In vitro reconstitution of branching microtubule nucleation
Figures
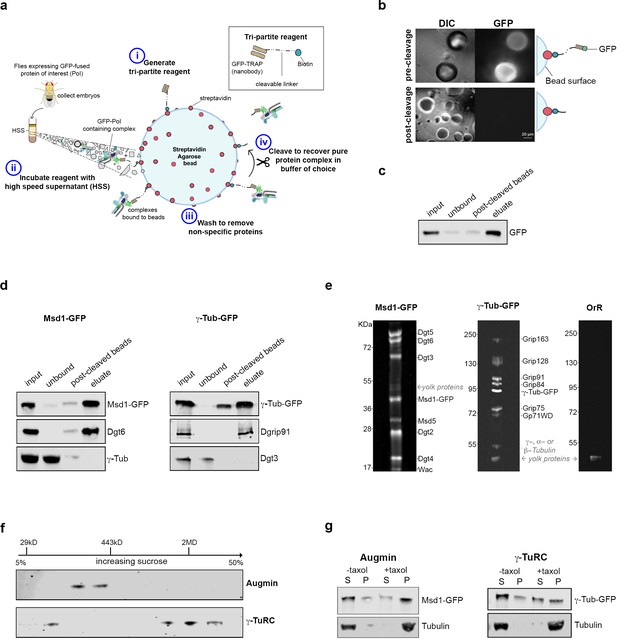
Isolation of functional γ-TuRC and Augmin using cleavable affinity purification.
(a) Sketch of purification methodology. (b) Images of GFP-TRAP-Sulfo beads after incubation with His-GFP, pre- and post-cleavage by 50 mM DTT. (c) Western blot demonstrating the effective isolation and cleavage of soluble His-GFP. (d) Western blots of cl-AP of Msd1-GFP and γ-Tubulin-GFP. The fusion proteins, present in embryo extracts (input), are efficiently depleted upon incubation with GFP-TRAP-Sulfo beads (unbound), released in 50 mM DTT (post-cleaved beads) and present in the eluate. Subunits of Augmin, but not γ-TuRC, co-elute with Msd1-GFP. Subunits of γ-TuRC, but not Augmin, co-elute with γ-Tubulin-GFP. (e). SYPRO-ruby stained gels of post-cleaved eluates from control embryos (OrR), or MG132-treated (mitotic) embryos expressing the Augmin subunit Msd1-GFP or γ-Tubulin-GFP. (f) Western blot of sucrose gradient fractionation of purified mitotic Augmin or γ-TuRC. Complexes sediment as expected for their molecular weights. (g) Western blots of in vitro MT co-sedimentation assays. In the absence of taxol (-), Tubulin, pure Augmin (Msd1-GFP) and pure γ-TuRC (γ-Tubulin-GFP) remain in the supernatant (S). In the presence of taxol (+), Tubulin polymerises and is present in the pellet (P). Augmin and γ-TuRC co-sediment.
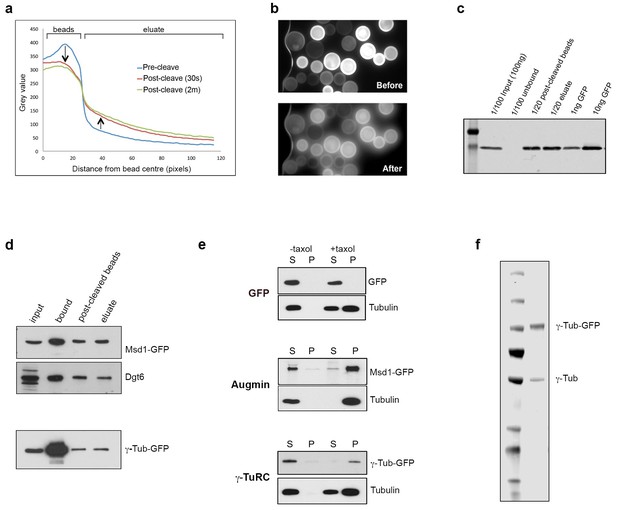
Isolation of functional γ-TuRC and Augmin using GFP-TRAP-PC beads.
(a) Graph showing the comparative decrease in GFP fluorescence on GFP-TRAP-PC beads, and increase in the surrounding eluate, following exposure to UV light (UV filter block on TE2000U fluorescence microscope). (b) Images of GFP-TRAP-PC beads after incubation with GFP, pre- and post-cleavage by 30 s exposure to UV light, used to generate data for (a). (c) Western blot to quantify the binding and release capacity of GFP-TRAP-PC beads. 100 ng of GFP is completely immobilised on the beads. In this case,~60% of GFP is cleaved via UV exposure, corresponding to 3 ng x 20 = 60 ng GFP. (d) Western blots demonstrating the isolation and cleavage of Msd1-GFP and γ-Tubulin-GFP using GFP-TRAP-PC beads. The proteins, present in embryo extracts (input), are efficiently captured onto beads (bound), with ~50% released following UV exposure (post-cleaved beads and eluate). (e) Western blots of in vitro MT co-sedimentation assays using GFP-TRAP-PC beads. In the absence of taxol (-), Tubulin, pure Augmin (Msd1-GFP) and pure γ-TuRC (γ-Tubulin-GFP) remain in the supernatant (S). In the presence of taxol (+), Tubulin polymerises and is present in the pellet (P). Augmin and γ-TuRC co-sediment. GFP alone does not co-sediment with MTs in this assay. (f) Western blot of purified γ-Tubulin-GFP eluate, probed with anti-γ-Tubulin antibody. Endogenously expressed, untagged γ-Tubulin is co-purified with γ-Tubulin-GFP, presumably as part of active γ-TuRCs, at a ratio of ~3:1 γ-Tubulin-GFP: γ-Tubulin (as quantified by LiCOR analysis).
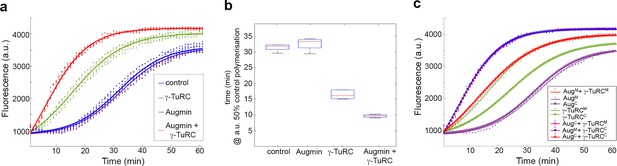
Pure Augmin enhances γ-TuRC-dependent MT nucleation in a cell cycle dependent manner.
(a) Tubulin polymerisation assays, where fluorescence is directly related to the amount of Tubulin polymer present. The curves are a sigmoidal fit to six replicates of the experiment (dots); three independent purification experiments, each undertaken in duplicate. (b) Plot of the x50 in relation to control polymerisation assay, showing the median (red line), interquartile ranges (blue box) and 95% confidence intervals of the median (notches) for each condition. The differences in the time taken for 50% polymerisation between all conditions differs significantly at p=0.001, except when comparing control to Augmin (ANOVA). (c) Fluorescent tubulin polymerisation assays undertaken with complexes isolated from MG132-treated (mitotic) or cycling embryos.
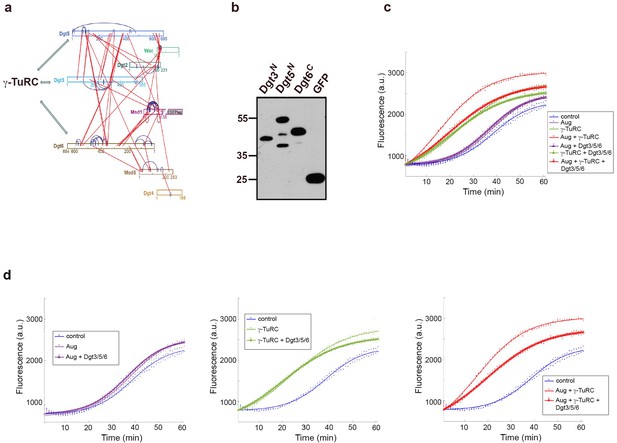
The synergistic effect of pure Augmin on γ-TuRC-dependent MT nucleation in vitro is dependent on the Augmin-γ-TuRC interface.
(a) Representation of the interactions between Augmin subunits, as determined by cross-linking mass spectrometry. The N-terminus of Dgt3 and Dgt5, and the C terminus of Dgt6 have all been previously shown to be responsible for localising the γ-TuRC to the mitotic spindle in Drosophila embryos. (b) Western blot showing the purification of MBP-Dgt3N, MBP-Dgt5N and MBP-Dgt6C, expressed in bacteria. (c) Fluorescent tubulin polymerisation assays undertaken with GFP-Augmin and GFP-γ-TuRC isolated from MG132-treated embryos, in the presence and absence of Dgt3N, Dgt5N and Dgt6C. Inclusion of the Dgt regions completely abrogates the ability of Augmin to affect γ-TuRC-dependent MT nucleation, without affecting γ-TuRC-dependent MT nucleation itself. (d) Individual datasets from (c). Note that inclusion of the Dgt regions with γ-TuRC alone does not affect γ-TuRC-dependent MT nucleation but does slightly reduce the total amount of MT polymer formed (middle graph). (a and b reproduced from Chen et al., 2017).
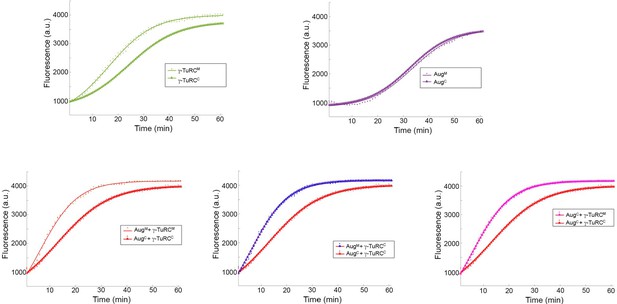
Individual sets of polymerisation curves using Augmin and γ-TuRC purified from either mitotic (M) or cycling (C) embryo extracts, taken from Figure 2c.
Mitotic γ-TuRC stimulates MT nucleation/polymerisation to a greater extent than γ-TuRC isolated from cycling embryo extracts. Neither cycling nor mitotic Augmin affects MT nucleation/polymerisation. Addition of mitotic Augmin to mitotic γ-TuRC stimulates γ-TuRC-dependent MT nucleation. This effect is reduced when cycling Augmin and cycling γ-TuRC are used. However, if either Augmin or γ-TuRC is mitotic, Augmin-dependent MT nucleation is active, even if the other complex is isolated from cycling embryos.
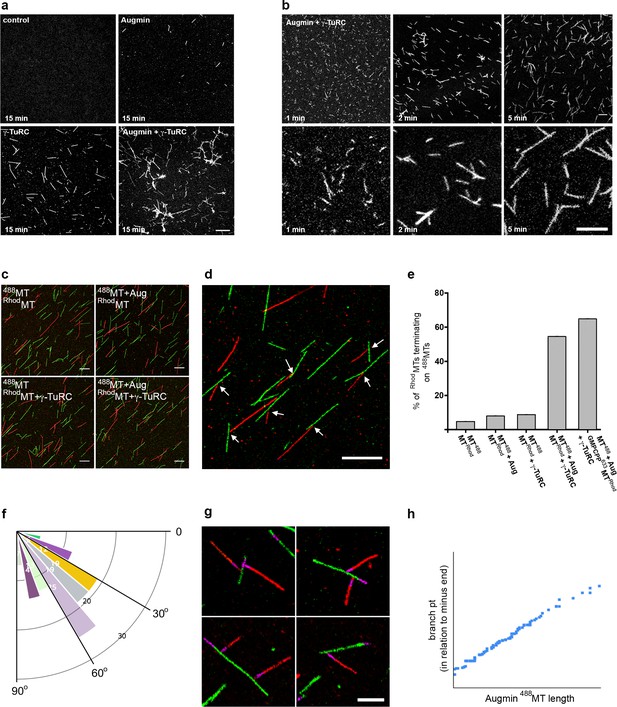
Reconstitution of the MT-Augmin-γ-TuRC-MT junction.
(a) Confocal images of fixed, fluorescent polymerisation assays at t = 15 mins for each condition. (b) Confocal images of fixed, fluorescent polymerisation assays at t = 1, 2 and 5 mins in the presence of purified Augmin and γ-TuRC. (c) Confocal images of taxol stabilised MTs, formed in the absence or presence of purified Augmin and γ-TuRC, and co-incubated. Incubation of Augmin-488MTs (green) with γ-TuRC-RhodMTs (red) leads to MT branches. (d) Higher magnification of the MT-Augmin-γ-TuRC-MT junctions (arrows). (e) Histogram of the percentage of RhodMTs that terminate precisely at a 488MT, generating a branchpoint. (f) Distribution of γ-TuRC-RhodMTs junction angles, in relation to Augmin-488MTs. (g) Confocal images of junctions, using GMPCPP seeds (purple) to distinguish the MT minus ends. Minus ends of γ-TuRC-RhodMTs (red) interact with Augmin-488MTs (green). (h) Pearson correlation coefficient plot demonstrating a strong positive correlation between the length of Augmin-488MTs and the position of γ-TuRC-RhodMT branch points. Scale bars, a-d, 5 μm; scale bar, g, 2 μm.
-
Figure 3—source data 1
Excel spreadsheets of the individual data points corresponding to Figure 3b (number and length of MTs per field of view at t = 2 and t = 5 mins); Figure 3d (Histogram of % of Rhod-MTs terminating on a 488-MT – i.e. % of γ-TuRC activity); Figure 3e (diagram of branch angles); Figure 3f (branchpoint distance from MT minus end, as assessed by GMPCPP seed).
- https://cdn.elifesciences.org/articles/49769/elife-49769-fig3-data1-v1.xlsx
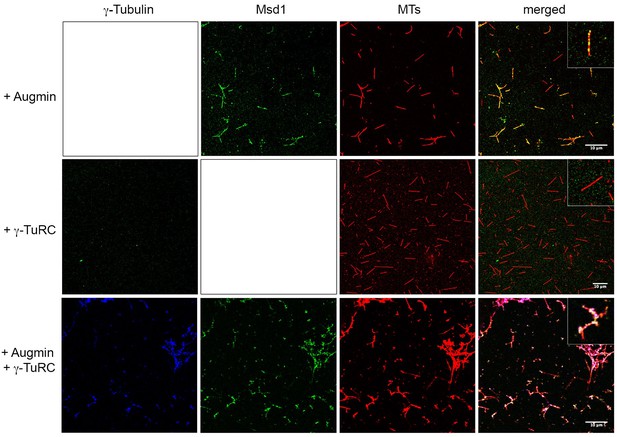
Augmin recruits γ-TuRC to MTs.
Samples were taken mid-way through in vitro polymerisation reactions, fixed and stained for γ-Tubulin, the Augmin Msd1 subunit or MTs. When present on its own, Augmin localises along the length of MTs. Note this localisation is not uniform; rather Augmin is present as punctae. When γ-TuRC is present on its own, γ-Tubulin is not observed on MTs, even though it stimulates MT nucleation (presumably the levels on the minus ends of MTs are too low to be resolved). However, when γ-TuRC is present with Augmin, γ-Tubulin co-localises with Augmin on MTs. Scale bar, 10 μm. See insets for higher magnified images.





