An arbitrary-spectrum spatial visual stimulator for vision research
Figures
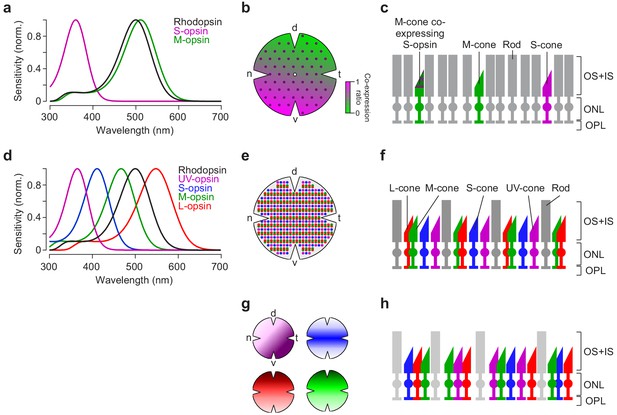
Photoreceptor types and distribution in mouse and zebrafish retina.
(a) Peak-normalised sensitivity profiles of mouse S- (magenta) and M-opsin (green) as well as rhodopsin (black; profiles were estimated following Stockman and Sharpe, 2000). (b) Schematic drawing of the distribution of cone photoreceptor (cone) types in the mouse; rod photoreceptors (rods) are homogeneously distributed (Jeon et al., 1998) (not shown here). Purple dots represent ‘true’ S-cones exclusively expressing S-opsin (Haverkamp et al., 2005); ratio of co-expression of S-opsin in M-cones (Applebury et al., 2000; Baden et al., 2013) is colour-coded from green to magenta (d, dorsal; t, temporal; v, ventral; n, nasal). (c) Illustration of mouse cone and rod arrangement (vertical view; OS+IS, outer and inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer). (d) Peak-normalised sensitivity profiles of zebrafish UV- (magenta), S- (blue), M- (green) and L-opsin (red) as well as rhodopsin (black). (e) Schematic illustration of the regular cone arrangement in adult zebrafish. Coloured dots represent UV-, S-, M- and L-cones. (f) Like (c) but for adult zebrafish retina. (g) Schematic drawing illustrating the distribution of cone types in zebrafish larvae (Zimmermann et al., 2018). Colours as in (d). (h) Like (c,f) for zebrafish larvae. Lighter colour of rods indicate that they are not functional at this age (7–9 dpf; Branchek and Bremiller, 1984; Morris and Fadool, 2005).
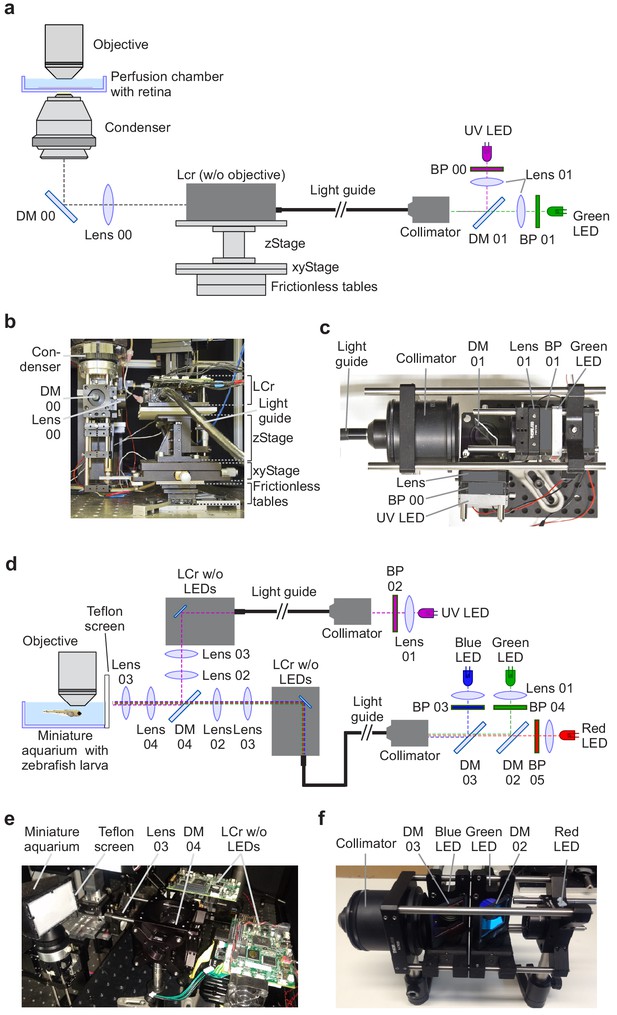
Visual stimulator design.
(a) Schematic drawing of the dichromatic stimulator for in vitro recordings of mouse retinal explants. The stimulator is coupled into the two-photon (2P) microscope from below the recording chamber with the retinal tissue (through-the-condenser; for alternative light paths (through-the-objective), see Figure 2—figure supplement 1). DM, dichroic mirror; BP, band-pass filter; LCr, lightcrafter; LED, light-emitting diode. For components, including custom-made parts, see Table 2. (b) LCr unit and substage portion of the 2P microscope in side-view. (c) External LED illumination unit in top-view. For details on mechanical parts, see Figure 2—figure supplement 6. (d) Schematic drawing of the tetrachromatic stimulator for in vivo recordings in zebrafish larvae. The optical pathways of two LCrs are combined and the stimulus is projected onto a UV-transmissive teflon screen at one side of the miniature aquarium. For components, see Table 3. (e) Side-view of tetrachromatic stimulation setup. (f) RGB external LED illumination unit of tetrachromatic stimulation setup. Band-pass (BP) filters 03, 04 and 05 as well as lenses 01 are not indicated due to space constraints.
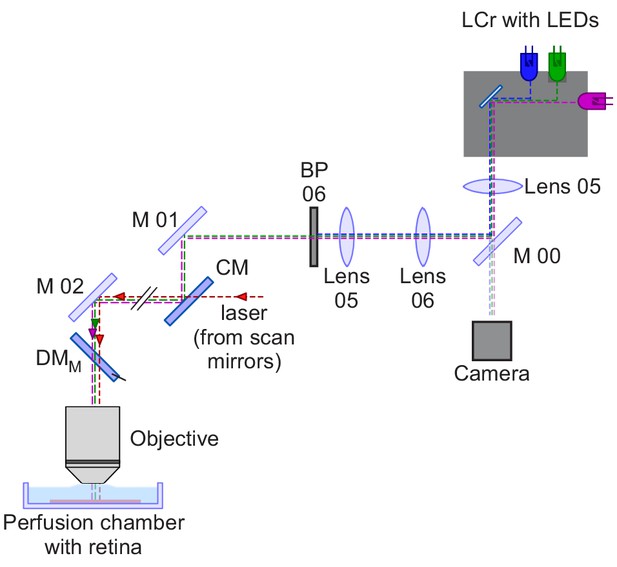
Optical pathway for a through-the-objective (TTO) mouse stimulator.
Schematic drawing of a TTO dichromatic stimulator for in vitro recordings of mouse retinal explants (cf. Euler et al., 2009). The light from the LCr with internal UV, blue and green LEDs is filtered by a dual-band filter transmitting UV and green light. Then, the light is coupled into the two-photon microscope using a cold mirror (CM). Using a beam-splitter (M 00), a small fraction of light is projected onto a camera to allow online visualisation of the visual stimulus. LCr, lightcrafter; LED, light-emitting diode; M, mirror; CM, cold mirror; DMM, dichroic mirror mouse; BP, band-pass filter. For specifications of the components, see Table 2 and Supplementary file 1.
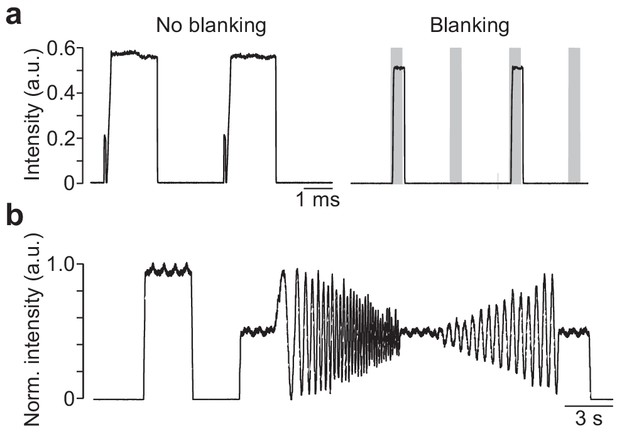
Intensity measurements of the LEDs of the mouse stimulator.
(a) Intensity of green LED measured with a PMT (at 250 kHz; for details, see Materials and methods) without (left) and with blanking (right); grey shading indicates blanking signal. (b) Smoothed (box smooth, box width: 100 ms) intensity profile of a full-field chirp stimulus recorded with a fast photodiode.
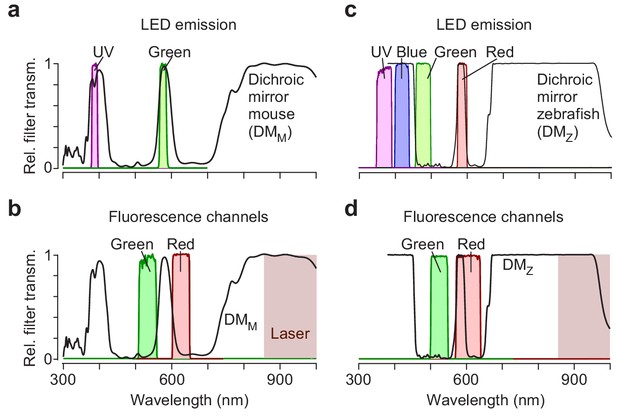
Spectral separation of visual stimulation and fluorescence detection.
(a) Relative transmission of filters in front of UV and green LED as well as of dichroic mirror on top of objective (cf. Euler et al., 2009). (b) Same as (a), for filters in front of PMTs (Materials and methods). Burgundy shading illustrates the wavelength range used for two-photon laser. (c, d) Like (a) and (b) but for zebrafish stimulator.
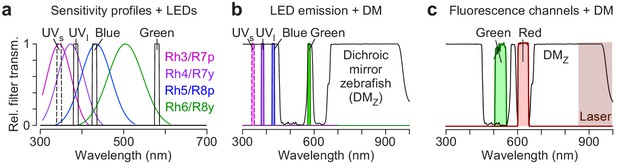
Suggestion for LED/filter design for a Drosophila visual stimulator.
(a) Spectral sensitivity of rhodopsins expressed in the four types of inner photoreceptors (Rh3: short-UV; Rh4: long-UV; Rh5: blue; Rh6: green) of Drosophila (data from Schnaitmann et al., 2018, based on Salcedo et al., 1999), with a possible combination of band pass-filtered LEDs for chromatic stimulation (black). Dotted line for the short-UV LED indicates that for wavelengths < 380 nm, additional optimisation of the optical parts within the LCr is required (see Discussion). (b) Suggested filter combination (black) from (a) can be combined with the dichroic mirror used for the zebrafish stimulator (DMZ; grey). (c) DMZ with suitable filters in front of PMTs for detection of green (535/50 BrightLine HC, AHF) and red (630/38 BrightLine HC, AHF) fluorescence. Burgundy shading marks wavelength range used for 2P laser.
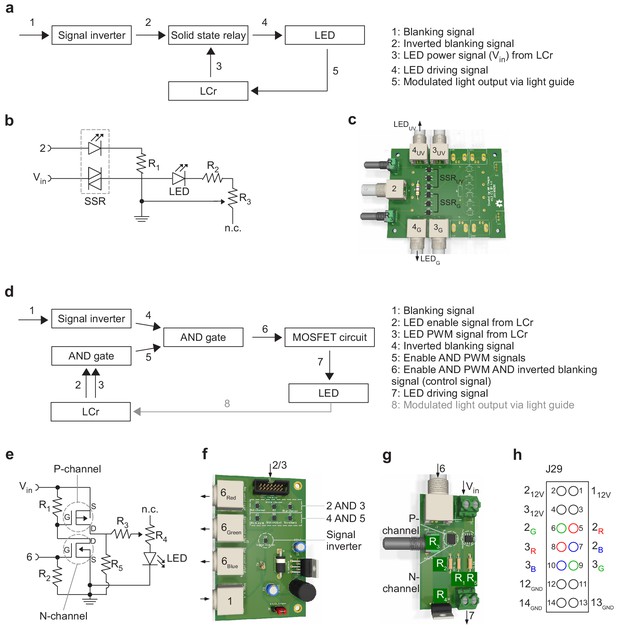
External LED control and temporal separation of stimulation and fluorescence detection.
(a) Schematic illustrating the circuit that ensures that the stimulator LEDs are only on during the microscope's scanner retrace (for details, see Results). The ‘laser blanking’ signal (1) generated by the scan software is inverted (2) and then used to drive solid-state relays that modulate the LED power signal generated by the LCr (3). This modulated power signal (4) drives the LEDs. The LED light (5) is fed to the LCr via a light guide. (b) Wiring diagram of the solid-state relay (SSR) circuit (signals like in (a); R1 = 220 Ω, the values for R2 and potentiometer R3 depend on the LEDs used and typically are in the range of 0–500 Ω). (c) Rendering of the custom-printed circuit board, which can accommodate up to four LED channels (only the components for two channels are soldered). (d) Schematic illustrating an alternative circuit to (a–c), where the LEDs are powered from an external supply. Here, the LCr LED control signals (2, 3) go through a logical AND operation and the resulting signal (5) is then combined with the inverted blanking signal (4). The resulting signal (6) is used to switch the LED power using a combination of P and N-channel MOSFETs (cf. (e)). Finally, the LED power signal (7) drives the internal or external LEDs. (e) Wiring diagram of the MOSFET circuit (signals as in (d); R1 = 220 Ω, R2 = 220 Ω, R3 = 0.5 Ω, potentiometer R4 = 25 Ω, R5 = 1 kΩ). (f,g) Rendering of two custom-printed boards responsible for combining the control signals (logic board), up to three LED channels per board (f) and switching the LEDs (driver board), just one LED channel per board (g). (h) Pinout of connector J29 (‘external LED driver connector’) on the LCr board. To disable the LCr’s internal LED drivers, jumper J30 must be installed, while J28 is used to choose between 3.3V or 1.8V supply voltage (see Table 1 for links to LCr instruction manuals).
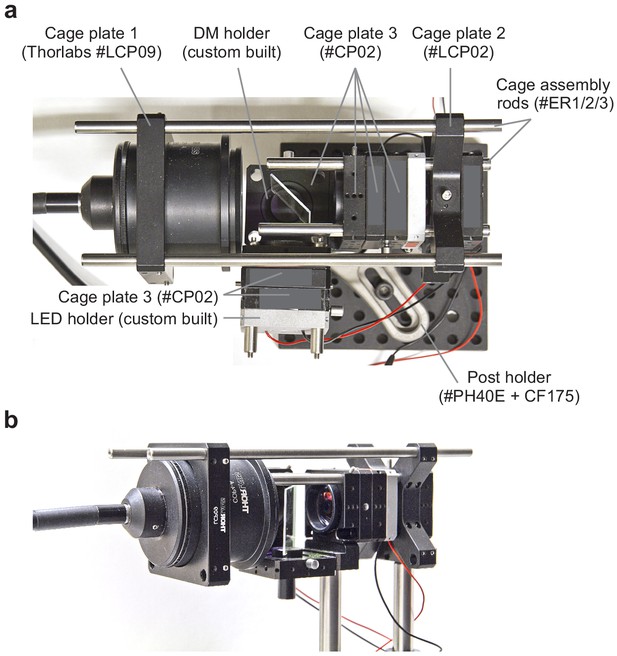
Detailed description of external LED unit of the mouse stimulator.
(a) Top-view of external LED illumination unit, with ordering numbers of all parts purchased from Thorlabs indicated (see also Table 2). Cage plates #LCP02 holds filters and lenses with a diameter of 0.5’’. DM (Nylon) and DM and LED holders (Aluminium) were custom-built by the University workshop (Table 2); the latter resemble Thorlab’s cage plates but with mounting holes for the LEDs. The LED holders dissipated the heat from the relatively low-power LEDs used for the mouse and zebrafish stimulator sufficiently, such that a cooling fan was not needed. (b) Side-view of external LED illumination unit.
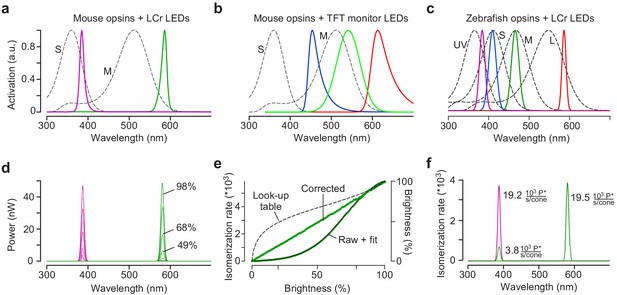
Calibration of the mouse stimulator.
(a) Sensitivity profiles of mouse S- and M-opsin (dotted black lines) and spectra of UV (magenta) and green LED/filter combinations used in the mouse stimulator. (b) Sensitivity profiles of mouse S- and M-opsin (dotted black line) and spectra of blue, green and red LED present in a standard TFT monitor. (c) Sensitivity profiles of zebrafish opsins (dotted black lines) and spectra of UV, blue, green and red LEDs used in the zebrafish stimulator. (d) Spectra (in nW) of UV and green LED obtained from measurements using increasing brightness levels; shown are spectra for 0, 9, 19, 29, 39, 49, 59, 68, 78, 88, and 98% brightness. (e) Non-linearised intensity curve (‘raw’) with sigmoidal fit (black), estimated gamma correction curve (black dotted line; ‘Look-up table’) and linearized intensity curve (‘corrected’) for green LED. (f) Photoisomerisation rates for maximal brightness of UV (19.2 and 3.8 photoisomerisations ·103 P*/second/cone for S- and M-opsin, respectively) and green LED (0 and 19.5 ·103 P*/second/cone for S- and M-opsin, respectively). Note that the UV LED also activates M-opsin due to its increased sensitivity in the short wavelength range (β-band, Discussion).
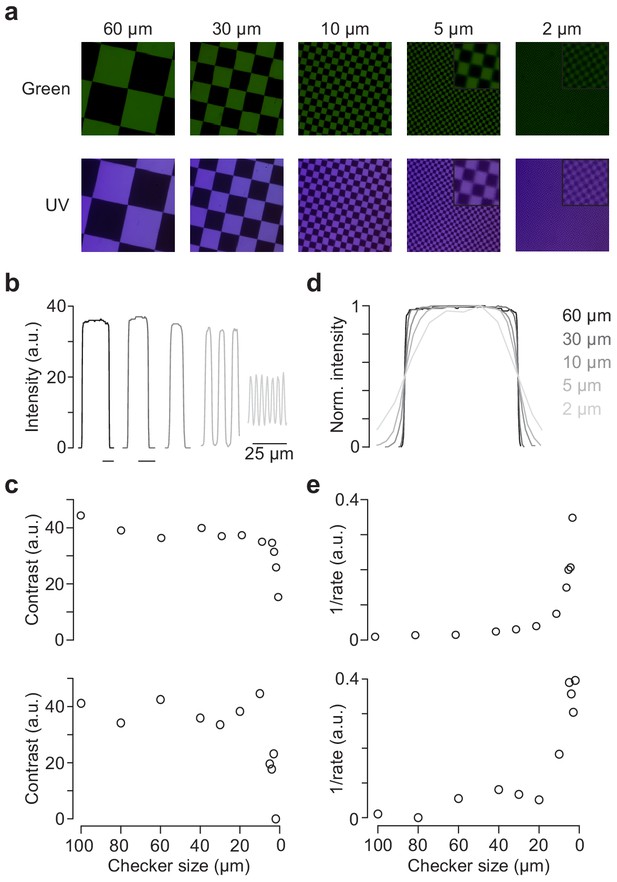
Spatial calibration of the mouse stimulator.
(a) Images of checkerboard stimuli with varying checker sizes projected through-the-objective (TTO) for illumination with green (top) and UV (bottom) LED, recorded by placing the sensor chip of a Raspberry Pi camera at the level of the recording chamber. Focus was adjusted for UV and green LED separately. Insets for 5 and 2 µm show zoomed in regions of the image. (b) Intensity profiles for five different checker sizes of green LED. (c) Contrasts () for checkerboards of varying checker sizes of the TTO (top) and through-the-condenser (TTC; bottom) configuration. (d) Peak-normalised intensity profiles of different checker sizes, scaled to the same half-maximum width. (e) 1/rate estimated from sigmoidal fits of normalised intensity curves like in (d) for varying checker sizes of the TTO (top) and TTC (bottom) configuration.
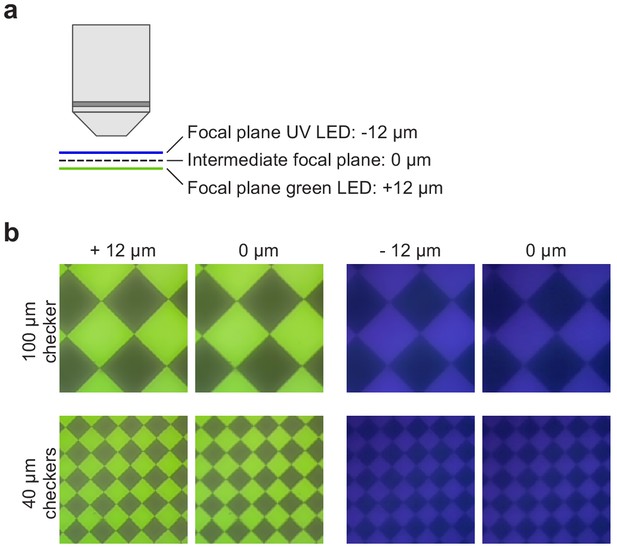
Chromatic aberration of the mouse stimulator.
(a) Schematic illustrating the chromatic aberration-related difference in focal planes of UV and green image in the TTO configuration. (b) Images of a 100 (top) and 40 (bottom) µm checkerboard stimulus using the green (left) and UV (right) LED, recorded by placing the sensor chip of a Raspberry Pi camera at the level of the recording chamber. Focus was set to intermediate focal plane (see (a); ±12 µm) or was adjusted for UV and green LED separately (0 µm).
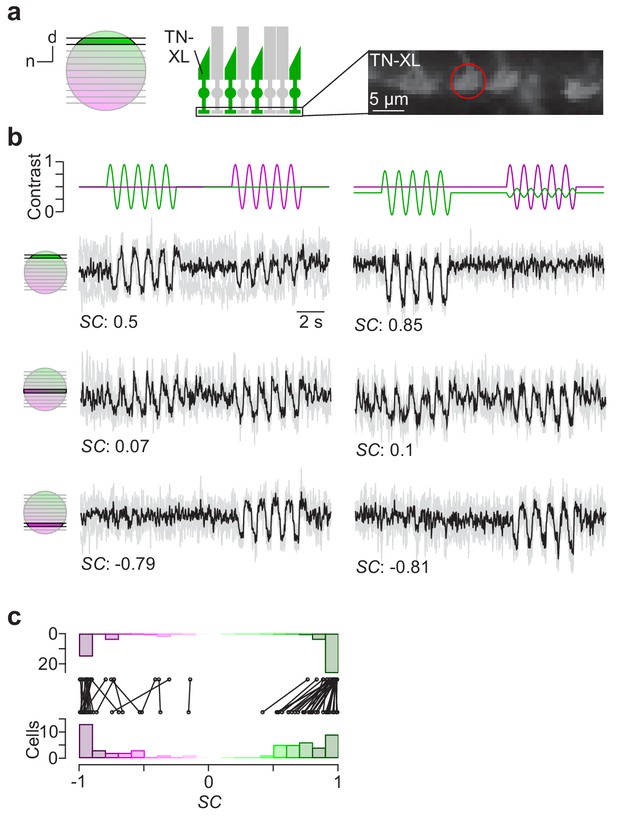
Cone-isolating stimulation of mouse cones.
(a) Dorsal recording field in the outer plexiform layer (OPL; right) shows labelling of cone axon terminals with Ca2+ biosensor TN-XL in the HR2.1:TN-XL mouse line (Wei et al., 2012). Schematic on the left illustrates retinal location of recorded slice. (b) Ca2+ traces (mean traces in black, n = 3 trials in grey) of cone axon terminals located in dorsal (top; cone axon terminal from (a)), medial (middle) and ventral (bottom) retina in response to 1 Hz sine modulation of green and UV LED, with spectral contrast (SC) indicated below. Colour substitution protocol (right) estimated from calibration data (Materials and methods). (c) Distribution and comparison of SC for sine modulation stimulus with (top) and without (bottom) silent substitution protocol (n = 55 cells, n = 12 scan fields, n = 1 mouse; p=9.31*10−9 for dorsal cells, n = 30; p=0.92 for ventral cells, n = 25; Wilcoxon signed-rank test).
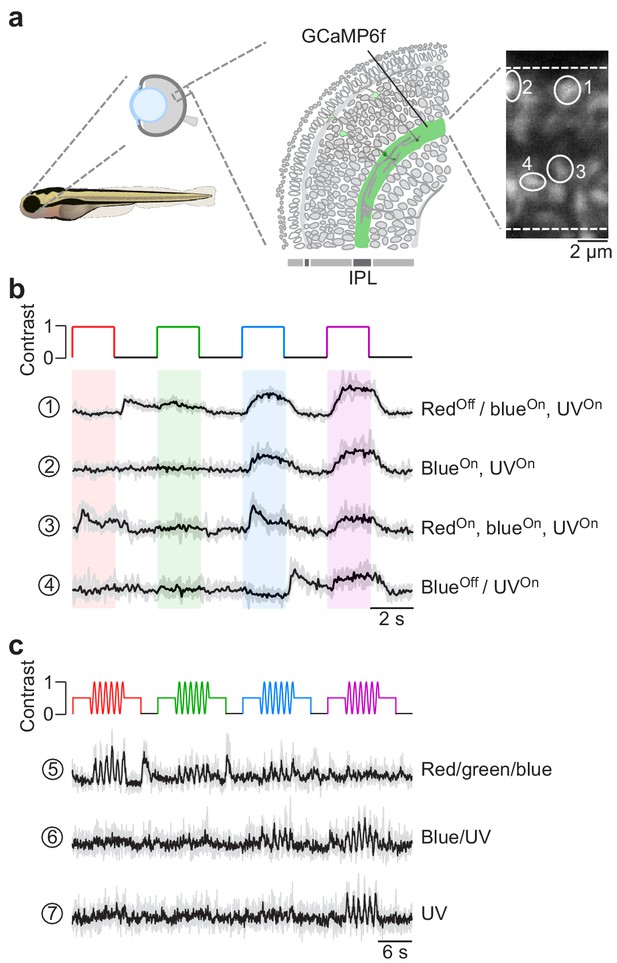
Chromatic responses in bipolar cells of in vivo zebrafish larvae.
(a) Drawing illustrating the expression of the genetically encoded Ca2+ biosensor SyGCaMP6f in bipolar cell terminals (left) of tg(1.8ctbp2:SyGCaMP6f) zebrafish larvae and scan field of inner plexiform layer (IPL; right), with exemplary regions-of-interest (ROIs) marked by white circles. (b) Mean Ca2+ traces (black; n = 6 trials in grey) in response to red, green, blue and UV full-field flashes (90 × 120 degrees visual angle, presented to the fish’s right side). (c) Mean Ca2+ traces (black; n = 4 trials in grey) in response to full-field sine modulation (at 1 Hz) of red, green, blue and UV LED.
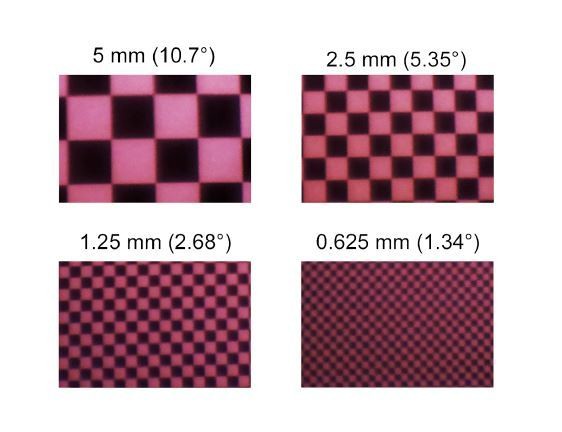
Images of checkerboard stimuli with varying sizes for UV LED of the zebrafish stimulator, recorded using a Raspberry Pi camera positioned between the LCrs and the teflon screen.
https://doi.org/10.7554/eLife.48779.022Tables
For detailed part lists, see Tables 2 and 3.
https://doi.org/10.7554/eLife.48779.003| Part | Links to online resources |
|---|---|
| TI DLP LightCrafter 4500 Evaluation Module (‘LCr’) | Product overview: http://www.ti.com/tool/dlplcr4500evm User guide: http://www.ti.com/lit/ug/dlpu011f/dlpu011f.pdf Programmer’s guide: http://www.ti.com/lit/ug/dlpu010g/dlpu010g.pdf DLP4500 data sheet w/DMD specs: http://www.ti.com/lit/ds/symlink/dlp4500.pdf Alternate DMD windows for increased UV transmission: http://www.ti.com/lit/an/dlpa031d/dlpa031d.pdf DMD reflectance w/o window: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20160010355.pdf |
| QDSpy – Open Source Visual stimulation software | Documentation: http://qdspy.eulerlab.de/ Python source code: https://github.com/eulerlab/QDSpy (Euler, 2019b; copy archived at https://github.com/elifesciences-publications/QDSpy) Information on ‘pattern mode’ with QDSpy: http://qdspy.eulerlab.de/lightcrafter.html#example-scripts |
| Chopper | For a ‘mechanical’ LED blanking solution (see Discussion), based on Thorlabs’ Optical Chopper System |
| open-visual-stimulator – Project GitHub repository | Contains spectral calibration scripts, 3D design files for printed parts, printed circuit board design files, bill of materials to populate boards, etc. https://github.com/eulerlab/open-visual-stimulator (Euler et al., 2019a; copy archived at https://github.com/elifesciences-publications/open-visual-stimulator) |
Parts list of the mouse visual stimulator (cf. Figure 2a–c and Figure 2—figure supplement 6).
https://doi.org/10.7554/eLife.48779.011| Part | Description (link) | Company | Item number |
|---|---|---|---|
| Parts for stimulator (except external illumination unit) | |||
| LCr | 0.45' ‘DLP Fiber couple E4500MKII Development Module FC/PC | EKB Technologies Ltd. | DPM-FE4500MKIIF |
| Condenser | C-C Achromat-Aplanat Condenser N.A. 1.40, oil | Nikon | MBL71400 |
| Objective | W Plan-Apochromat 20x/1.0 DIC (UV) VIS-IR | Zeiss | 421452-9880-000 |
| DM 00 | Beamsplitter 900DCXXR | AHF Analysetechnik AG | F73-903 |
| Lens 00 | Achromatic Doublet, f = 75 mm | Thorlabs | AC254-075-A-ML |
| Light guide | Liquid light guide 5 mm Core, 1.2 m length | Thorlabs | LLG05-4H |
| z stage | 13 mm Travel Vertical Translation Stage | Thorlabs | MVS005/M |
| x-y stage | XY Stage, 13 mm Travel | Thorlabs | XYT1/M |
| Frictionless tables | Type NK frictionless table (rollers/balls) | Schneeberger GmbH | NK2-50 (x2) |
| Perfusion chamber | Quick release magnetic imaging chamber | Warner Instruments | 64–1943 |
| Parts for the external illumination unit | |||
| Cage plate 1 | Cage Plate with Ø2.2’ Double Bore | Thorlabs | LCP09/M |
| Cage plate 2 | 30 mm to 60 mm Cage Plate Adapter | Thorlabs | LCP02/M |
| Cage plate 3 | SM1-Threaded Standard Cage Plates | Thorlabs | CP02/M (x7) |
| Cage assembly rods | ER Assembly Rods for 30 mm and 60 mm Cage Systems | Thorlabs | ER1/2/3 (x4) |
| Post holders | Pedestal Post Holders | Thorlabs | PH40E (x2) |
| Post holders | Clamping Forks and Base Adapters | Thorlabs | CF175 (x2) |
| Collimator | 5 mm LLG Collimating Adapter, Zeiss Axioskop | Thorlabs | LLG5A4-A |
| BP 00 | 387/11 BrightLine HC | AHF | F39-387 |
| BP 01 | 576/10 BrightLine HC | AHF | F37-576 |
| UV LED | 385 nm, 320 mW at 350 mA, +- 75° | Roithner | H2A1-H385-r2 |
| Green LED | 590 nm, 8–10 mW at 350 mA, +- 30° | Roithner | M3L1-HY-30 |
| DM 01 | Beamsplitter HC BS 495 | AHF | F38-495 |
| Lens 01 | 1’’ N-BK7 Plano-Convex Lens, f = 25.4 mm | Thorlabs | LA1951-A-ML (x2) |
| DM holder | DM_holder, Nylon | Custom-made (University workshop) | |
| LED holder | LED_holder, Aluminium | Custom-made (University workshop) | (x2) |
Parts list of the zebrafish visual stimulator (cf. Figure 2d,e; italic entries are not shown in figure).
https://doi.org/10.7554/eLife.48779.012| Part | Description (link) | Company | Item number |
|---|---|---|---|
| Parts for stimulator (except external illumination unit) | |||
| LCr | 0.45' ‘DLP Fiber couple E4500MKII Development Module FC/PC | EKB Technologies Ltd. | DPM-FE4500MKIIF (x2) |
| DM 04 | Beamsplitter T 400 LP | AHF | F79-100 |
| Lens 02 | Mounted N-BK7 Bi-Convex Lens, f = 50.0 mm | Thorlabs | LB1844-ML (x2) |
| Lens 03 | Air-Spaced Achromatic Doublet, f = 50 mm | Thorlabs | ACA254-050-A (x3) |
| Lens 04 | Achromatic Doublet, f = 100 mm | Thorlabs | AC508-100-A-ML |
| Light guide | Liquid light guide 5 mm Core, 1.2 m length | Thorlabs | LLG05-4H (x2) |
| z stage | 13 mm Travel Vertical Translation Stage | Thorlabs | MVS005/M (x2) |
| x-y stage | 13 mm Translation Stage | Thorlabs | MT1B/M (x4) |
| Mount plate | Aluminium Breadboard | Thorlabs | MB2530/M |
| Lens holder | 30 mm to 60 mm Cage Plate Adapter | Thorlabs | LCP02/M (x4) |
| Optical Post | Optical Post 12.7 mm diam | Thorlabs | TR100/M (x2) |
| Post Holder | Post holder 12.7 mm diam 100 mm | Thorlabs | PH100/M (x2) |
| Post clamp | Clamping Fork, 1.24’ Counterbored Slot | Thorlabs | CF125-P5 (x2) |
| Metal rods | Thorlabs | ER-12 (x4) | |
| Metal rods | Cage Assembly Rod, 3’ Long, Ø6 mm | Thorlabs | ER-3 (x8) |
| Dichroic holder | Kinematic Cage Cube Platform for C4W/C6W, Metric | Thorlabs | B4C/M |
| Dichroic holder | Cage compatible rectangular filter mount | Thorlabs | FFM1 |
| Cage cube | 60 mm cube cage | Thorlabs | LC6W |
| Lens holder | Thorlabs | CP02/M (x2) | |
| LCr Lens holder | LCr lens holder adapter | 3D printed part | (x2) |
| Fish aquarium | 3D printed part(s) | ||
| Teflon screen | PTFE (Teflon) glass fibre high temperature coating cloth, 0.15 mm | Artistore | |
| Parts for the external illumination units (RGB + UV) | |||
| Collimator | Thorlabs | LLG5A4-A (x2) | |
| Lens 01 | 1’’ N-BK7 Plano-Convex Lens, f = 25.4 mm | Thorlabs | LA1951-A-ML (x4) |
| DM 02 | Laser Beamsplitter H 560 LPXR superflat | AHF | F48-559 |
| DM 03 | AHF | F48-450 | |
| BP 02 | AHF | F39-370 | |
| BP 03 | AHF | F47-420 | |
| BP 04 | AHF | F49-480 | |
| BP 05 | AHF | F39-587 | |
| UV LED | 365 nm 2.4–6.0 mW 20 mA 15° | Roithner | XSL-365-5E |
| Blue LED | 420 nm 420 mW 350 mA 20° | Roithner | SMB1N-420H-02 |
| Green LED | 470 nm 70 mW 350 mA 20° | Roithner | SMB1N-D470-02 |
| Red LED | 588 nm 13.5 cd 20 mA 8° | Roithner | B5B-434-TY |
| Filter/LED/lens holder | Thorlabs | CP02/M (x4) | |
| Collimator holder | Thorlabs | LCP09 (x2) | |
| Vertical holder | 60 mm to 30 mm Cage System Right-Angle Adapter | Thorlabs | LCP30 (x3) |
| Dichroic frame | Dichroic frame | 3D printed part | (x2) |
| Frame holder | 3D printed part | (x2) | |
| Horizontal holder | 3D printed part | (x2) | |
| Metal rods | Thorlabs | ER-8 (x4) | |
| Metal rods | Cage Assembly Rod, 3’ Long, Ø6 mm | Thorlabs | ER-3 (x10) |
| Optical Post | Optical Post 12.7 mm diam | Thorlabs | TR100/M (x4) |
| Post Holder | Post holder 12.7 mm diam 100 mm | Thorlabs | PH100/M (x4) |
| Post clamp | Clamping Fork, 1.24’ Counterbored Slot | Thorlabs | CF125-P5 (x4) |
Different commercially available UV-enabled projectors.
https://doi.org/10.7554/eLife.48779.018| Part | Description (link) | Company |
|---|---|---|
| DPM-E4500UVBGMKII | DLP LightCrafter E4500 MKII with 3 LEDs: UV (385 or 405 nm), blue (460 nm), and green (520 nm) | EKB Technologies Ltd., Bat Yam, Israel https://www.ekbtechnologies.com/ |
| 3DLP9000 UV Light Engine | DLP-based light engine that can be equipped with one arbitrary LED, including UV (365, 385, or 405 nm) | DLi Digital Light innovations, Austin, TX https://www.dlinnovations.com |
| DLP660TE – 4K UHD | 4K-enabled projector, with flexible light sources, using Texas Instruments’ DLP660TE chipset | VISITECH Engineering GmbH Wetzlar, Germany https://visitech.no/ |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | HR2.1:TN-XL | Wei et al., 2012 | Dr. Bernd Wissinger (Tübingen University) | |
| Genetic reagent (Danio rerio) | tg(1.8ctbp2:SyGCaMP6f) | Rosa et al., 2016 | Dr. Leon Lagnado (Sussex University) | |
| Software, algorithm | KiCad EDA | http://kicad-pcb.org/ | Electronics design software | |
| Software, algorithm | OpenSCAD | http://www.openscad.org | 3D CAD software |
Additional files
-
Supplementary file 1
Parts list of the through-the-objective mouse stimulator (cf. Figure 2—figure supplement 1; entries in grey are not shown).
- https://doi.org/10.7554/eLife.48779.019
-
Transparent reporting form
- https://doi.org/10.7554/eLife.48779.020





