Ternary structure of the outer membrane transporter FoxA with resolved signalling domain provides insights into TonB-mediated siderophore uptake
Figures
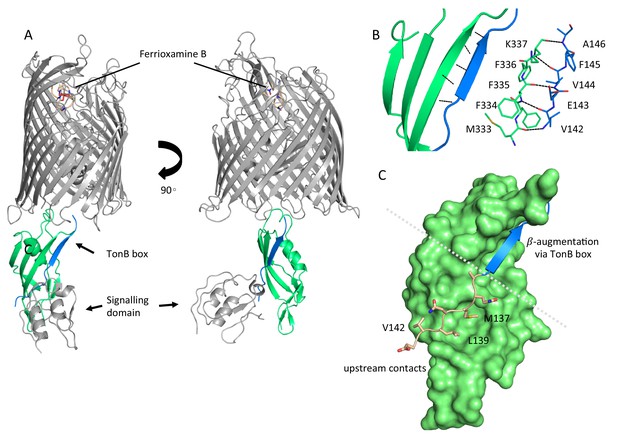
Complex formation between ferrioxamine B-bound FoxA and TonB is driven by multiple binding sites.
(A) Overview of the ferrioxamine B-bound FoxA-TonBCt complex. TonBCt (green) interacts with the TonB box (blue) of FoxA (grey). (B) Parallel β–strands formed between the TonB box of FoxA (blue) and TonBCt (green) through β-augmentation. All contacts are mediated predominantly by backbone hydrogen bonds between the two proteins. (C) Polypeptide stretch (pink) upstream of the TonB box (blue) forms additional contacts with the surface of TonB (green).
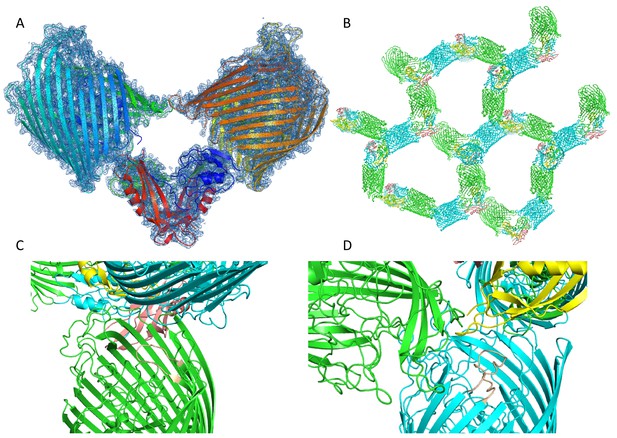
Crystal packing of the FoxA-ferrioxamine B-TonBCt complex.
(A) The contents of the asymmetric unit of the complex with electron density contoured at 1 σ. (B) Crystal packing of the complex. (C) and D) show examples of crystal packing between the FoxA complexes showing little effect of crystal packing on loop conformations between the two monomers of FoxA and the neighbouring molecules.
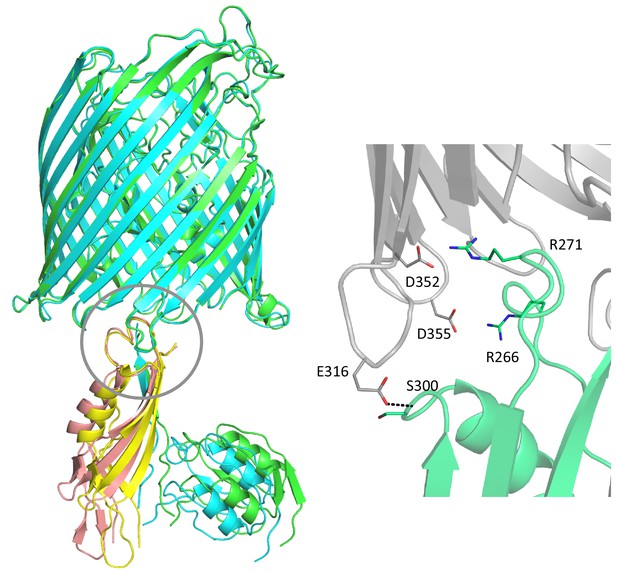
Flexibility in the FoxA-TonB complex.
Overlay of the two individual complexes found in the asymmetric unit reveals that the TonB fragment and the signalling domain along with the TonB box are shifted by roughly 9° in the distal part of the complex. The protein region closest to the membrane and the barrel lumen exhibit limited movement, highlighting the importance of the secondary tethering site in FoxA. Secondary contacts between FoxA (grey) and TonBCt (green) are mediated through electrostatic interactions and hydrogen bonds.
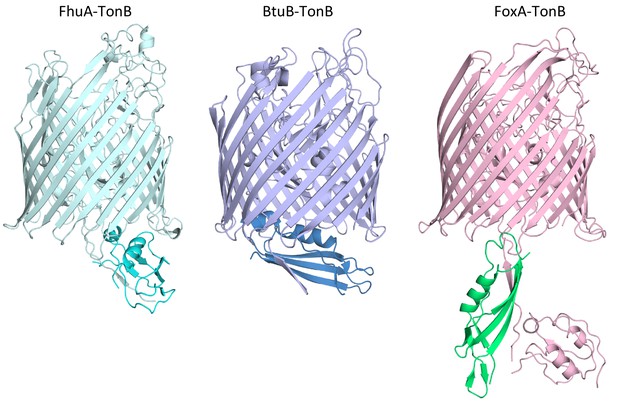
Comparison of different TBDT-TonB complex structures reveals distinct mechanisms of TonB capture and positioning.
Shown are the structures of FhuA-TonB (PDBID: 2GRX) and BtuB-TonB (PDBID: 2GSK).
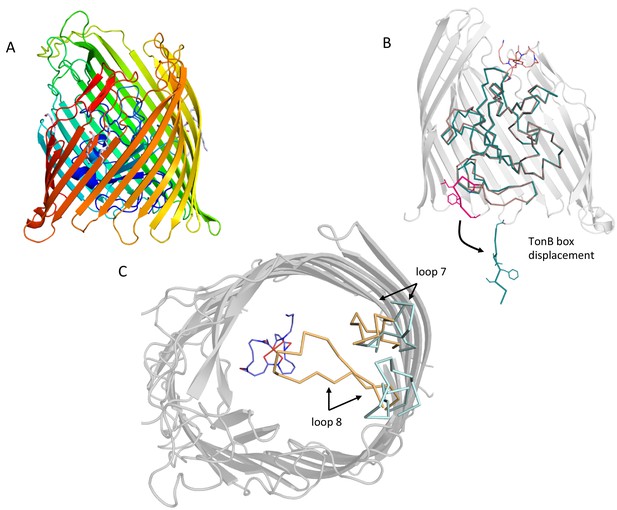
Conformational changes in the plug domain and extracellular loops of FoxA in response to ferrioxamine B and TonB binding.
(A) The overall fold of apo FoxA consists of a 22-stranded β-barrel lined by the small globular plug domain within the lumen. The structure is colour-coded from blue (N-terminus) to red (C-terminus). (B) Structural rearrangements within the plug domain necessary to accommodate interactions with TonBCt. The region of the polypeptide being part of the TonB box common to both FoxA structures is highlighted in pink. This region is displaced by approximately 22 Å into the periplasm. Slight conformational changes are also observed throughout the rest of the plug domain (blue: ternary complex/brown: apo state). (C) Loops 7 and 8 enclose the bound siderophore within the hydrophobic cavity to prevent its dissociation and reduce permeation across the bacterial membrane during the process of siderophore uptake. Loop closure is only evident once the FoxA is bound with ferrioxamine B and the TonBCt fragment (loops coloured brown), indicative of allosteric communication between the extracellular and periplasmic regions of the transporter (blue loops correspond to the apo FoxA).
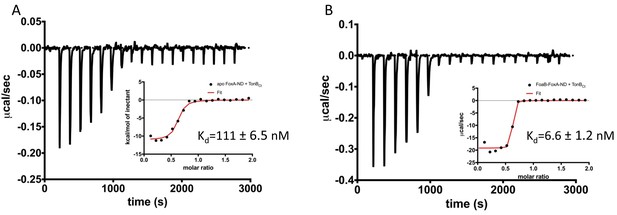
Two distinct modes of association between FoxA and TonB as revealed by the thermodynamics of complex formation.
ITC profiles showing titration of TonBCt (150 μM) into 15 μM apo FoxA nanodisc complexes (A) and 15 μM ferrioxamine B-FoxA nanodisc complexes (B). Insets show the integrated heats of binding with a single-site fit to the data.
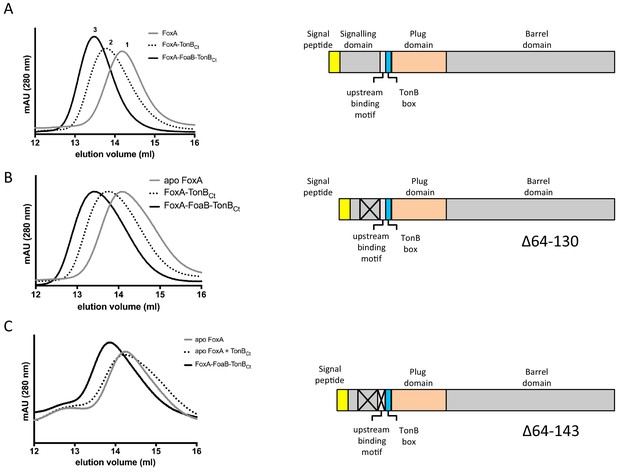
Delineation of the constitutive TonB-binding motif in FoxA using analytical size-exclusion chromatography.
(A) Addition of TonBCt (100 μM) results in the earlier elution of the main peak when mixed with purified apo FoxA (10–20 μM). In the presence of ferrioxamine B (0.5 mM) the main peak shifts to even earlier elution volumes. (B) Deletion of residues 64–130 in FoxA does not affect the elution profiles of the complexes with and without ferrioxamine B. (C) Truncation of residues 64–143 from the full-length FoxA abrogates constitutive mode of binding between the receptor and TonBCt, however the proteins can still associate in the presence of ferrioxamine B.
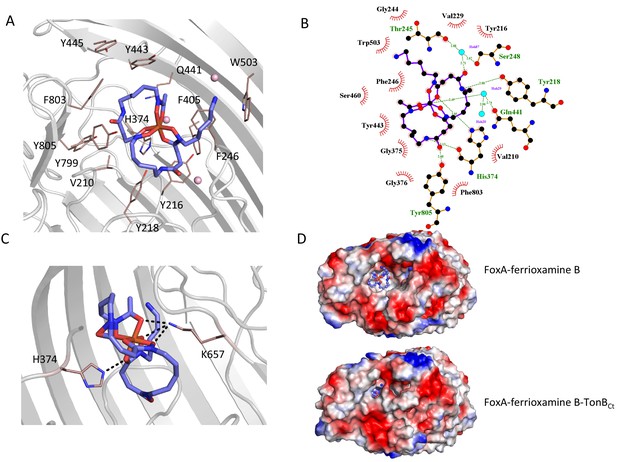
Ferrioxamine B interactions with FoxA reveal the basis for high-affinity siderophore capture.
(A) and B) Interaction of ferrioxamine B by hydrophobic and aromatic residues lining the binding pocket of FoxA. Several hydrogen bonds are also observed between ferrioxamine B and residues His374 and a Gln441-H2O network, respectively. (C) The closure of loop eight results in additional hydrogen bonds between ferrioxamine B and the ε-amino group of Lys657 facing the siderophore, which becomes locked from both sides by hydrogen bonds. (D) Large hydrophobic cavity facing the extracellular milieu occupied by ferrioxamine B. In the apo/ferrioxamine B structures the cavity and ferrioxamine B are solvent exposed (top), whereas in the ternary complex (bottom) the loop closure sequesters ferrioxamine B inside the barrel.
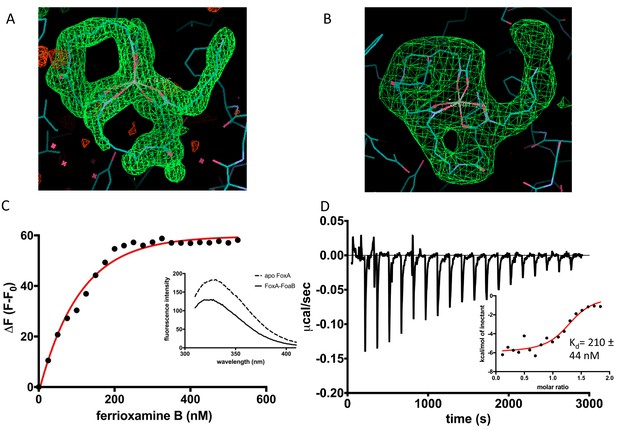
Characterisation of ferrioxamine B binding to FoxA.
(A) Polder omit maps of ferrioxamine B in the ferrioxamine B-bound crystal structure of FoxA contoured at 3 σ. (B) Polder omit map of ferrioxamine B in the ternary complex contoured at 7.2 σ. (C) Titration of ferrioxamine B into purified FoxA (100 nM protein) leads to quenching of tryptophan fluorescence. The difference in fluorescence is plotted (black circles) and the data are fitted to a single-site binding model (red curve) yielding a dissociation constant (Kd) of 100 ± 10 nM. Inset: fluorescence spectra of 100 nM FoxA purified in nonyl glucopyranoside in the absence and in the presence of saturating amounts of ferrioxamine B (500 nM). (D) ITC experiment measuring ferrioxamine B titration (250 μM) into 15 μM FoxA-ND. Inset shows the integrated heats fitted to a single-site binding model with the calculated Kd of 210 nM.
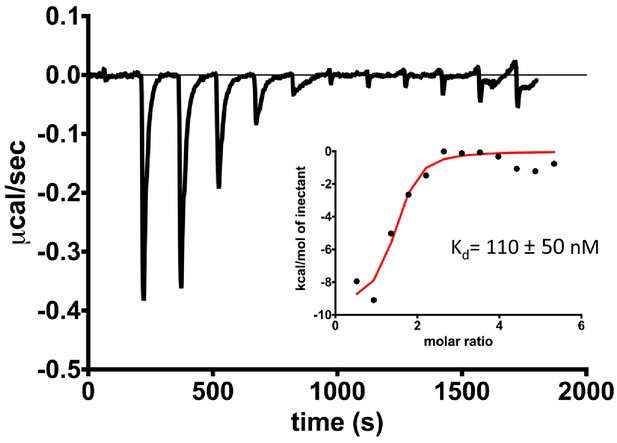
ITC measurement titrating 500 μM ferrioxamine B into 15–20 μM of pre-assembled FoxA-TonBCt in nanodiscs shows that constitutive binding of TonBCt does not lead to the closure of extracellular loops in apo FoxA.
https://doi.org/10.7554/eLife.48528.012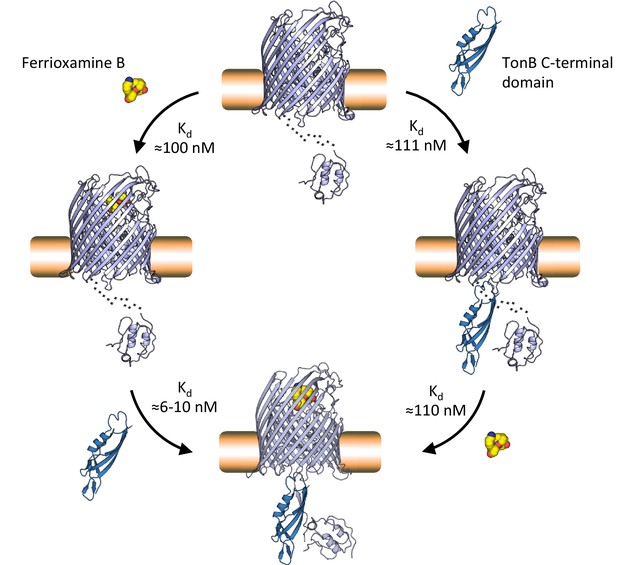
Proposed mechanism of TonB-mediated ferrioxamine B uptake via the FoxA transporter.
Our studies suggest that FoxA can exist in several states depending on the abundance of ferrioxamine B in the environment and the occupancy of TonB by other TBDRs. FoxA is able to interact with ferrioxamine B or engage with TonB with near-equal affinity (Kd≈100 nM) in a constitutive fashion. The presence of ferrioxamine B, however, is necessary for the expulsion of the TonB box into the periplasm and the formation of the full, translocation-competent ternary complex through β-augmentation (Kd≈6–10 nM). This very high-affinity interaction provides the necessary contacts in the complex for subsequent steps of the plug domain re-modelling or expulsion, necessary for siderophore translocation through the lumen of the barrel.
Videos
Visualising the conformational changes occurring in FoxA in response to ferrioxamine B and TonBCt binding.
We observe the closure of extracellular loops 7 and 8 as a prerequisite for translocation of ferrioxamine B. At the periplasmic side, TonB box is expelled from the plug domain in order to make contacts with the TonB molecule.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Pseudomonas aeruginosa) | FoxA (Pseudomonas aeruginosa strain PAO1) | PA2466 | ||
| Strain, strain background (E. coli) | Lemo 21 | New England Biolabs | C2528J | |
| Strain, strain background (E. coli) | BL21 Gold (DE3) | Agilent | 230132 | |
| Chemical compound, drug | desferrioxamine B | Sigma-Aldrich | D9533 | |
| Chemical compound, drug | octyl glucopyranoside (OG) | Anatrace | O311 | |
| Chemical compound, drug | nonyl glucopyranoside (NG) | Anatrace | N324 | |
| Chemical compound, drug | C8E4 | Anatrace | T350 | |
| Software, algorithm | XDS | (Kabsch, 2010) | http://xds.mpimf-heidelberg.mpg.de/ | |
| Software, algorithm | AIMLESS | (Evans, 2011) | http://www.ccp4.ac.uk/html/aimless.html | |
| Software, algorithm | Phaser | (McCoy et al., 2007) | http://www.ccp4.ac.uk/html/phaser.html | |
| Software, algorithm | PHENIX 1.14 | (Adams et al., 2010) | https://www.phenix-online.org/ | |
| Software, algorithm | Coot 0.8.9.1 | (Emsley et al., 2010) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ | |
| Software, algorithm | REFMAC5 | (Murshudov et al., 2011) | http://www.ccp4.ac.uk/html/refmac5.html | |
| Software, algorithm | Buster-TNT | (Blanc et al., 2004) | https://www.globalphasing.com/buster/ |
Additional files
-
Supplementary file 1
Supplementary tables.
- https://doi.org/10.7554/eLife.48528.014
-
Transparent reporting form
- https://doi.org/10.7554/eLife.48528.015





