Kv1.1 contributes to a rapid homeostatic plasticity of intrinsic excitability in CA1 pyramidal neurons in vivo
Figures
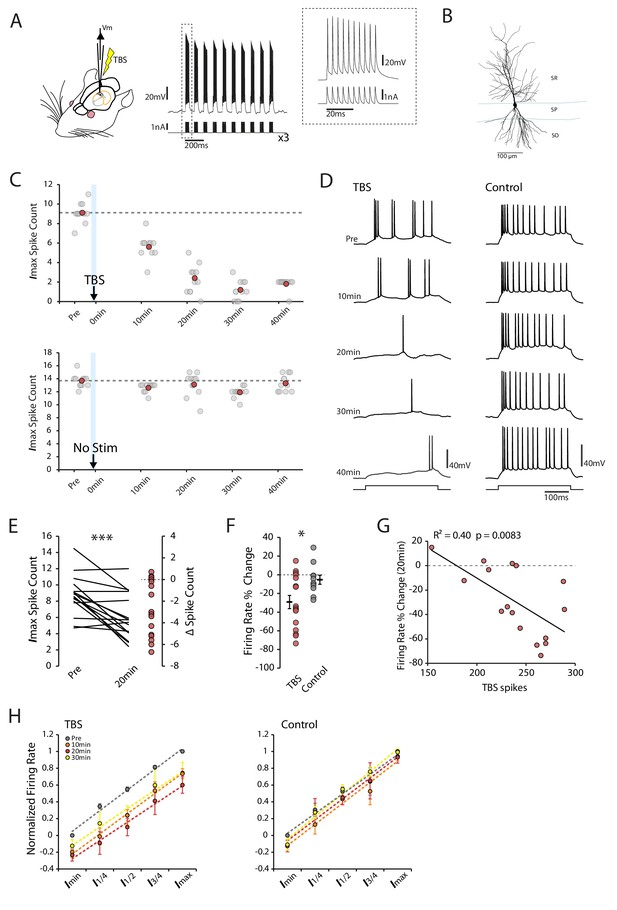
Theta-burst stimulation reduces intrinsic excitability.
(A) Left In vivo whole-cell recordings were made from CA1 pyramidal neurons. The TBS was applied through the patch pipette. Right An example of TBS. EPSP-like waveforms were applied in bursts of 10 (at 100Hz, inset), these bursts were repeated 10 times at theta frequency (5Hz) and the process was repeated three times with an interval of 15 seconds. (B) Morphology of a recorded cell. SR stratum radiatum, SP stratum pyramidale, SO stratum oriens. (C-D) Examples of a TBS and a control cell. The plots show the response to each of the maximum current injections at different times during the testing protocol (grey circles). The red circles mark the mean response at each timepoint. The traces shown in (D) are marked by dark grey borders at their respective timepoints. (E) Left Mean number of spikes evoked in response to the maximal current injection prior to and 20 minutes post-TBS for each cell. Right Distribution of changes in spike count (F) Comparison of change in firing rate at 20 minutes between TBS and control groups. (G) Correlation between the number of spikes evoked during TBS and the change in firing rate. (H) Firing rate vs injected current for the populations of TBS and control cells. The current range is normalized to correct for differences in the range of current amplitudes used. In the TBS group there was a significant reduction (p < 0.05) in the intercept at 10 min (green) and 20 min (red) post-TBS but no change in the gain of the fits. Error bars show mean ± SEM. TBS n = 16 (30 min n = 14), Control n = 11 (30 min n = 6).
-
Figure 1—source data 1
Spike count in TBS and control conditions.
- https://cdn.elifesciences.org/articles/49915/elife-49915-fig1-data1-v1.xlsx

Change in excitability is not related to inital state of the cells.
(A) Correlation with the input resistance prior to TBS. (B) Correlation with membrane potential immediately after breaking in. (C) Correlation with initial AP threshold. (D) Correlation with burstiness. n = 16
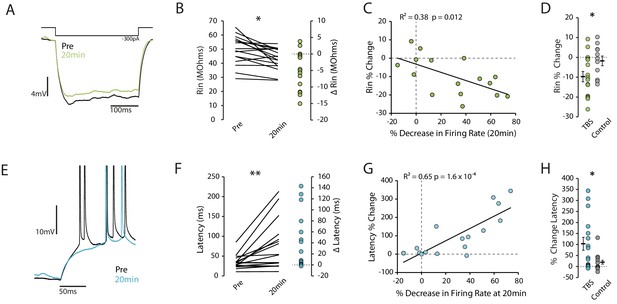
Reduction in excitability is associated with decreased Rin and increased latency to first spike.
(A) Example traces showing a reduction in input resistance. Each trace is the mean of 10 traces. Rin was calculated on hyperpolarizing steps only and the voltage was taken as the minimum within the first 150ms. (B) Input resistance prior to and 20 minutes post-TBS. (C) Correlation between the change in input resistance and the change in firing rate. (D) Comparison of change in input resistance at 20 minutes between TBS and control groups. (E) Example trace showing increase in latency after TBS. (F) Latency to first spike prior to and 20 minutes post-TBS. (G) Correlation between the change in latency and the change in firing rate. (H) Comparison of change in latency at 20 minutes between TBS and control groups. Error bars show mean ± SEM. TBS n = 16, Control n = 11.
-
Figure 2—source data 1
Input resistance and first spike latency in TBS and control conditions.
- https://cdn.elifesciences.org/articles/49915/elife-49915-fig2-data1-v1.xlsx
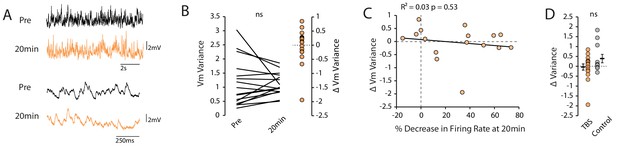
TBS did not change background synaptic activity.
(A) Examples of synaptic activity prior to testing excitability before and 20 minutes post-TBS. (B) Membrane potential variance prior to and 20 minutes post-TBS. (C) Scatter plot showing no correlation between the change in membrane potential variance and the change in firing rate. (D) Comparison of change in variance of the membrane potential at 20 minutes between TBS and control groups.
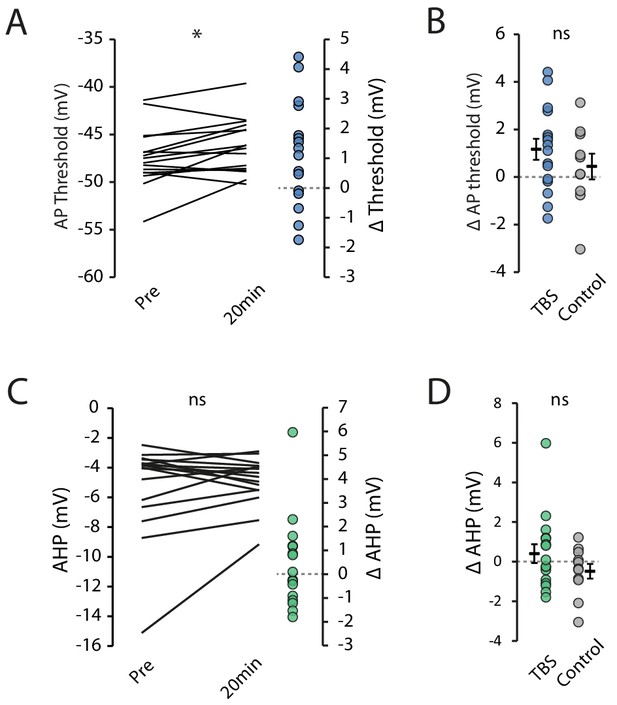
Action potential threshold and medium AHP.
(A) Action potential threshold prior to and 20 minutes post-TBS. (B) Comparison of change in threshold between TBS and control cells. (C) AHP amplitude prior to and 20 minutes post-TBS. (D) Comparison of change in AHP between TBS and control cells. Error bars show mean ± SEM. TBS n = 16, Control n = 11.
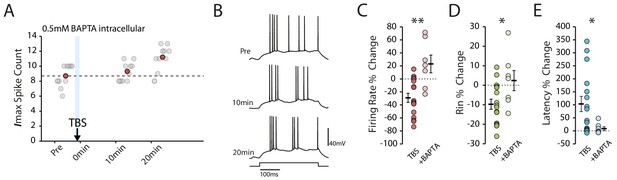
Calcium chelation prevents the TBS-induced decrease in excitability.
(A-B) Example cell showing that the decrease in excitability is blocked with 0.5mM BAPTA inside the patch pipette. Scatter plots show the response to each of the maximum current injections during the testing protocol (grey circles). The red circles mark the mean response at each timepoint. Dark grey borders mark the current steps depicted in (B). (C-E) Comparisons of changes in firing rate (C), input resistance (D) and latency (E) between TBS alone (n = 16) and TBS with BAPTA (n = 7). Error bars show mean ± SEM.
-
Figure 3—source data 1
Spike count, input resistance and first spike latency in TBS condition with BAPTA.
- https://cdn.elifesciences.org/articles/49915/elife-49915-fig3-data1-v1.xlsx
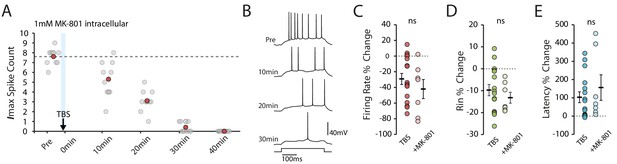
NMDAR block does not prevent the TBS-induced decrease in excitability.
(A-B) Example cell showing that 1mM MK-801 inside the pipette does not prevent the decrease in excitability. Scatter plot shows the response to each of the maximum current injections during the testing protocol (grey circles). The red circles mark the mean response at each timepoint. Dark grey borders mark the current steps depicted in (B). (C-E) Comparisons of changes in firing rate (C), input resistance (D) and latency (E) between TBS alone (n = 16) and TBS with MK-801 (n = 7). Error bars show mean ± SEM.
-
Figure 4—source data 1
Spike count, input resistance and first spike latency in TBS condition with MK801.
- https://cdn.elifesciences.org/articles/49915/elife-49915-fig4-data1-v1.xlsx
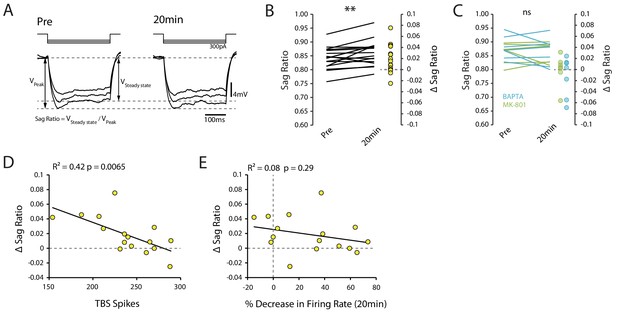
Changes in Ih do not account for decrease in excitability.
(A) Example traces showing the voltage sag caused by Ih pre and 20 min post-TBS. Each trace is the mean of 10 trials. (B) Sag ratio prior to and 20 minutes post-TBS (n = 16). (C) Sag ratio prior to and 20 minutes post-TBS with 0.5mM BAPTA (n = 7) or 1mM MK-801 (n = 7) in the patch pipette. (D) Correlation between the change in sag ratio and the number of spikes evoked during TBS. (E) Scatter plot showing that there was no strong relationship between the change in sag ratio and the decrease in firing rate.
-
Figure 5—source data 1
Sag ratio in TBS condition without drugs and with BAPTA and MK801.
- https://cdn.elifesciences.org/articles/49915/elife-49915-fig5-data1-v1.xlsx
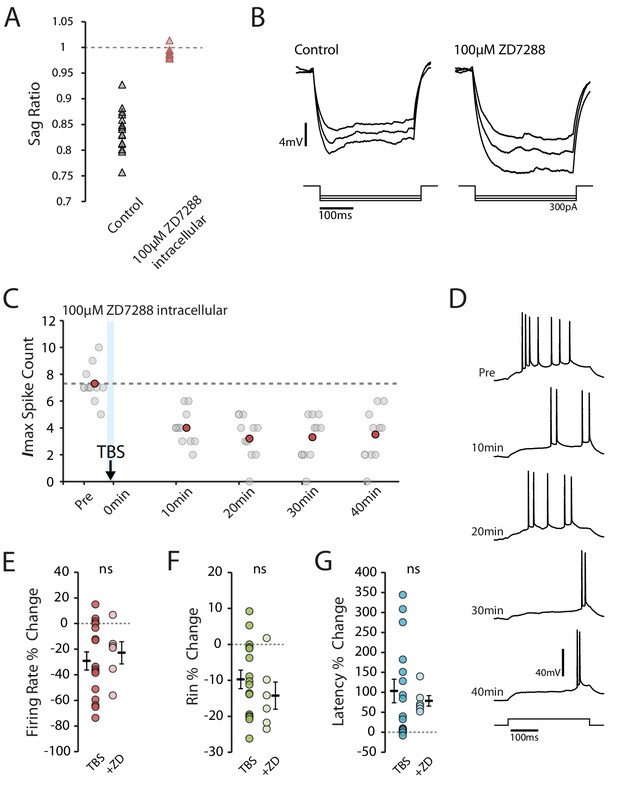
Block of Ih does not prevent reduction in excitability.
(A-B) Inclusion of ZD7288 in the patch pipette blocked Ih, abolishing the voltage sag. Each trace shown is the mean of 10 responses. (C-D) Example cell showing the effect of TBS in the presence of ZD7288 inside the patch pipette. Scatter plot shows the response to each of the maximum current injections during the testing protocol (grey circles). The red circles mark the mean response at each timepoint. Dark grey borders mark the current steps depicted in (D). (E-F) Comparisons of changes in firing rate (E), input resistance (F) and latency (G) between TBS alone (n = 16) and TBS with ZD7288 (n = 6). Error bars show mean ± SEM.
-
Figure 6—source data 1
Spike count, input resistance and first spike latency in TBS condition with ZD7288.
- https://cdn.elifesciences.org/articles/49915/elife-49915-fig6-data1-v1.xlsx
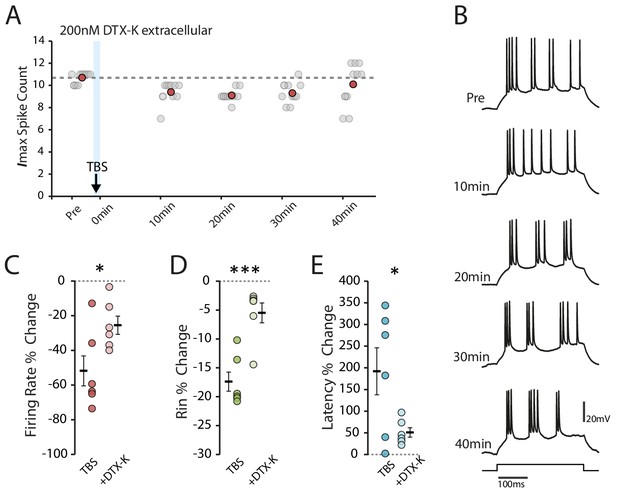
Dendrotoxin-K reduces the effect of TBS on excitability.
(A-B) Example cell recorded after the application of DTX-K. Scatter plot shows the response to each of the maximum current injections during the testing protocol (grey circles). The red circles mark the mean response at each timepoint. Dark grey borders mark the current steps depicted in (B). (C-E) Comparisons of changes in firing rate (C), input resistance (D) and latency (E) between TBS alone (n = 6) and with DTX-K (n = 6) for cells where TBS evoked at least 250 spikes. Error bars show mean ± SEM.
-
Figure 7—source data 1
Spike count, input resistance and first spike latency in TBS condition with DTX-K.
- https://cdn.elifesciences.org/articles/49915/elife-49915-fig7-data1-v1.xlsx
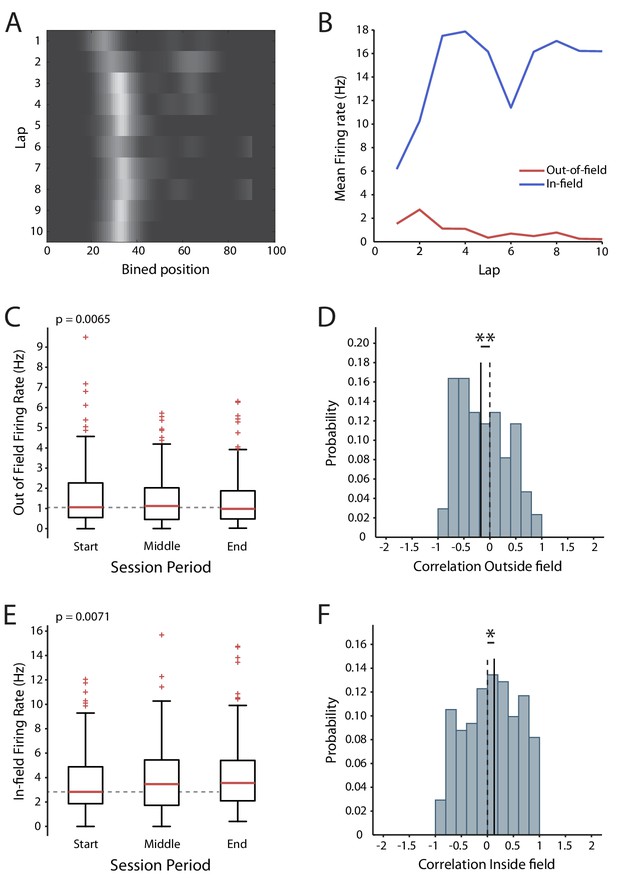
Changes in excitability during exploration of a novel virtual reality track.
(A) Firing rate map of one cell. Lighter colors indicate higher rate. (B) In-field and out-of-field firing rate by lap for the example cell. (C) Mean firing rates outside the place field across the session. (D) Distribution of the out-of-field firing rate correlations. (E) Mean in-field firing rates across the session. (F) Distribution of the in-field firing rate correlations. Box and whisker plots show median, 25-75th and 5-95th percentiles, n = 171
-
Figure 8—source data 1
In-field, out-of-field firing rates and correlations across laps for place cells in new condition.
- https://cdn.elifesciences.org/articles/49915/elife-49915-fig8-data1-v1.xlsx
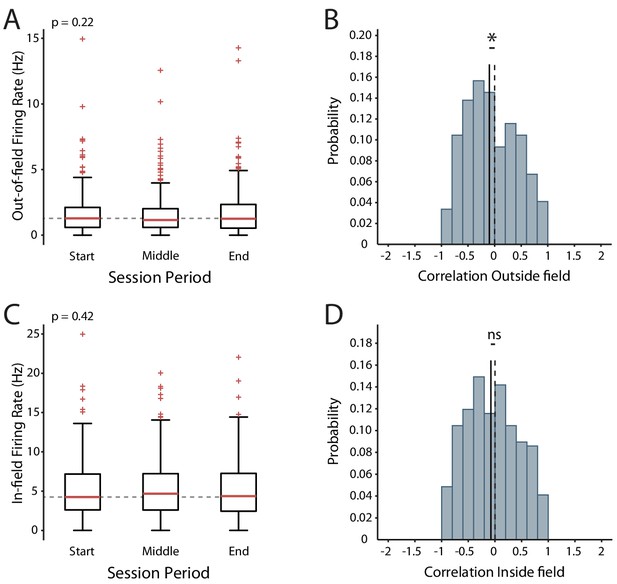
Changes in excitability during exploration of a familiar environment.
(A) Mean firing rates outside the place field across the session. (B) Distribution of the out-of-field firing rate correlations. (C) Mean in-field firing rates across the session. (D) Distribution of the in-field firing rate correlations. Box and whisker plots show median, 25-75th and 5-95th percentiles, n = 268.
-
Figure 8—figure supplement 1—source data 1
In-field, out-of-field firing rates and correlations across laps for place cells in familiar condition.
- https://cdn.elifesciences.org/articles/49915/elife-49915-fig8-figsupp1-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Chemical compound, drug | ZD7288 | Tocris Bioscience | Cat. No. 1000 | |
| Chemical compound, drug | Dendrotoxin K | Sigma Aldrich | D4183 | |
| Chemical compound, drug | MK-801 | Tocris Bioscience | Cat. No. 0924 |





