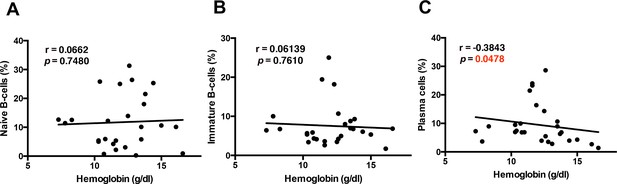
Correlations of other B-cell subsets with hemoglobin levels in P. falciparum patients.
Non-parametric Spearman correlation analysis of relevant B-cells subsets from the PBMC of P. falciparum patients: (A) naïve B-cells (CD27–CD21+CD10–), (B) immature B-cells (CD10+), and (C) plasma cells (CD27+CD21–CD20–) compared with hemoglobin levels.
-
Figure 4—figure supplement 6—source data 1
Source data for Figure 4—figure supplement 6.
- https://doi.org/10.7554/eLife.48309.026
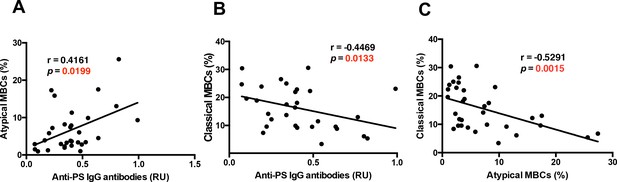
Anti-PS IgG antibodies show distinct correlations with classical and atypical MBC subsets in P. falciparum patients.
Correlation analysis of levels of (A) atypical and classical (B) MBCs with anti-PS IgG antibody levels from the plasma of P. falciparum patients. (C) Correlation analysis of atypical and classical MBC levels. Significance was assessed by non-parametric Spearman correlation analysis.
-
Figure 5—source data 1
Source data for Figure 5.
- https://doi.org/10.7554/eLife.48309.035
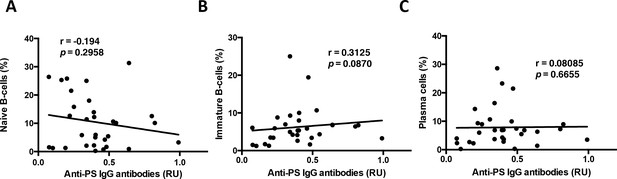
Anti-PS IgG antibodies do not correlate with other B-cell subsets in P. falciparum patients.
Non-parametric Spearman Correlation analysis of (A) naïve B-cells (CD27–CD21+CD10–), (B) immature B-cells (CD10+), and (C) plasma cells (CD27+CD21–CD20–) with anti-PS IgG antibody levels from the plasma of P. falciparum patients.
-
Figure 5—figure supplement 1—source data 1
Source data for Figure 5—figure supplement 1.
- https://doi.org/10.7554/eLife.48309.030
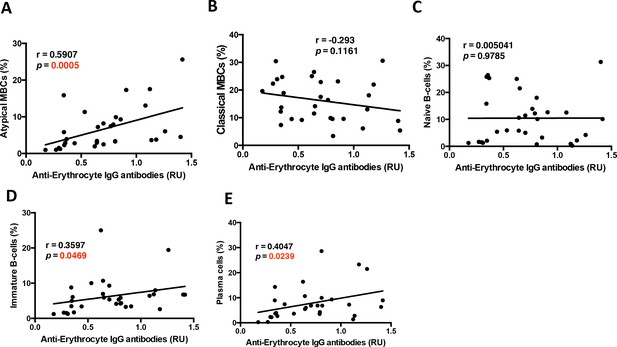
Correlations of anti-RBC IgG antibodies with B-cell subsets in P. falciparum patients.
Non-parametric Spearman Correlation analysis of (A) atypical MBCs (CD27–CD21–FcRL5+), (B) classical MBCs (CD27+CD21+), (C) naïve B-cells (CD27–CD21+CD10–), (D) immature B-cells (CD10+), and (E) plasma cells or plasmablasts (CD27+CD21–CD20–) with anti-erythrocyte lysate IgG antibody levels from the plasma of P. falciparum patients.
-
Figure 5—figure supplement 2—source data 1
Source data for Figure 5—figure supplement 2.
- https://doi.org/10.7554/eLife.48309.032
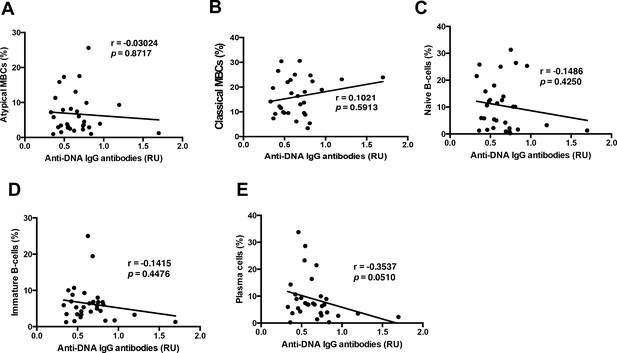
Anti-DNA IgG antibodies do not correlate with the B-cell subsets analyzed in P. falciparum patients.
Non-parametric Spearman correlation of (A) atypical MBCs (CD27–CD21–FcRL5+), (B) classical MBCs (CD27+CD21+), (C) naïve B-cells (CD27–CD21+CD10–), (D) immature B-cells (CD10+), and (E) plasma cells or plasmablasts (CD27+CD21–CD20–) with anti-DNA lysate IgG antibody levels from the plasma of P. falciparum patients.
-
Figure 5—figure supplement 3—source data 1
Source data for Figure 5—figure supplement 3.
- https://doi.org/10.7554/eLife.48309.034
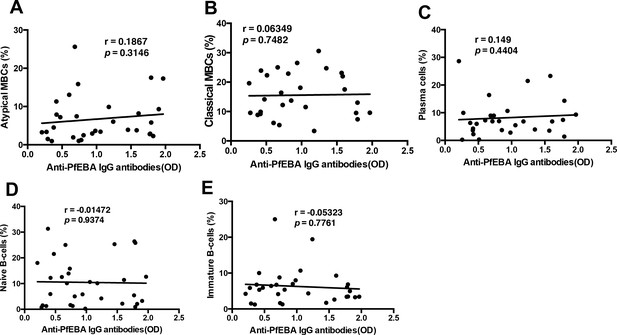
There is no significant correlation of anti-parasite PfEBA antibodies with relevant B-cell subsets from P. falciparum patients.
Correlation analysis of (A) atypical MBCs, (B) classical MBCs, (C) plasma cells, (D) naïve B-cells, and (E) immature B-cells with anti-P. falciparum (PfEBA) IgG antibody levels from the plasma of P. falciparum patients. Significance was assessed by non-parametric Spearman correlation analysis.
-
Figure 6—source data 1
Source data for Figure 6.
- https://doi.org/10.7554/eLife.48309.037
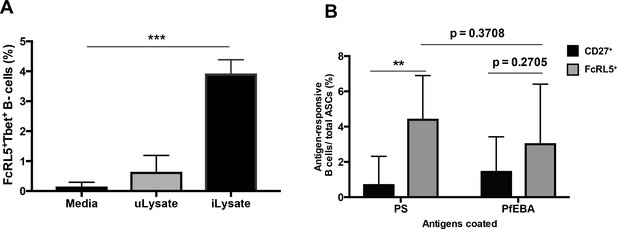
P. falciparum drives the expansion of human FcRL5+ T-bet+ B-cells that secrete anti-PS antibodies in vitro.
(A) Percentage of T-bet+FcRL5+ B-cells that expanded from the PBMCs of a healthy naïve donor after in-vitro exposure to either uninfected erythrocyte lysate (uLysate) or P. -falciparum-infected erythrocyte lysate (iLysate). (B) ELISPOT of enriched populations for either FcRL5 (gray bars) or CD27 (black bars) from PBMCs of healthy naïve US donors after in-vitro exposure to P.-falciparum-infected erythrocyte lysate (iLysate) (N = 3). ASC, antibody-secreting cells. Significance assessed by unpaired Student's t test. **p≤0.01, ***p≤0.001.
-
Figure 7—source data 1
Source data for Figure 7.
- https://doi.org/10.7554/eLife.48309.041
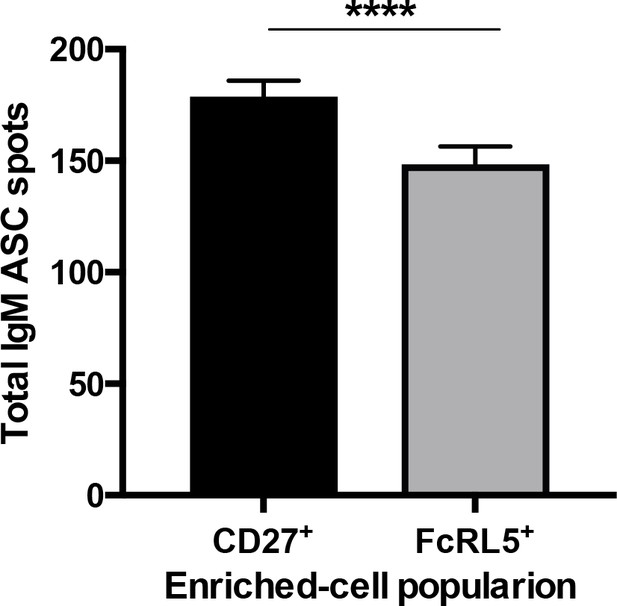
Total antibody-secreting cells among the CD27+- and FcLR5+-enriched PBMC.
Total number of anti-IgM spots of antibody-secreting cells (ASCs) from either CD27+- or FcRL5+-enriched PBMC that were stimulated with P.-falciparum-infected erythrocyte lysate. Significant assessed by unpaired Student's t test, ****p<0.0001.
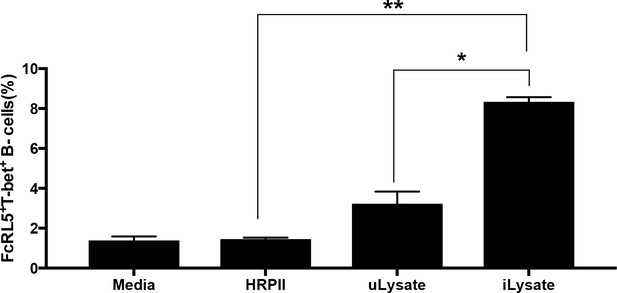
Stimulation with P. falciparum Histidine Rich Protein II (HRPII) does not stimulate the expansion of atypical MBCs in vitro.
Stimulation in vitro of naïve PBMC from healthy US donors (n = 3) with medium, P. falciparum HRPII, uninfected erythrocyte lysate (uLysate) or P.-falciparum-infected erythrocyte lysate (iLysate). Significance was assessed by one-way Anova. *p<0.05, **p<0.01.
Tables
Clinical information from P.-falciparum-infected returned German travelers.
https://doi.org/10.7554/eLife.48309.002| Subject ID | Day of sampling& | Hemoglobin (g/dl)* | Hemoglobin (g/dl)** | Thrombocyte count (1000/µl)* | Parasite count/µl **, # | Red blood cell (RBC) count (million/µl)** | Sex | Age | Type of patient$ | Country of infection |
|---|---|---|---|---|---|---|---|---|---|---|
| 100 | 3 | 10.3 | 13.4 | 135 | 132,000 | 4.6 | m | 51 | VFR | Nigeria |
| 31 | ND | 13.4 | ND | 132,000 | 4.6 | m | 51 | VFR | Nigeria | |
| 101 | 8 | 7.3 | 12.1 | 270 | 1,860,000 | 3.89 | f | 56 | T | Gambia |
| 24 | 8.3 | 12.1 | 589 | 1,860,000 | 3.89 | f | 56 | T | Gambia | |
| 102 | 6 | 13.5 | 16.2 | 140 | <52,800 | 5.28 | m | 63 | T | Uganda |
| 27 | ND | 16.2 | ND | <52,800 | 5.28 | m | 63 | T | Uganda | |
| 103 | 2 | 13.8 | 14.4 | 54 | 46,900 | 4.69 | m | 38 | VFR | Guinea |
| 104 | 3 | 12.6 | 13.6 | 130 | 470,000 | 4.7 | m | 56 | T | Madagascar |
| 7 | 13.7 | 13.6 | 321 | 470,000 | 4.7 | m | 56 | T | Madagascar | |
| 105 | 5 | 7.8 | 8.1 | 135 | 1,050,000 | 3.5 | f | 53 | VFR | Kenya |
| 106 | 2 | 10.8 | 11.9 | 9 | 63,900 | 4.26 | m | 43 | VFR | Ghana |
| 107 | 3 | 11.6 | 12.4 | 48 | >430,000 | 4.3 | m | 52 | VFR | Ghana |
| 12 | 11.4 | 12.4 | 475 | >430,000 | 4.3 | m | 52 | VFR | Ghana | |
| 108 | 0 | 10.3 | 10.3 | 156 | 176 | 3.83 | m | 50 | VFR | Benin |
| 109 | 2 | 10.5 | 10.8 | 98 | 16 | 3.73 | m | 62 | VFR | Ghana |
| 110 | 3 | 12.3 | 13.5 | 128 | 366,800 | 5.24 | f | 20 | VFR | Tanzania |
| 111 | 1 | 13.5 | 14.4 | 23 | 144,600 | 4.82 | m | 35 | T | Nigeria |
| 112 | 3 | 13.1 | 13.7 | 70 | 160,200 | 5.34 | m | 26 | VFR | Benin |
| 113 | 4 | 10.7 | 13.7 | 74 | 340,000 | 4.25 | m | 39 | VFR | Unknown |
| 114 | 3 | 16.6 | 18.7 | 53 | 4840 | 5.96 | m | 26 | T | Ghana |
| 115 | 0 | 12.6 | 12.6 | 53 | 14,762 | 4.84 | m | 62 | VFR | Ghana |
| 116 | 2 | 12.5 | 12.9 | 80 | 26,917 | 4.58 | f | 43 | VFR | Cameroon |
| 4 | 10.4 | 12.9 | 106 | 26,917 | 4.58 | f | 43 | VFR | Cameroon | |
| 117 | 4 | 15 | 19.5 | 32 | 492,800 | 6.16 | m | 46 | T | Nigeria |
| 118 | 1 | 11 | 12.1 | 20 | 296,100 | 4.23 | m | 39 | T | Uganda |
| 119 | 1 | 11.9 | 11.9 | 123 | 29,800 | 4.02 | f | 25 | VFR | Ivory Coast |
| 3 | 11.6 | 11.9 | 116 | 29,800 | 4.02 | f | 25 | VFR | Ivory Coast | |
| 120 | 0 | 16.1 | 16.1 | 102 | 7896 | 5.36 | m | 38 | VFR | Guinea Bissau |
| 121 | 2 | 14.4 | 15.7 | 119 | 496 | 5.48 | m | 34 | VFR | Nigeria |
| 122 | 2 | ND | 13.5 | ND | 74 | 5.15 | m | 18 | VFR | Togo |
| 123 | 2 | 12.8 | 14.2 | 60 | 49,800 | 4.98 | m | 52 | VFR | Ghana |
-
&Days since treatment start to sampling, *Measurement at day of sampling, **Measurement at day of presentation, #Parasitemia expressed in infected erythrocytes per µl of blood, $Tourist (T), Visiting Friend or Relative (VFR). ND, not determined.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Biological sample (Homo sapiens) | CPD backed cells | Interstate Blood bank | ||
| Antibody | Anti-human CD20 (mouse monoclonal) | Biolegend | 302304 | 1:100 |
| Antibody | Anti- T-bet (mouse monoclonal) | Biolegend | 644810 | 1:100 |
| Antibody | Anti-human CD11c (mouse monoclonal) | Biolegend | 301604 | 1:100 |
| Antibody | Anti-human CD27 (mouse monoclonal) | Biolegend | 302806 | 1:100 |
| Antibody | Anti-human CD21 (mouse monoclonal) | Biolegend | 354910 (FITC) 354906 (APC) | 1:100 |
| Antibody | Anti-human FcRL5 (mouse monoclonal) | Biolegend | 340306 | 1:100 |
| Antibody | Anti-human CD10 (mouse monoclonal) | Biolegend | 312210 | 1:100 |
| Antibody | Anti-human CD19 (mouse monoclonal) | Biolegend | 30228 | 1:100 |
| Antibody | Anti-human IgM- HRP (goat polyclonal) | Millipore | AP114P | 1:2000 |
| Antibody | Anti-human IgG-HRP (goat polyclonal) | GE Healthcare | NA933 | 1:2000 |
| Antibody | Anti-human FcRL5-biotin (mouse monoclonal) | Miltenyi Biotec | 130-105-993 | 1:100 |
| Antibody | Anti-human IgM unlabeled (mouse monoclonal) | Biolegend | 314–502 | 15 μg/ml |
| Antibody | Anti-human IgM-biotin(mouse monoclonal) | EMD Millipore | 411543 | 1 μg/ml |
| Peptide, recombinant protein | P. falciparum Erythrocyte Binding Antigen | BEI Resources MR-4 | #MRA-1162 | 15 μg/ml |
| Commercial assay or kit | True-Nuclear Transcription Factor Buffer Set | Biolegend | 424401 | |
| Commercial assay or kit | MycoAlert Mycoplasma Detection Kit | Lonza | LT07-118 | |
| Commercial assay or kit | TMB substrate | BD Biosciences | 555214 | |
| Commercial assay or kit | CD27 Microbeads human | Miltenyi Biotec | 130-051-601 | |
| Chemical compound, drug | Ionomycin | Life technologies | I24222 | 2.5 µM |
| Chemical compound, drug | Ficoll-Paquee Plus | GE Life Sciences | 17144002 | |
| Software, algorithm | GraphPad PRISM | GraphPad PRISM | ||
| Other | Phosphatidylserine | Sigma-Aldrich | P7769 | 20 μg/ml |
| Other | Calf Thymus DNA | Sigma-Aldrich | D4522 | 10 μg/ml |
| Other | Stop buffer | Biolegend | 423001 | |
| Other | Annexin V | Biolegend | 640902 | 0.5 µM |
| Other | X- VIVO 15 media | Lonza | 04-418Q | |
| Other | Human AB healthy plasma | Sigma-Aldrich | H4522 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.48309.042





