Negative regulation of autophagy by UBA6-BIRC6–mediated ubiquitination of LC3
Figures
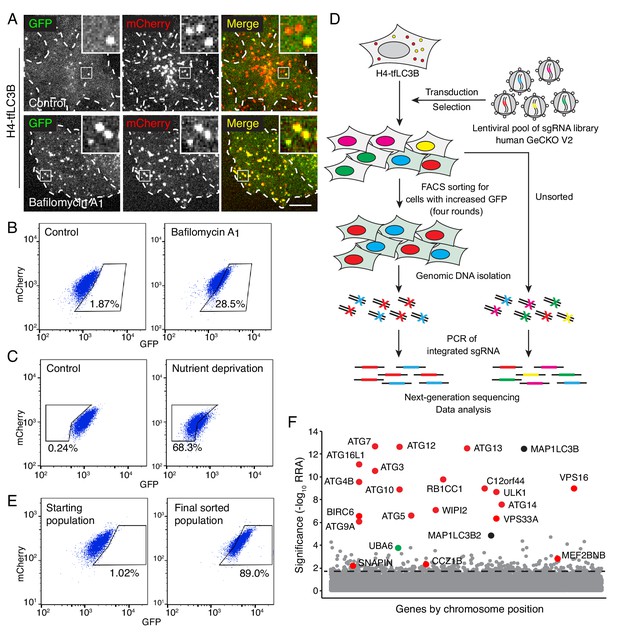
Genome-wide CRISPR/Cas9 screen for the identification of autophagy genes.
(A) Live-cell imaging of H4 cells expressing GPF-mCherry-LC3B (H4-tfLC3B). Cells were incubated in the absence (control) or presence of 50 nM bafilomycin A1 for 2 hr prior to imaging. Single-channel images are shown in grayscale. Cell edges are outlined. Scale bar: 10 μm. Insets are 3.6x magnifications of the boxed areas. (B) H4-tfLC3B cells were incubated without (control) or with 50 nM bafilomycin A1 for 2 hr, and GFP and mCherry fluorescence was measured by FACS. (C) H4-tfLC3B cells were incubated in regular medium (control) or amino-acid- and serum-free medium for 4 hr (nutrient deprivation), and then analyzed by FACS. (D) Schematic representation of the genome-wide CRISPR/Cas9 screen. H4-tfLC3B cells were mutated with a pooled lentiviral GeCKO v2 library. Cells with high GFP:mCherry ratio were sorted and propagated; after four rounds of sorting, genomic DNA was isolated. The sequences of sgRNAs were determined by next-generation sequencing. (E) FACS analysis of cells infected with the lentiviral pool (starting population) and cells after four rounds of sorting (final sorted population). In B, C and E, the percentages of cells in the boxed areas are indicated. (F) Ranking of genes from the CRISPR/Cas9 screen based on the RRA (Robust Ranking Aggregation) algorithm score calculated using the MAGeCK method. Genes known to participate in autophagy are labeled in red; MAP1LC3B genes (i.e., LC3B and LC3B2) are labeled in black; a gene not previously implicated in autophagy, UBA6, is labeled in green. The identification of LC3B and LC3B2 may be due to the synthesis of a truncated GPF-mCherry-LC3B that cannot be degraded. The genes above the horizontal dotted line were tested in the secondary screen.
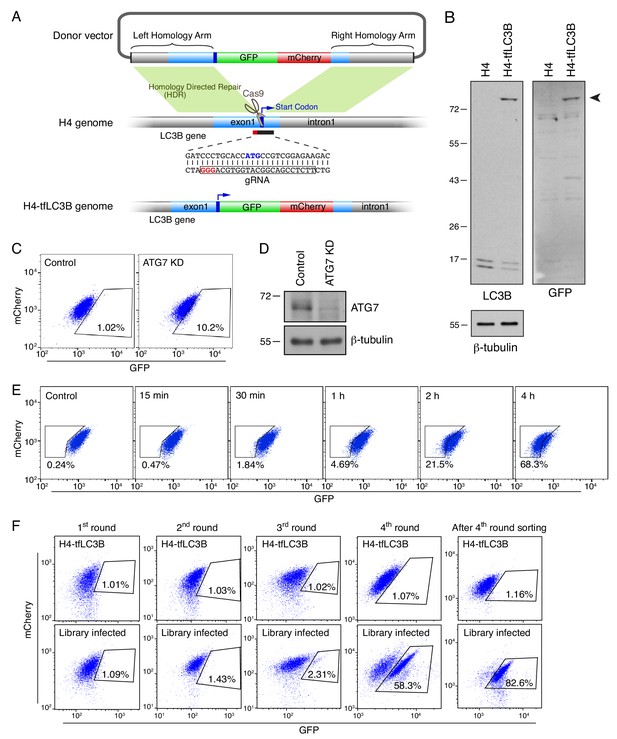
Generation, analysis and mutant selection of H4 cells expressing endogenously-tagged GFP-mCherry-LC3B.
(A) Schematic representation of the tagging of endogenous LC3B with GFP-mCherry in H4 neuroglioma cells (H4-tfLC3B) by CRISPR/Cas9-mediated genome editing. A donor vector for HDR (homology-directed repair) was constructed by inserting DNA fragments encoding the left homology arm, GFP-mCherry, and the right homology arm into the pCI-neo vector (E1841, Promega). A double-strain break on the genomic DNA was generated by Cas9 using a gRNA targeting the first exon of the LC3B-encoding gene. The break was repaired by HDR using donor vector as a template. (B) SDS-PAGE and immunoblotting of H4 and H4-tfLC3B cells with antibodies to LC3B, GFP and β-tubulin (loading control). The arrowhead indicates the position of the GFP-mCherry-LC3B protein. (C) H4-tfLC3B cells were transfected with control or ATG7 siRNAs. After 48 hr, GFP and mCherry fluorescence was measured by FACS. Notice the increased GFP fluorescence in the ATG7-KD cells. (D) SDS-PAGE and immunoblotting showing the reduction of ATG7 expression in ATG7 siRNA-transfected H4-tfLC3B cells. (E) H4-tfLC3B cells were deprived of amino acids and serum for the indicated periods and analyzed by FACS. Notice the decline of the GFP signal over time, reflecting the activation of autophagy during starvation. (F) H4-tfLC3B cells were infected with a genome-wide CRISPR/Cas9 KO lentiviral library, and cells with increased GFP signal were collected by cell sorting. Sorted cells were propagated and subjected to additional rounds of sorting. The naïve H4-tfLC3B cells were used to determine gating in each round of sorting. Notice the gradual enrichment of the population of library-infected high-GFP cells after each round of sorting. In B and D, the positions of molecular mass markers (in kDa) are indicated on the left.
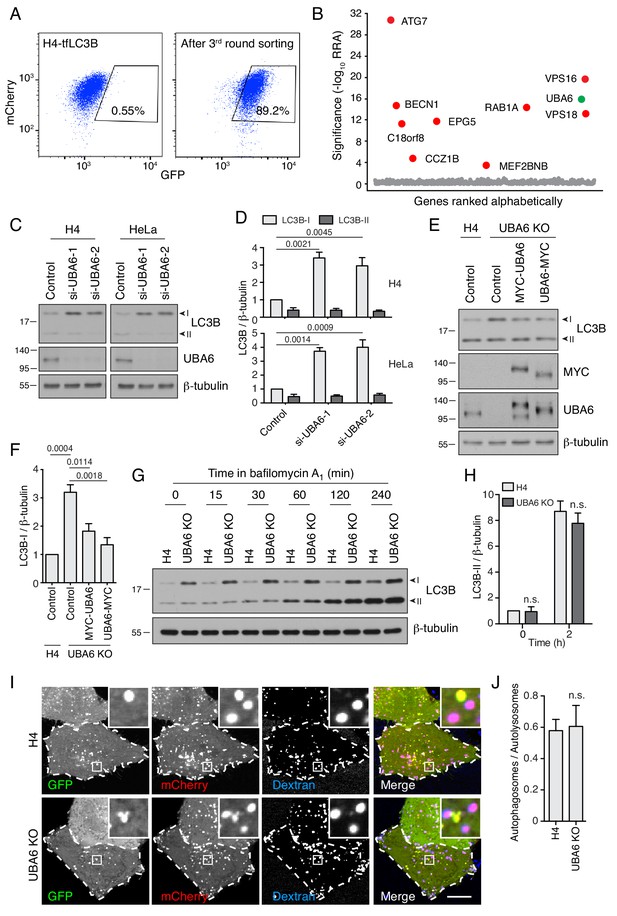
UBA6-KO cells accumulate LC3B-I but not LC3B-II.
(A) FACS analysis of cells from the secondary screen showing enrichment of library-infected H4 cells with increased GFP fluorescence after three rounds of sorting. The percentages of cells in the boxed areas are indicated. (B) Ranking of genes in the CRISPR/Cas9 screen based on RRA. Genes highlighted in red were previously reported to function in autophagy. The novel autophagy regulator UBA6 is highlighted in green. (C) H4 or HeLa cells were transfected with control or either of two UBA6 siRNAs. After 48 hr, cells were analyzed by SDS-PAGE and immunoblotting for LC3B, UBA6 and β-tubulin (control). In this and all other relevant figures, the positions of the I and II forms of LC3B are indicated. (D) Quantification of the ratio of LC3B-I and -II to β-tubulin. The ratio for control siRNA was arbitrarily set at 1. Values are the mean ± SEM from three independent experiments such as that shown in C. The indicated p-values relative to the control were calculated using a two-way ANOVA with Tukey's multiple comparisons test. (E) WT or UBA6-KO H4 cells were transfected with control plasmid or plasmids encoding MYC-UBA6 or UBA6-MYC. The cells were analyzed by SDS-PAGE and immunoblotting with antibodies to the antigens on the right. (F) Quantification of ratio of LC3B-I to β-tubulin. The ratio for the control plasmid transfection in WT H4 cells was arbitrarily set at 1. Values are the mean ± SEM from three independent experiments. The indicated p-values were calculated using a one-way ANOVA with Dunnett’s multiple comparisons test. (G) WT and UBA6-KO H4 cells were incubated with 50 nM bafilomycin A1 for the indicated periods prior to SDS-PAGE and immunoblotting with antibodies to LC3B and β-tubulin. In C, E and G, the positions of molecular mass markers (in kDa) are indicated on the left. (H) Quantification of the ratio of LC3B-II to β-tubulin. The ratio of WT H4 cells not treated with bafilomycin A1 was arbitrarily set at 1. Values are the mean ± SEM from three independent experiments such as that shown in G. p-values were calculated using two-way ANOVA with Tukey's multiple comparisons tests. n.s.: not significant. (I) WT and UBA6-KO H4 cells were transfected with a plasmid encoding GFP-mCherry-LC3B and allowed to internalize Alexa Fluor 647-conjugated dextran for 16 hr at 37°C to label late endosomes, lysosomes and autolysosomes. GFP (green), mCherry (red) and Alexa Fluor 647 (blue) fluorescence was visualized by live-cell imaging. Single-channel images are shown in grayscale. Cell edges are outlined. Scale bar: 10 μm. Insets are 4.6x magnifications of the boxed areas. (J) The ratio of autophagosomes (red-green–positive puncta) to autolysosomes (red-blue–positive puncta) was determined. Bars represent the mean ± SEM of the ratio in 20 cells from three independent experiments. N.s.: not significant, according to an unpaired Student’s t test.
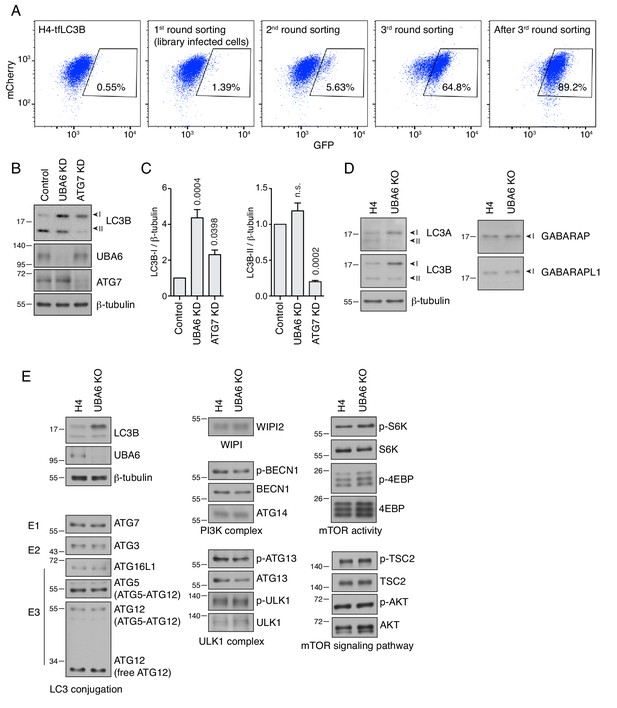
Secondary screen for autophagy mutants and expression of autophagy proteins in WT and UBA6-KO H4 cells.
(A) H4-tfLC3B cells were infected with a secondary lentiviral library targeting the top 432 genes in the primary screen. The cells with increased GFP signal were collected and enriched to 89.2% by three rounds of sorting and propagation. (B) SDS-PAGE and immunoblotting of H4 cells transfected with control, UBA6 or ATG7 siRNAs. (C) Quantification of the ratio of LC3B-I and LC3-II to β-tubulin. The ratio for control siRNA was arbitrarily set at 1. Values are the mean ± SEM from three independent experiments such as that shown in B. The indicated p-values were calculated using a one-way ANOVA with Dunnett’s multiple comparisons test. Notice that depletion of ATG7 caused accumulation of LC3B-I and decrease of LC3B-II. In contrast, UBA6 KD increased the levels of LC3B-I without changing the levels of LC3B-II, indicative of the different roles of UBA6 and ATG7 in autophagy. (D) WT and UBA6-KO H4 cells were lysed in 1xLDS sample buffer and analyzed by SDS-PAGE and immunoblotting for Atg8 family proteins. Notice how LC3A-I and LC3B-I levels were increased in the UBA6-KO cells. In contrast, the levels of GABARAP-I and GABARAPL1-I were not altered in UBA6-KO cells. LC3C and GABARAPL2 were not tested because of lack of good antibodies. (E) WT and UBA6-KO H4 cells were subjected to SDS-PAGE and immunoblotting to determine the total levels and/or phosphorylation status (indicated by the p prefix) of proteins involved in autophagy. Notice that, with the exception of LC3B-I, the levels of these proteins were largely unaltered in UBA6-KO cells. In B, D and E, the positions of molecular mass markers (in kDa) are indicated on the left.
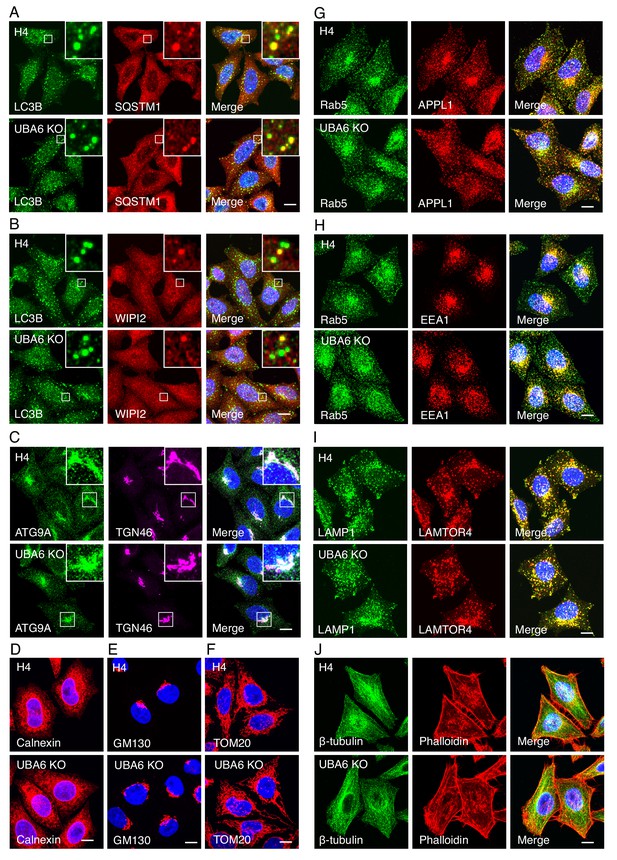
Distribution of organellar proteins in WT and UBA6-KO H4 cells.
(A) Confocal fluorescence microscopy of WT and UBA6-KO H4 cells immunostained with antibodies to LC3B and SQSTM1. Scale bar: 10 μm. Punctate structures and colocalization of LC3B with SQSTM1 were observed in WT as well as UBA6-KO cells. (B) Confocal fluorescence microscopy showing colocalization of LC3B and WIPI2 in WT and UBA6-KO H4 cells. Scale bar: 10 μm. Nascent phagophores were positive for both proteins, while mature autophagosomes were only positive for LC3B. (C) Confocal fluorescence microscopy showing the distribution of ATG9A and TGN46 in WT and UBA6-KO H4 cells. Scale bar: 10 μm. In both cell lines, the majority of ATG9A localized to trans-Golgi network (TGN) and peripheral vesicles. (D–J) Confocal fluorescence microscopy of WT and UBA6-KO cells immunostained with antibodies to calnexin (endoplasmic reticulum) (D), GM130 (Golgi apparatus) (E), TOM20 (mitochondria) (F), Rab5, APPL1, EEA1 (early endosomes) (G, H), LAMP1, LAMTOR4 (late endosomes and lysosomes) (I) and β-tubulin (microtubules) (J), and with phalloidin (F-actin) (J). Scale bars: 10 μm. No obvious differences in staining for these markers were observed in UBA6-KO cells relative to WT cells.
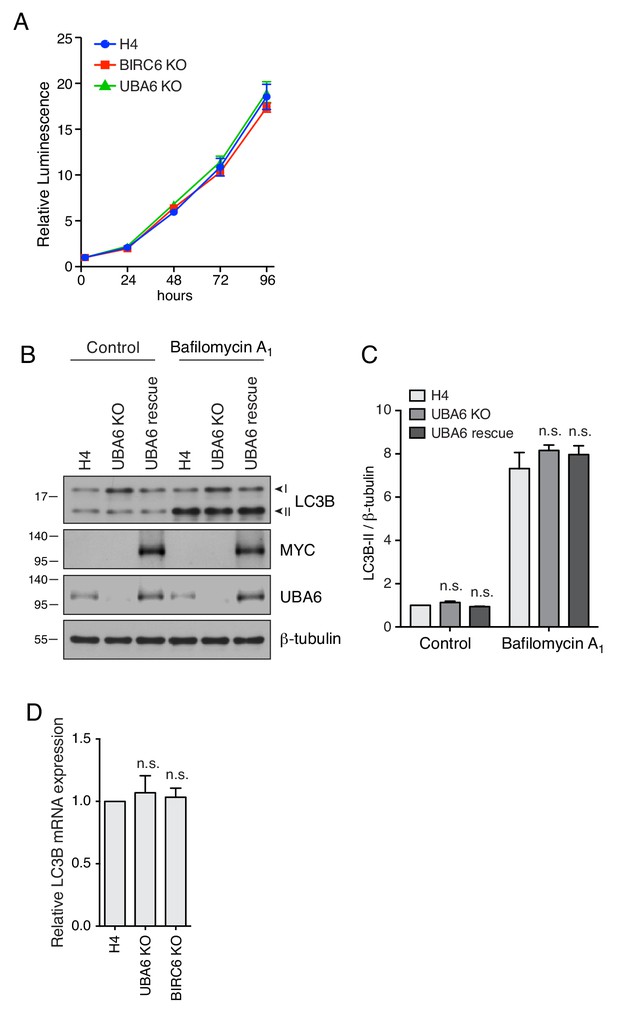
Proliferation rate and LC3B mRNA expression in UBA6-KO and BIRC6-KO cells.
(A) Four-thousand WT, UBA6-KO and BIRC6-KO H4 cells were seeded into each well of a 96-well plate in quadruplicate for each time point in regular culture medium. After 2, 24, 48, 72 and 96 hr, the number of cells per well was measured using a CellTiter-Glo 2.0 Cell Viability Assay (G9242, Promega). The luminescence from each well represented the number of cells. The luminescence of WT H4 cells at 2 hr was set as 1. Values are the mean ± SEM from three independent experiments. The results show that WT, UBA6-KO and BIRC6-KO H4 cells proliferated at the same rates. (B) WT, UBA6-KO and UBA6-rescue cells (UBA6-KO cells transiently transfected with a plasmid encoding UBA6-MYC) were incubated with 50 nM bafilomycin A1 for 2 hr prior to SDS-PAGE and immunoblotting with antibodies to LC3B and β-tubulin. The positions of molecular mass markers (in kDa) are indicated on the left. (C) Quantification of the ratio of LC3B-II to β-tubulin. The ratio of untreated WT H4 cells was arbitrarily set at 1. Values are the mean ± SEM from three independent experiments such as that shown in B. The p-values were calculated using two-way ANOVA with Tukey's multiple comparisons tests. n.s.: not significant. (D) Real-time quantitative PCR (RT-qPCR) was performed to measure the mRNA levels of LC3B in WT, UBA6-KO and BIRC6-KO H4 cells. The fold expression of LC3B relative to GAPDH was determined by the 2-ΔΔCt method (Schmittgen and Livak, 2008). The relative LC3B mRNA level in H4 cells was arbitrarily set at 1. Values are the mean ± SEM from three independent experiments. The p-values were calculated using a one-way ANOVA with Dunnett’s multiple comparisons test. n.s.: not significant. Notice the similar levels of LC3B mRNA in all the cell lines.
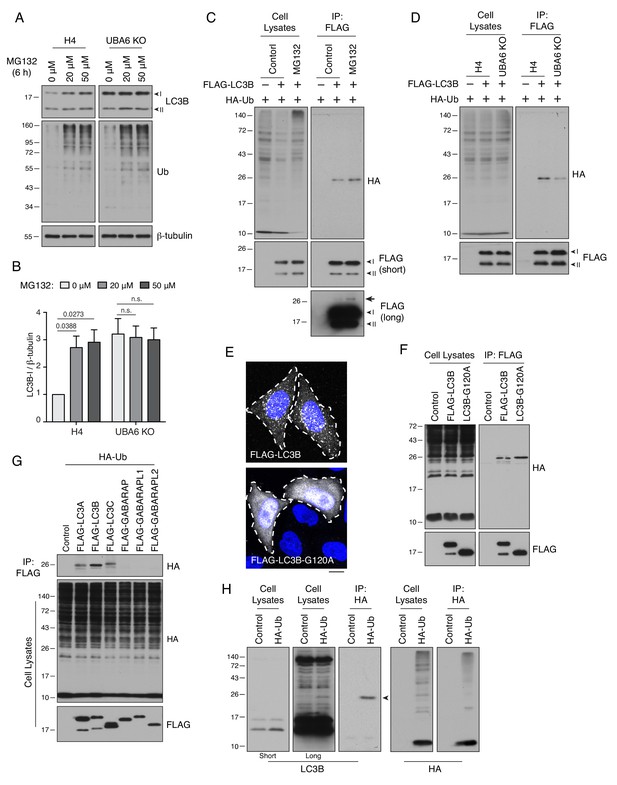
UBA6 participates in monoubiquitination of LC3B.
(A) WT and UBA6-KO H4 cells were incubated with 0, 20 or 50 μM MG132 for 6 hr, and analyzed by SDS-PAGE and immunoblotting with antibodies to LC3B, Ub and β-tubulin (loading control). (B) The ratio of LC3B-I to β-tubulin was determined from experiments such as that in A. The ratio in WT H4 cells without MG132 treatment was arbitrarily set at 1. Bars represent the mean ± SEM of the ratio from three independent experiments. The indicated p-values were calculated using two-way ANOVA with Tukey's multiple comparisons tests. (C) WT and UBA6-KO cells were transfected with plasmids encoding HA-Ub and FLAG-LC3B. Cell lysates were immunoprecipitated (IP) with antibody to the FLAG epitope, and cell lysates and immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with antibodies to the HA and FLAG epitopes. Ubiquitinated FLAG-LC3B can be seen as a faint band on the long exposure of the anti-FLAG blot (arrow). (D) H4 cells were transfected with plasmids encoding HA-Ub and FLAG-LC3B. Cells were incubated with 20 μM MG132 for 6 hr before lysis and immunoprecipitation with antibody to the FLAG epitope. Cell lysates and immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with antibodies to the HA and FLAG epitopes. (E) Immunofluorescence microscopy showing the localization of FLAG-LC3B and FLAG-LC3B-G120A mutant in WT H4 cells. DAPI (blue) was used to stain the nucleus. Cell edges are outlined. Scale bar: 10 μm. (F) H4 cells expressing HA-Ub together with FLAG-LC3B or FLAG-LC3B-G120A mutant were analyzed by immunoprecipitation with antibody to the FLAG epitope, followed by SDS-PAGE and immunoblotting with antibodies to the HA and FLAG epitopes. (G) H4 cells were transfected with plasmids encoding FLAG-tagged Atg8-family proteins and HA-Ub. Cell lysates were subjected to immunoprecipitation with antibody to the FLAG epitope, and cell lysates and immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with antibodies to the HA and FLAG epitopes. (H) H4 cells were transfected with control or HA-Ub-encoding plasmids. Ubiquitinated proteins in the cell lysates were enriched by immunoprecipitation with antibody to the HA epitope. Cell lysates and immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with antibodies to LC3B or the HA epitope. In A, C, D, G, F and H, the positions of molecular mass markers (in kDa) are indicated on the left.
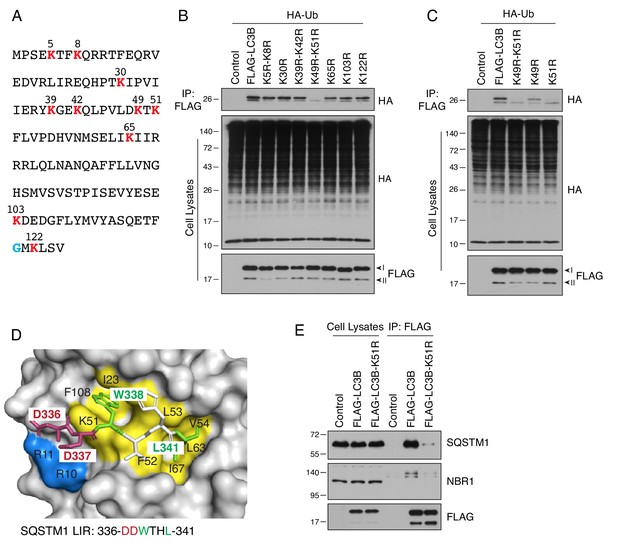
LC3B is ubiquitinated on lysine-51.
(A) Sequence of human LC3B. Lysine residues are highlighted in red. A glycine residue that becomes conjugated to phosphatidylethanolamine is highlighted in blue. (B,C) H4 cells were transfected with plasmids encoding WT and mutant LC3B constructs and HA-Ub. The ubiquitination of LC3B was examined as described for Figure 3C. (D) Binding of the LIR motif of SQSTM1 (DDWTHL) (stick representation) to LC3B (surface representation) (PDB code: 2ZJD). Yellow and green indicate residues involved in electrostatic interactions; blue and red indicate residues forming hydrogen bonds. (E) H4 cells were transfected with plasmids encoding control, FLAG-LC3B or FLAG-LC3-K51R mutant plasmids. Cell lysates were immunoprecipitated with antibody to the FLAG epitope. Cell lysates and immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with antibodies to SQSTM1, NBR1 and the FLAG epitope. In B, C and E, the positions of molecular mass markers (in kDa) are indicated on the left.
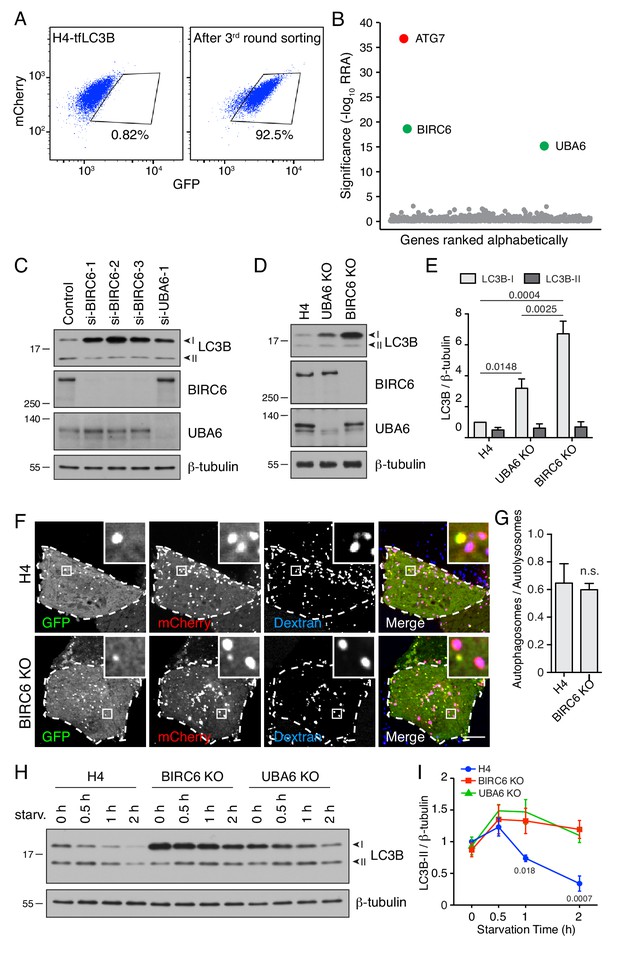
BIRC6 depletion increases the level of LC3B-I.
(A) H4-tfLC3B cells were mutated with a CRISPR/Cas9 lentiviral library targeting ubiquitination-related genes. The figure shows a FACS analysis of the enrichment of library-infected cells with increased GFP fluorescence after three rounds of sorting and expansion. (B) Ranking of genes based on RRA. ATG7 (control) is highlighted in red. UBA6 and BIRC6 are highlighted in green. (C) H4 cells were transfected with control, BIRC6 (three different siRNAs) or UBA6 siRNAs. Cells were analyzed by SDS-PAGE and immunoblotting for LC3B, BIRC6, UBA6 and β-tubulin (loading control). (D) SDS-PAGE and immunoblotting of lysates from WT, UBA6-KO and BIRC6-KO H4 cells with antibodies to the proteins on the right. (E) Quantification of the ratio of LC3B-I and -II proteins to β-tubulin. The value of LC3B-I/β-tubulin in WT H4 cells was arbitrarily set at 1. Values are the mean ± SEM from three independent experiments such as that shown in D. The indicated p-values were calculated using a two-way ANOVA with Tukey's multiple comparisons test. (F) WT and BIRC6-KO H4 cells were transfected with a plasmid encoding tfLC3B and allowed to internalize Alexa Fluor 647-conjugated dextran for 16 hr at 37°C. GFP (green), mCherry (red) and Alexa Fluor 647 (blue) fluorescence was visualized by live-cell imaging. Single-channel images are shown in grayscale. Scale bar: 10 μm. Insets are 4.6x magnifications of the boxed areas. (G) The ratio of the number of autophagosomes (red-green–positive puncta) to autolysosomes (red-blue–positive puncta) was determined. Bars represent the mean ± SEM of the ratio in 10 cells from three independent experiments. n.s., not significant, according to an unpaired Student’s t test. (H) WT, BIRC6-KO and UBA6-KO H4 cells were starved of amino acids and serum for the indicated periods, and analyzed by SDS-PAGE and immunoblotting with antibodies to LC3B and β-tubulin. In C, D and H, the positions of molecular mass markers (in kDa) are indicated on the left. (I) Ratio of LC3B-II to β-tubulin at different times relative to the ratio in fed WT H4 cells (set at 1). Points represent the mean ± SEM of the ratio from three independent experiments such as that in H. The indicated p-values were calculated using a two-way ANOVA with Tukey's multiple comparisons test.
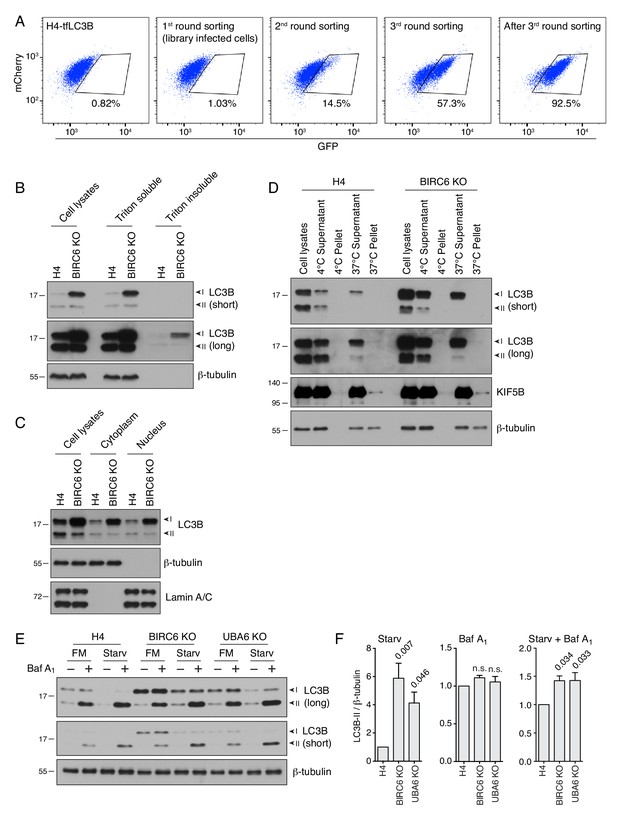
Screening of a ubiquitination library, increased autophagic flux in UBA6-KO and BIRC6-KO cells, and analysis of LC3B in aggregates, nuclei and microtubules.
(A) H4-tfLC3B cells were infected with a lentiviral library targeting 661 major ubiquitination-related genes. Cells with increased GFP signal were collected by FACS and subjected to several rounds of propagation and FACS selection. Notice, that after three rounds, GFP-positive cells were enriched from 1.03% to 92.5%. (B) Analysis of LC3B association with aggregates. WT and BIRC6-KO H4 cells were extracted in Triton X-100 buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA and 1% Triton X-100 supplemented with protease inhibitor cocktail) and centrifuged. Supernatants were collected as the Triton-soluble fraction, while the pellets were resuspended in 5% SDS buffer (Triton X-100 buffer supplemented with 5% SDS) as the Triton-insoluble fraction. Fractions were analyzed by SDS-PAGE and immunoblotting with antibodies to LC3B and β-tubulin. Short and long exposures are shown. Notice that most LC3B is in the soluble fraction in both WT and BIRC6-KO cells, and that only a small amount of aggregated LC3B can be detected upon long exposure of the blots (higher in BIRC6-KO than in WT cells, as in the soluble fraction). (C) Analysis of LC3B presence in a nuclear fraction. WT and BIRC6-KO H4 cells were lysed in hypotonic buffer and centrifuged at 750 g as described in the Materials and methods section. Supernatants were collected as the cytoplasm. Pellets were washed and resuspended in hypotonic buffer, and defined as the nuclear fraction. Samples were analyzed by SDS-PAGE and immunoblotting with antibodies to LC3B, β-tubulin (marker for cytosol) and Lamin A/C (marker for nuclei). Notice the presence of similar amounts of LC3B in the cytoplasmic and nuclear fractions, as previously described (Huang et al., 2015). BIRC6 KO increased the levels of LC3B in both fractions. (D) Analysis of LC3B association with microtubules. Cell lysates from WT and BIRC6-KO H4 cells were analyzed using an in vitro microtubule co-sedimentation assay as described in the Materials and methods section. Incubations were performed at 4°C (control for no microtubule polymerization) and 37°C (microtubule polymerization). Supernatants and pellets were analyzed by SDS-PAGE and immunoblotting with antibodies to LC3B, β-tubulin (indicating the polymerization of microtubules) and KIF5B (positive control for a microtubule-binding protein). Notice the absence of any LC3B associated with the 37°C pellet (i.e., microtubules) in both WT and BIRC6-KO cells. (E) Effects of starvation on LC3B levels in WT, UBA6-KO and BIRC6-KO H4 cells treated with bafilomycin A1. Cells were incubated with bafilomycin A1, nutrient-depleted medium (i.e., starvation), or a combination of both, for 2 hr. Cells were then lysed in 1xLDS sample buffer and analyzed by SDS-PAGE and immunoblotting. FM: fed medium; Starv: starvation medium lacking amino acids and serum. (F) Bar graphs showing LC3B levels for each condition normalized to the levels in WT cells (defined as 1). Values are the mean ± SEM from three independent experiments. The indicated p-values were calculated using a one-way ANOVA with Dunnett’s multiple comparisons test. Notice the presence of more LC3B-II in UBA6-KO and BIRC6-KO cells after starvation or a combination of starvation and bafilomycin A1 treatment, indicative of increased autophagy flux under these conditions. In B, C, D and E, the positions of molecular mass markers (in kDa) are indicated on the left.
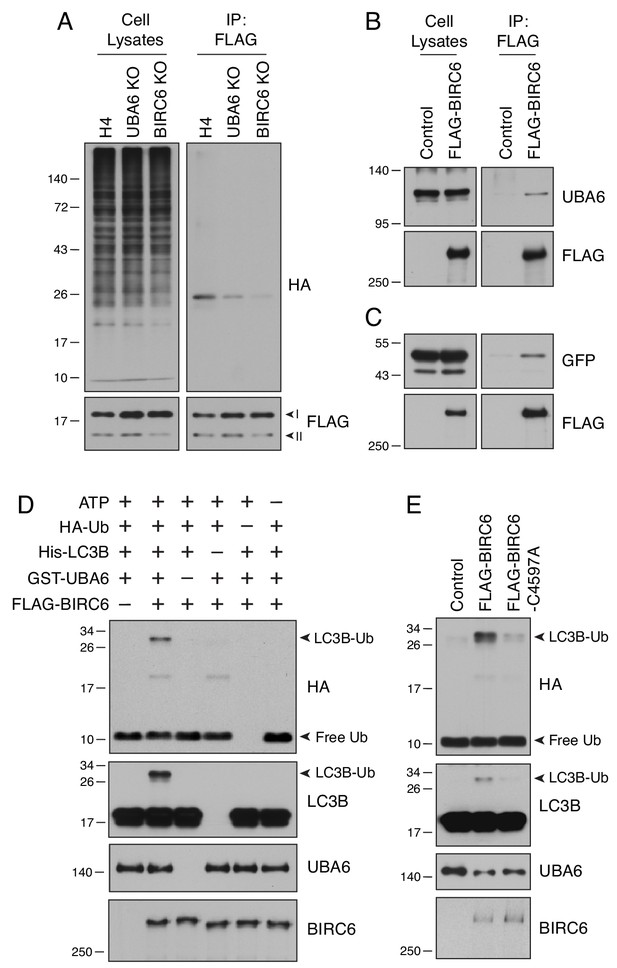
UBA6 and BIRC6 function as E1 and E2/E3 enzymes, respectively, for LC3B monoubiquitination.
(A) WT, UBA6-KO and BIRC6-KO H4 cells were transfected with plasmids encoding HA-Ub and FLAG-LC3B. Cell lysates were analyzed by immunoprecipitation with antibody to the FLAG epitope, followed by SDS-PAGE and immunoblotting with antibodies to the HA and FLAG epitopes. (B) WT H4 cells were transfected with control or FLAG-BIRC6 plasmids. Cell lysates were analyzed by immunoprecipitation with antibody to the FLAG epitope, followed by SDS-PAGE and immunoblotting with antibodies to the FLAG epitope and UBA6. (C) Plasmids encoding GFP-LC3B and FLAG-BIRC6 were transfected into H4 cells. Cell lysates were analyzed by immunoprecipitation with antibody to the FLAG epitope, followed by SDS-PAGE and immunoblotting with antibodies to GFP and the FLAG epitope. (D) FLAG-BIRC6 immunopurified from 107 HEK293T cells transfected with FLAG-BIRC6 plasmid was incubated with recombinant HA-Ub, 6xHis-LC3B and GST-UBA6 in a reaction buffer containing ATP at 37°C for 30 min. Samples were analyzed by SDS-PAGE and immunoblotting with antibodies to the antigens on the right. Notice the ubiquitination of LC3B (LC3B-Ub) only when all the components are present in the mix. (E) WT and C4597A-mutant FLAG-BIRC6 were analyzed for their ability to ubiquitinate 6His-LC3B as in D. In all the panels, the positions of molecular mass markers (in kDa) are indicated on the left.

Mapping regions of BIRC6 that are required for binding to LC3B, and interaction of BIRC6 with Atg8 family members.
(A) Schematic representation of full-length and truncated BIRC6 constructs. The BIR [baculoviral IAP (inhibitor of apoptosis) repeat] domain is colored in red, and the UBC (ubiquitin-conjugating) domain is colored in green. Amino-acid numbers and results of interaction with LC3B are indicated. +, interaction; -, no interaction. (B) Analysis of LC3B interaction with BIRC6 constructs. HEK293T cells were cotransfected with plasmids encoding GFP-LC3B and full-length or truncated FLAG-BIRC6 deletion mutants. Immunoprecipitation was performed using an antibody to the FLAG epitope. Cell lysates and immunoprecipitates were analyzed by immunoblotting with the indicated antibodies. The results indicate that the 2201–2800, 3301–3800 and 3801–4300 segments of BIRC6 participate in the interaction with LC3B. (C) Interaction of BIRC6 with Atg8 family proteins. H4 cells were transfected with plasmids encoding GFP-tagged Atg8-family proteins and FLAG-BIRC6. Cell lysates were subjected to immunoprecipitation with antibody to the FLAG epitope, and cell lysates and immunoprecipitates were analyzed by SDS-PAGE and immunoblotting with antibodies to GFP and the FLAG epitope. Notice that BIRC6 interacts with LC3A, LC3B and LC3C, and weakly or not at all with GABARAP, GABARAPL1 and GABARAPL2. (D) Effect of the K51R mutation in LC3B on interaction with BIRC6. Plasmids encoding FLAG-BIRC6 and GFP-LC3B (WT or K51R mutant) were cotransfected into H4 cells. Cell lysates were analyzed by immunoprecipitation with antibody to the FLAG epitope, followed by SDS-PAGE and immunoblotting with antibodies to GFP and the FLAG epitope. Notice that the LC3B-K51R mutant still interacts with BIRC6. In B, C and D, the positions of molecular mass markers (in kDa) are indicated on the left.
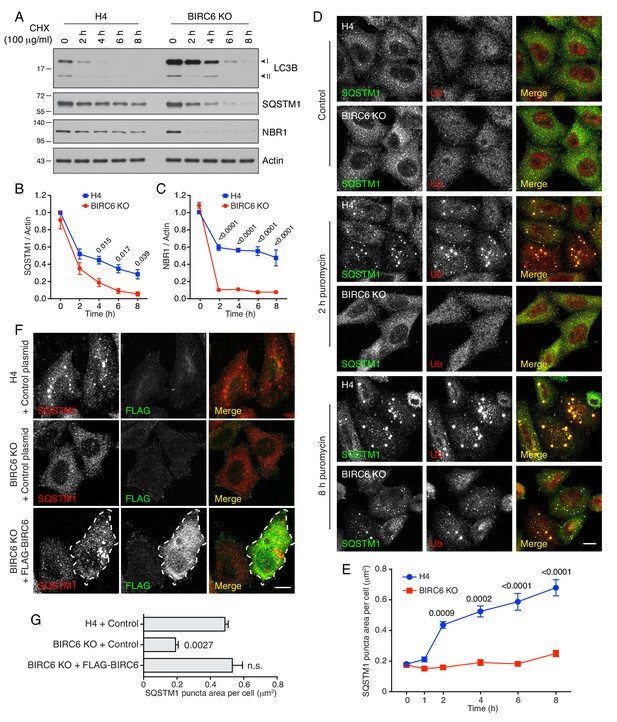
BIRC6 KO reduces the accumulation of puromycin-induced ALIS.
(A) WT and BIRC6-KO H4 cells were incubated with 100 μg/ml cycloheximide (CHX) for the indicated periods. Cells were lysed and analyzed by SDS-PAGE and immunoblotting for the proteins indicated on the right. (B,C) Quantification of the ratio of SQSTM1 (B) and NBR1 (C) to actin at different times after the addition of CHX. Values were normalized to the ratio at time 0 (set at 1). Values are the mean ± SEM from three independent experiments such as that shown in A. (D) WT and BIRC6-KO H4 cells were incubated without (control) or with 5 μg/ml puromycin for 2 and 8 hr. Cells were subsequently immunostained for SQSTM1 and Ub, and examined by confocal microscopy. Scale bar: 10 μm. (E) Quantification of the area (in μm2) of SQSTM1-positive puncta per cell determined from cells such as those shown in D. Values represent the mean ± SEM of the puncta area in 30 cells from three independent experiments. (F) WT or BIRC6-KO cells were transfected with control plasmid or plasmid encoding FLAG-BIRC6 as indicated in the figure, and incubated with 5 μg/ml puromycin for 4 hr prior to immunostaining with antibodies to SQSTM1 and the FLAG epitope. Scale bar: 10 μm. A FLAG-BIRC6–expressing cell is outlined. In D and F, single-channel images are shown in grayscale. (G) Quantification of the area of SQSTM1 positive puncta per cell. Values represent the mean ± SEM of the puncta area in 30 cells from three independent experiments. The p-values shown in panels B, C, E and G were calculated using a one-way ANOVA with Dunnett’s multiple comparisons test.
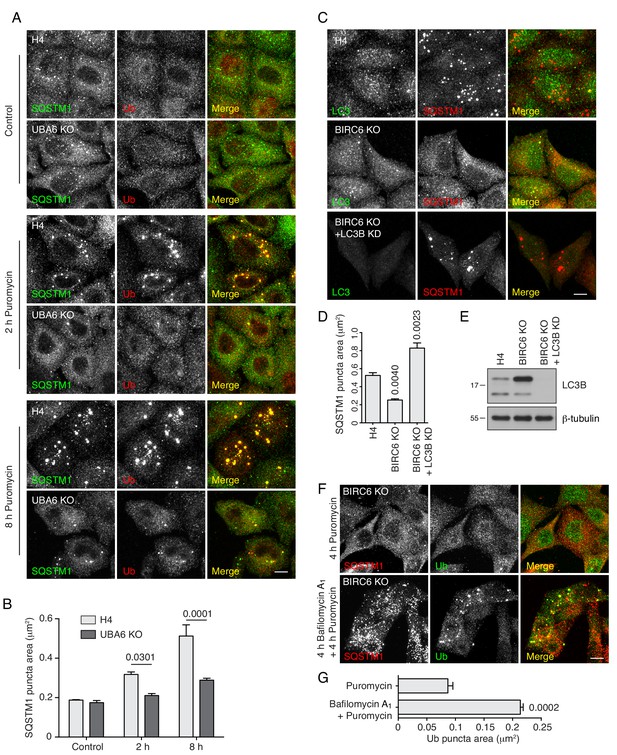
Analyses of ALIS formation in UBA6-KO and BIRC6-KO cells.
(A) WT and UBA6-KO H4 cells were incubated with 5 μg/ml puromycin for 2 and 8 hr. Cells were fixed and immunostained with antibodies to SQSTM1 and Ub. Scale bar: 10 μm. As shown in Figure 7, puromycin induced formation of ALIS (aggresome-like induced structures) in WT H4 cells after a 2 hr incubation. In contrast, ALIS emerged after an 8 hr incubation in UBA6-KO cells. (B) Quantification of the area of SQSTM1-positive puncta in cells such as those shown in A. Values represent the mean ± SEM of the puncta area in 30 cells from three independent experiments. The indicated p-values were calculated using a two-way ANOVA with Tukey's multiple comparisons test. (C) BIRC6-KO cells were transfected with siRNA oligonucleotides targeting LC3B (Cell Signaling Technology, 6212), and then incubated with 5 μg/ml puromycin for 4 hr prior to immunofluorescent staining. Scale bar: 10 μm. As also shown in Figure 7, puromycin did not accumulate ALIS in BIRC6-KO as compared to WT H4 cells. However, LC3B depletion by siRNA treatment of BIRC6-KO cells increased the accumulation of ALIS by puromycin. (D) Quantification of the area of SQSTM1-positive puncta in cells such as those shown in C. Values represent the mean ± SEM of the puncta area in 30 cells from three independent experiments. The indicated p-values were calculated using a one-way ANOVA with Dunnett’s multiple comparisons test. (E) SDS-PAGE and immunoblotting of WT, BIRC6-KO and BIRC6-KO–LC3B-KD H4 cells. Notice the drastic reduction of LC3B levels in siRNA-transfected BIRC6-KO cells. The positions of molecular mass markers (in kDa) are indicated on the left. (F) BIRC6-KO cells were pretreated without or with 50 nM Bafilomycin A1 for 4 hr prior to ALIS induction by 4 hr puromycin treatment. Cells were immunostained with antibodies to SQSTM1 and Ub. Scale bar: 10 μm. Notice that bafilomycin A1 pretreatment caused accumulation of SQSTM1 puncta in BIRC6-KO cells, indicating that lysosomal degradation was successfully inhibited and that the failure of the mutant cells to accumulate ALIS in the absence of bafilomycin A1 is due to their higher degradative capacity relative to WT cells. (G) Quantification of the area of Ub-positive puncta in cells such as those shown in F. Values represent the mean ± SEM of the puncta area in 30 cells from three independent experiments. The indicated p-values were calculated using Student’s t test.
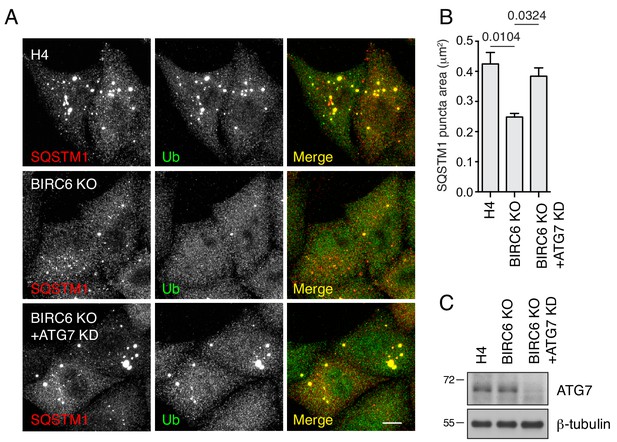
Analyses of ALIS formation in BIRC6-KO cells transfected with ATG7 siRNA.
(A) WT and BIRC6-KO H4 cells were transfected with control or ATG7 siRNA for 48 hr prior to ALIS induction with puromycin. Cells were immunostained with antibodies to SQSTM1 and Ub. Scale bar: 10 μm. Notice that ATG7 depletion resulted in accumulation of ALIS in BIRC6-KO cells, indicating that the increased clearance of protein aggregates in BIRC6-KO cells was dependent on autophagy. (B) Quantification of the area of SQSTM1-positive puncta in cells such as those shown in H. Values represent the mean ± SEM of the puncta area in 30 cells from three independent experiments. The indicated p-values were calculated using a one-way ANOVA with Dunnett’s multiple comparisons test. (C) Immunoblotting of WT, BIRC6-KO and BIRC6-KO–ATG7-KD H4 cells showing the reduction of ATG7 levels in BIRC6-KO–ATG7-KD cells. The positions of molecular mass markers (in kDa) are indicated on the left.
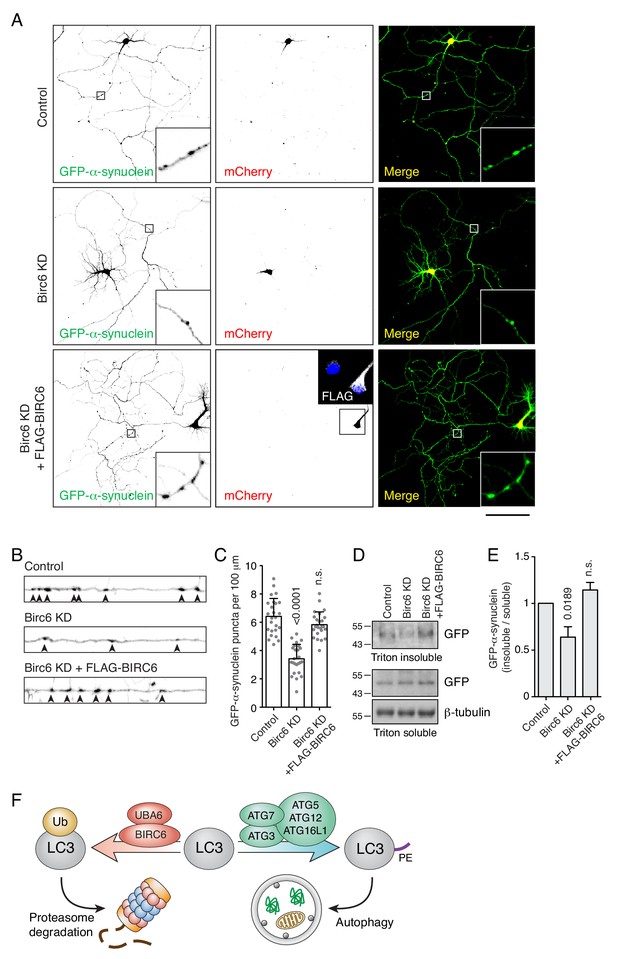
Birc6 depletion promotes the clearance of α-synuclein aggregates in neurons.
(A) Confocal fluorescence microscopy of cultured rat hippocampal neurons co-transfected with plasmids encoding GFP-α-synuclein A53T mutant, mCherry transfection control, rat Birc6 shRNA-mCherry, and/or shRNA-resistant FLAG-tagged human BIRC6 (rescue), as indicated in the figure. Transfections were performed at day-in-vitro 3 (DIV3) and neurons were fixed for immunofluorescence microscopy at DIV7. Single-channel images are shown in inverted grayscale. Scale bar: 100 μm. Insets on the left and right columns are 7-fold magnified views from the axons in the boxed area. The inset in the middle bottom row is a 2.2-fold magnified view of FLAG-BIRC6 expression in the boxed area. (B) Magnified and straightened axons from control, Birc6-KD and FLAG-BIRC6-rescue neurons shown in A. Arrowheads indicate α-synuclein aggregates in the axon. (C) Quantification of the number of α-synuclein puncta (i.e., aggregates) per 100 μm of axon. Values are the mean ± SEM from 25 neurons from three independent experiments. The indicated p-values were calculated using a one-way ANOVA with Dunnett’s multiple comparisons test. (D) Cultured rat hippocampal neurons were transfected as in A. Neurons were extracted in Triton X-100 buffer and centrifuged. Supernatants were collected as the Triton-soluble fraction, while the pellets were resuspended in 5% SDS buffer as the Triton-insoluble fraction. Fractions were analyzed by SDS-PAGE and immunoblotting with antibodies to GFP (to detect GFP-α-synuclein) and β-tubulin (loading control). The positions of molecular mass markers (in kDa) are indicated on the left. (E) Quantification of the ratio of Triton-insoluble and -soluble GFP-α-synuclein. The value in control-shRNA–transfected neurons was set at 1. Values are the mean ± SEM from three independent experiments such as that in panel D. The indicated p-values were calculated using a one-way ANOVA with Dunnett’s multiple comparisons test. (F) Schematic representation of the role of UBA6 and BIRC6 in LC3 ubiquitination and targeting for degradation, decreasing the amount of LC3 that can be modified with PE for its function in autophagy.

Aggregation of α-synuclein and verification of Birc6 KD in rat hippocampal neurons.
(A) Rat hippocampal neurons in primary culture were cotransfected with plasmids encoding GFP-α-synuclein A53T mutant and HA-Ub or mCherry-LC3B as indicated. Transfections were performed at day-in-vitro 3 (DIV3), and neurons were fixed for immunofluorescence microscopy at DIV7. Single-channel images are shown in inverted grayscale (left panels). Scale bar: 100 μm. Axons indicated by arrowheads were straightened and enlarged for better appreciation of protein colocalization (right panels). Merged images show that α-synuclein aggregates colocalize with Ub, SQSTM1 and LC3B, indicating that α-synuclein aggregates are ubiquitinated and recognized for selective autophagy. (B) Alignment of the shRNA-target sequence of genes encoding rat Birc6 and human BIRC6. The human BIRC6 gene has a two-nucleotide mismatch as compared to the shRNA designed for rat Birc6. (C) BIRC6-H2R (human to rat) plasmid was generated by substituting the shRNA-targeting site in human BIRC6 with the homologous rat Birc6 sequence. H4 cells were transfected with plasmids encoding BIRC6 or BIRC6-H2R together with the plasmid encoding Birc6 shRNA. Cells were lysed in 1xLDS sample buffer and analyzed by immunoblotting with antibodies to the indicated proteins. Notice the decrease in BIRC6-H2R levels by Birc6 shRNA, verifying that the Birc6 shRNA was able to silence the expression of rat Birc6 gene. The positions of molecular mass markers (in kDa) are indicated on the left.
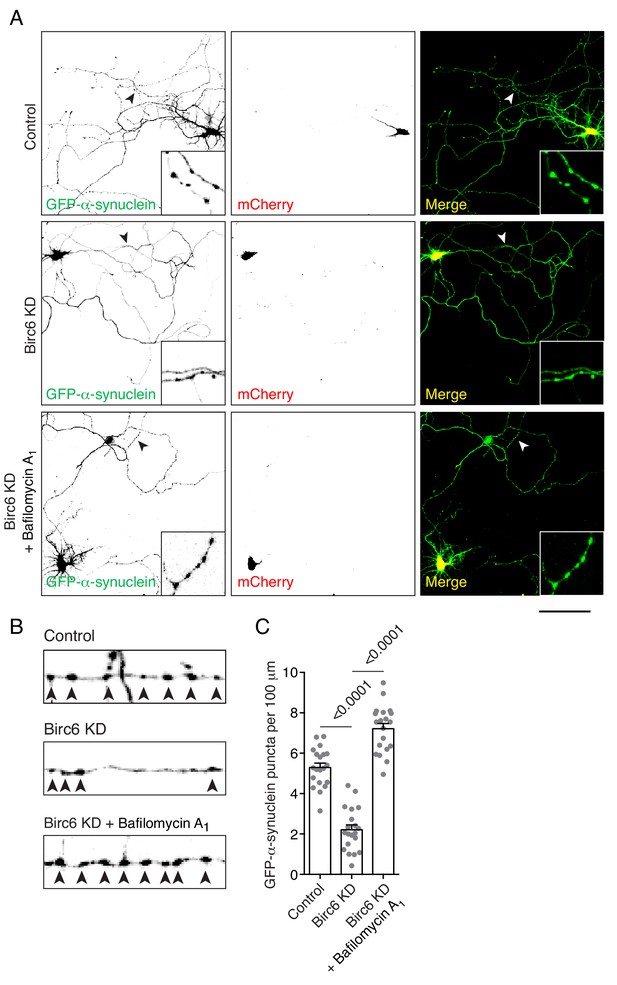
Autophagy dependence of α-synuclein aggregate clearance in rat hippocampal neurons.
(A) Confocal fluorescence microscopy of cultured rat hippocampal neurons cotransfected at DIV3 with plasmids encoding GFP-α-synuclein A53T mutant, control shRNA-mCherry or rat Birc6 shRNA-mCherry as indicated in the figure. At DIV6, Birc6 shRNA-mCherry transfected neurons were incubated with 10 nM bafilomycin A1 for 16 hr. Neurons were fixed for immunofluorescence assay at DIV7. Single-channel images are shown in inverted grayscale. Scale bar: 100 μm. Insets show magnified views of the axons indicated by arrowheads to better appreciation of α-synuclein aggregates. (B) Magnified and straightened axons from control, Birc6-KD and Birc6-KD–bafilomycin A1 neurons shown in A. Arrowheads indicate mutant α-synuclein aggregates in the axon. (C) Quantification of the number of α-synuclein aggregates per 100 μm of axon. Values are the mean ± SEM from 25 neurons from three independent experiments. The indicated p-values were calculated using a one-way ANOVA with Dunnett’s multiple comparisons test. Notice that Birc6 KD decreased GFP-α-synuclein aggregate formation and that bafilomycin A1 treatment reversed this effect of Birc6 KD.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | LC3B (rabbit, monoclonal) | Cell Signaling Technology | 3868 | IB: 1:2000 |
| Antibody | UBA6 (rabbit polyclonal) | Cell Signaling Technology | 13386 | IB: 1:1000 |
| Antibody | β-tubulin (rabbit polyclonal) | Cell Signaling Technology | 2146 | IB: 1:1000 |
| Antibody | MYC epitope (rabbit polyclonal) | Santa Cruz Biotechnology | sc-789 | IB: 1:5000 |
| Antibody | Ub (mouse monoclonal) | Thermo Fisher Scientific | 13–1600 | IB: 1:1000 |
| Antibody | Ub (mouse monoclonal) | Enzo Life Sciences | BML-PW8805-0500 | IF: 1:500 |
| Antibody | HA epitope (mouse monoclonal) | BioLegend | 901501 | IB: 1:2000 |
| Antibody | FLAG epitope (mouse monoclonal) | Sigma-Aldrich | F1804 | IB: 1:1000 |
| Antibody | SQSTM1 (mouse monoclonal) | BD Biosciences | 610833 | IB: 1:1000 |
| Antibody | SQSTM1 (rabbit polyclonal) | Enzo Life Sciences | BML-PW9860-0100 | IF: 1:500 |
| Antibody | NBR1 (rabbit monoclonal) | Cell Signaling Technology | 9891 | IB: 1:1000 |
| Antibody | BIRC6 (rabbit monoclonal) | Cell Signaling Technology | 8756 | IB: 1:1000 |
| Antibody | GFP (rabbit polyclonal) | Thermo Fisher Scientific | A11122 | IB: 1:1000 |
| Antibody | Actin (mouse monoclonal) | Cell Signaling Technology | 3700 | IB: 1:1000 |
| Chemical compound, drug | Bafilomycin A1 | Sigma-Aldrich | B1793 | |
| Chemical compound, drug | MG132 | Sigma-Aldrich | M7449 | |
| Chemical compound, drug | Cycloheximide | Sigma-Aldrich | C4859 | |
| Chemical compound, drug | Puromycin | Sigma-Aldrich | P8833 | |
| Chemical compound, drug | Polybrene | Sigma-Aldrich | H9268 | |
| Chemical compound, drug | MgATP | Boston Biochem | B-20 | |
| Chemical compound, drug | Fibronectin | Sigma-Aldrich | F2006 | |
| Cell line (Homo sapiens) | H4 | ATCC | HTB-148 | |
| Cell line (Homo sapiens) | HeLa | ATCC | CCL-2 | |
| Cell line (Homo sapiens) | HEK293T | ATCC | CRL-11268 | |
| Recombinant DNA | HA-Ub | Addgene; gift from Edward Yeh | 18712 | |
| Recombinant DNA | GFP-α-synuclein A53T | Addgene; gift from David Rubinsztein | 40823 | |
| Recombinant DNA | GFP-LC3B | Jia et al., 2017 | ||
| Recombinant DNA | GFP-mCherry-LC3B | Jia et al., 2017 | ||
| Recombinant DNA | pSpCas9 (BB)−2A-GFP | Addgene; gift from Feng Zhang | 40823 | |
| Recombinant DNA | FLAG-BIRC6 | Gift from Mikihiko Naito | Kawasaki Hospital, Kobe, Japan | |
| Recombinant DNA | pMD2.G | Addgene; gift from Didier Trono | 12259 | |
| Recombinant DNA | psPAX | Addgene: gift from Didier Trono | 12260 | |
| Recombinant DNA | lentiCRISPR v2 | Addgene; gift from Feng Zhang | 52961 | |
| Sequence-based reagent | UBA6 siRNA | Thermo Fisher Scientific | 4392420-s30516 | |
| Sequence-based reagent | UBA6 siRNA | Thermo Fisher Scientific | 4392420-s30517 | |
| Sequence-based reagent | BIRC6 siRNA | Thermo Fisher Scientific | 4427037-s33037 | |
| Sequence-based reagent | BIRC6 siRNA | Thermo Fisher Scientific | 4427037-s33038 | |
| Sequence-based reagent | BIRC6 siRNA | Thermo Fisher Scientific | 4427037-s33039 | |
| Sequence-based reagent | Non-targeting siRNA | Eurofins Scientific | UGGUUUACAUGUCGACUAAUUU | |
| Peptide, recombinant protein | GST-UBA6 | Boston Biochem | E-307 | |
| Peptide, recombinant protein | 6xHis-LC3B | ProSpec | PRO-076 | |
| Peptide, recombinant protein | HA-Ub | Boston Biochem | U-110 | |
| Commercial assay or kit | Q5 Site-Directed Mutagenesis Kit | New England Biolabs | E0552 | |
| Commercial assay or kit | Gibson AssemblyMaster Mix | New England Biolabs | E2611 | |
| Commercial assay or kit | Human CRISPR KO Pooled Library (GeCKO v2) | Addgene; gift from Feng Zhang | 1000000048 | |
| Commercial assay or kit | Endura ElectroCompetent cells | Lucigen | 60242 | |
| Software, algorithm | Prism 7 | GraphPad Software | https://www.graphpad.com | |
| Software, algorithm | MAGeCK | https://sourceforge.net/p/ mageck/wiki/Home | ||
| Software, algorithm | ImageJ | National Institutes of Health | https://imagej.nih.gov/ij/ |
-
IB: Immunoblotting; IF immunofluorescence
Additional files
-
Supplementary file 1
Summary of next generation sequencing reads in the primary genome-wide screen.
- https://doi.org/10.7554/eLife.50034.020
-
Supplementary file 2
MAGeCK of results from the primary genome-wide screen.
- https://doi.org/10.7554/eLife.50034.021
-
Supplementary file 3
List of sgRNAs used in the secondary CRISPR library.
- https://doi.org/10.7554/eLife.50034.022
-
Supplementary file 4
Summary of next generation sequencing reads in the secondary screen.
- https://doi.org/10.7554/eLife.50034.023
-
Supplementary file 5
MAGeCK of results from the secondary screen.
- https://doi.org/10.7554/eLife.50034.024
-
Supplementary file 6
List of sgRNAs used in the ubiquitination CRISPR library.
- https://doi.org/10.7554/eLife.50034.025
-
Supplementary file 7
Summary of next generation sequencing reads in the ubiquitination screen.
- https://doi.org/10.7554/eLife.50034.026
-
Supplementary file 8
MAGeCK of results from the ubiquitination screen.
- https://doi.org/10.7554/eLife.50034.027
-
Transparent reporting form
- https://doi.org/10.7554/eLife.50034.028





