Control of cell death/survival balance by the MET dependence receptor
Figures
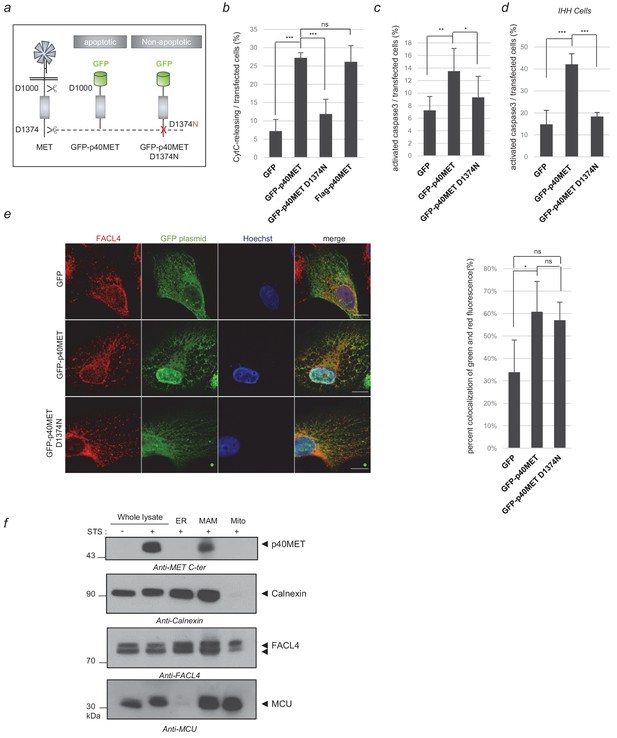
Locating the p40MET fragment by immunofluorescence and subcellular fractionation.
(a) Schematic representation of MET receptor cleavage by caspase during apoptosis, with besides representation of the GFP-p40MET fragment and of the GFP-p40MET D1374N fragment which still possesses the C-terminal tail. (b, c, d) MCF10A (b–c) or IHH (d) cells were transiently transfected with a vector expressing GFP, GFP-p40MET, GFP-p40MET D1374N, or flag-p40MET. After 24 hr transfection for MCF10A and 48 hr for IHH, the cells were fixed and labeled with an appropriate antibody: anti-flag when transfected with the vector expressing flag-p40MET, anti-cytochrome C or anti-cleaved caspase 3 antibody to evaluate apoptosis. The percentage of cytochrome C release or of cleaved-caspase-3-positive cells was determined with respect to the number of GFP- or flag-positive cells. At least 150 cells per well (n = 6;± S.D.) (b), 200 cells per well (n = 6;± S.D.) (c) and 60 cells per well (n = 4;± S.D.) (d) were counted. (e) MCF10A epithelial cells were transfected with a vector expressing GFP, GFP-p40MET, or GFP-p40MET D1374N. Twenty-four hours after transfection, the nuclei were stained with Hoechst (blue staining) and immunofluorescence staining was performed with anti-FACL4 to label the MAMs (red staining). Cells were observed by fluorescence confocal microscopy. Weighted colocalization coefficients were determined by means of Manders coefficients for green staining (of GFP, GFP-p40MET, or GFP-p40MET D1374N) and FACL4 staining (red) on the basis of the fluorescence confocal microscopy images (n = 30;±S.D.). (f) MCF10A cells were starved overnight and treated for 4 hr with 1 µM staurosporine (STS). After treatment, the cells were fractionated into ER, MAM and mitochondrial fractions. Proteins from whole-cell lysates (50 µg) and from the different fractions (20 µg) were analyzed by western blotting with antibodies against the MET kinase domain, the reticular protein calnexin, the MAM protein FACL4, and the inner mitochondrial membrane protein MCU. The positions of prestained molecular weight markers are indicated. Arrows indicate the positions of p40MET, calnexin, FACL4 and MCU; scale bar = 10 µm, ns, nonsignificant; *, p<0.05; **, p<0.01; ***, p<0.001 as determined by Student’s t test.
-
Figure 1—source data 1
Source data of Figure 1b–c–d and Figure 1—figure supplement 1d reporting counting of GFP, active caspase 3, and cytochrome C release positive cells, calculation of the percentage, mean and SD, diagram conception and statistical analyses; source data of Figure 1e reporting the coefficient of fluorescence colocalisation, calculation of the mean, SD and statistical analyses.
- https://cdn.elifesciences.org/articles/50041/elife-50041-fig1-data1-v1.xlsx
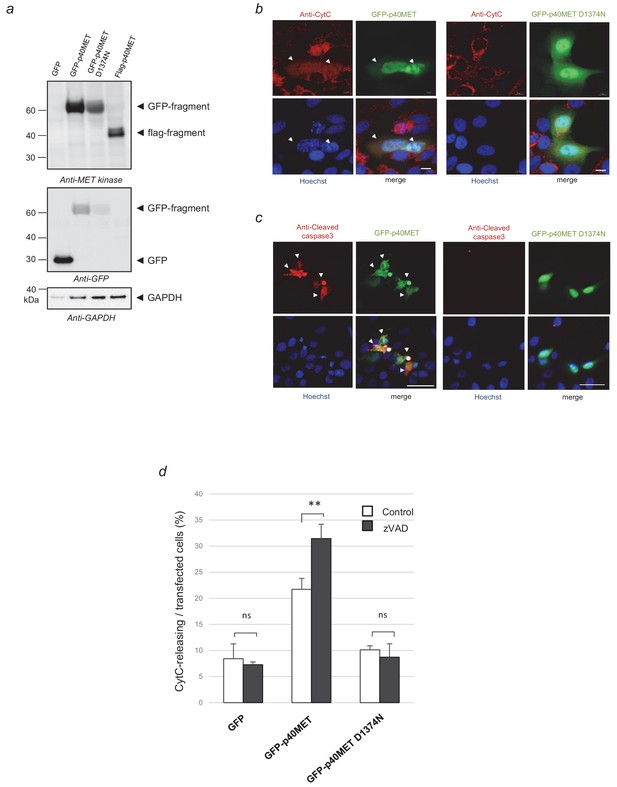
Validation of the vectors expressing GFP-p40MET and GFP-p40MET D1374N.
(a) HEK 293 cells were transfected with a vector expressing GFP, GFP-p40MET, GFP-p40MET D1374N or Flag-p40MET. Twenty-four hours after transfection, the cells were lysed. The protein mixture was resolved by 4–12% SDS-PAGE and analyzed by western blotting with antibodies against the MET kinase domain, GFP, and GAPDH. (b–c) Representative pictures of transfected cells immuno-labeled with a cytochrome-c (b) or cleaved-caspase 3 antibody (c) are shown. White arrowheads indicate transfected cells positive for cytochrome-c release or cleaved caspase 3; scale bars = 10 µm (b) and 50 µm (c). (d) MCF10A epithelial cells were transiently transfected with a vector expressing GFP, GFP-p40MET or GFP-p40MET D1374N and treated with zVAD (20 µM). Twenty-four hours after transfection, the cells were fixed and labeled with anti-cytochrome C antibody. The percentage of cells displaying cytochrome C release was determined with respect to the number of GFP-positive cells. At least 60 cells were counted per well (n = 3; mean ± S.D; **p<0.01 as determined by Student’s t test).
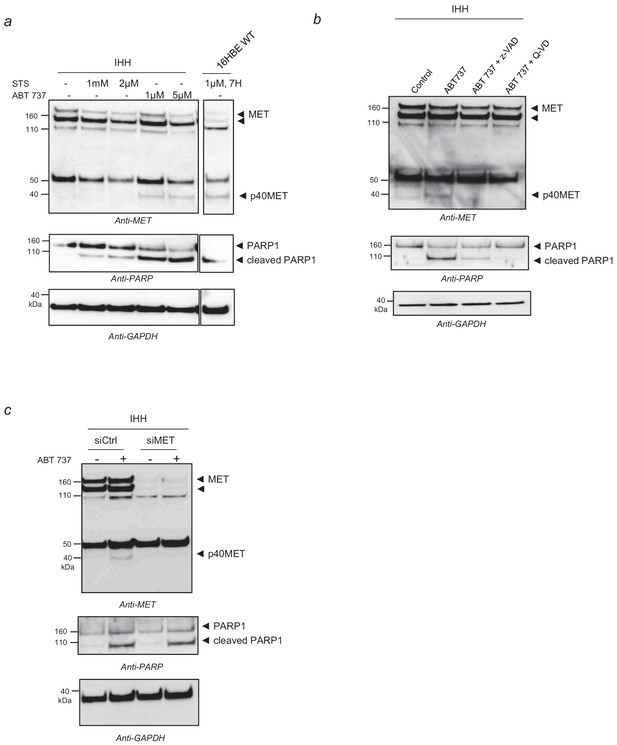
p40MET fragment generation in IHH cells.
(a) IHH hepatocyte cells were cultured for 24 hr on 6 well plates coated with collagen. Cells were then starved overnight in a serum-free medium and treated 7 hr with staurosporine (STS) or ABT 737 at the indicated concentration before cell lysis. As a positive control, 16HBE epithelial pulmonary cells were starved overnight in a serum-free medium and treated 7 hr with staurosporine (STS) at the indicated concentration before cells lysis. (b), IHH cells were cultured for 24 hr on 6 well plates coated with collagen. Then cells were starved overnight in a serum-free medium and treated 7 hr with 1 µM ABT 737 with or without the pan-caspase inhibitor zVAD-FMK or Q-VD (20 µM). (c) IHH cells were transfected with a control siRNA or a pool of three MET-targeting siRNAs for 48 hr on 12 well plates coated with collagen. Cells were then treated 7 hr with ABT 737 (1µM) before cells lysis. The same amount of proteins was resolved by SDS-PAGE and analyzed by immunoblotting with antibodies against the MET kinase domain, PARP, to assess apoptosis induction, and GAPDH, to assess loading.
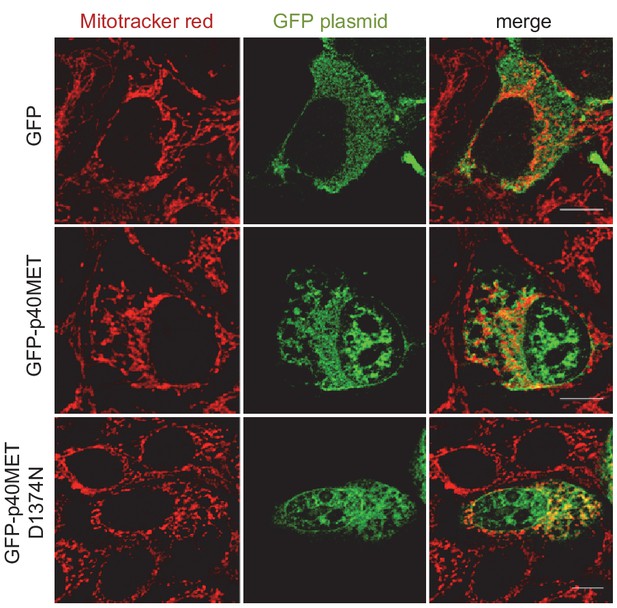
Partial overlap between GFP-p40MET and mitotracker signals acquired by immunofluorescence.
MCF10A epithelial cells were transfected with a vector expressing GFP, GFP-p40MET, or GFP-p40MET D1374N. Twenty-four hours after transfection the mitochondria were stained with MitoTracker (red), fixed, and observed by fluorescence confocal microscopy.
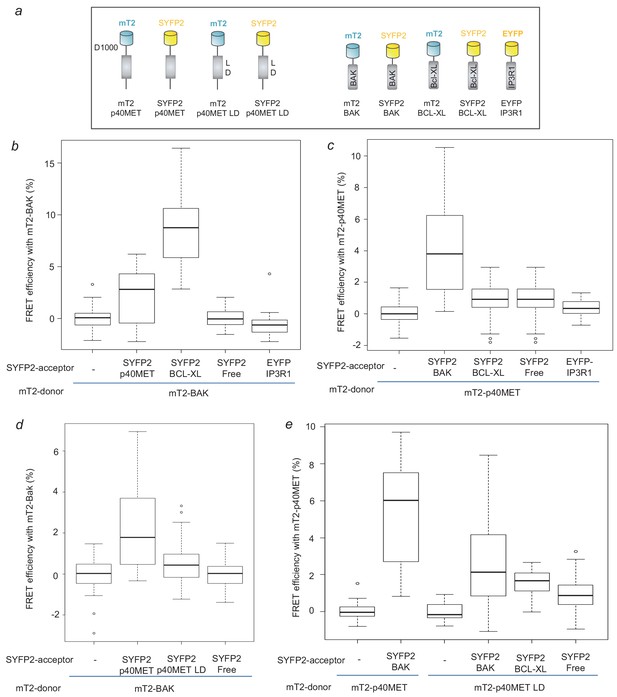
FRET measurement to analyze p40MET and BAK interaction.
(a) Schematic representation of the mTurquoise2- or SYFP2-tagged proteins used for FRET analysis. (b) Cells were co-transfected with a vector expressing BAK-mT2 as FRET donor and with a vector expressing free SYFP2, BCL-XL-SYFP2, EYFP-IP3R1 or p40MET-SYFP2 as FRET acceptor. The fluorescence lifetime of BAK-mT2 was measured by Time-Correlated Single Photon Counting (TCSPC) and the FRET efficiency was calculated with respect to the donor-alone condition. At least 35 cells were counted for each condition. (c) Cells were co-transfected with a vector expressing p40MET-mT2 as FRET donor and a vector expressing free SYFP2, BAK-SYFP2, EYFP-IP3R1 or BCL-XL-SYFP2 as FRET acceptor. The fluorescence lifetime of p40MET-mT2 was measured by TCSPC and the FRET efficiency was calculated with respect to the donor-alone condition. At least 40 cells were counted for each condition. (d and e) Cells were co-transfected with a vector expressing BAK-mT2 as FRET donor and with a vector expressing free SYFP2, p40MET-SYFP2 mutated or not at the L and D residues of the putative BH3-like domain as FRET acceptor (d). Inversely, cells were co-transfected also with vectors expressing p40MET-mT2 mutated or not on the L and D residues as FRET donor and a vector expressing free SYFP2, BAK-SYFP2, or BCL-XL-SYFP2 as FRET acceptor (e). The fluorescence lifetime of p40MET-mT2 and BAK-mT2 were measured by TCSPC and the FRET efficiency was calculated with respect to the donor-alone condition. At least 30 cells were counted for each condition.
-
Figure 2—source data 1
Source data of Figure 2b-e reporting FRET source data and diagram conception.
- https://cdn.elifesciences.org/articles/50041/elife-50041-fig2-data1-v1.xlsx
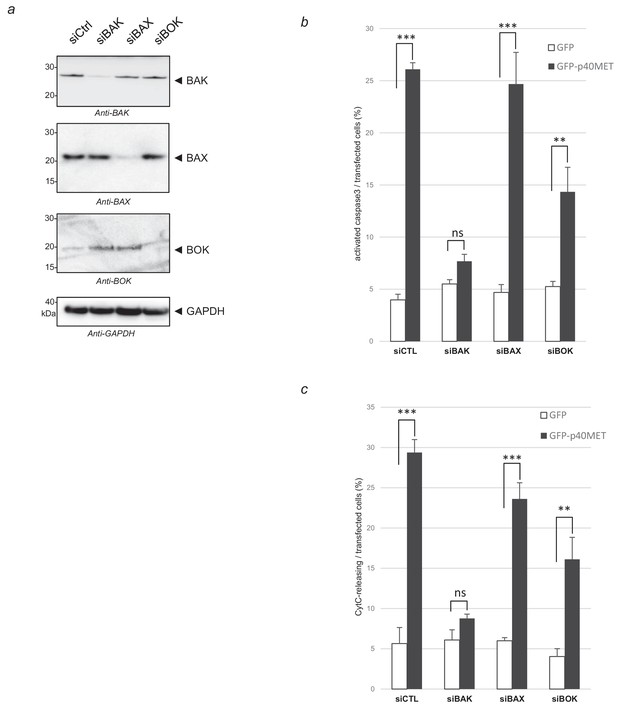
Effect of BAK, BAX and BOK silencing on p40MET-induced apoptosis in MCF10A cells.
One day before transfection with a vector expressing either GFP or GFP-p40MET, MCF10A cells cultured on 12 well plates were transfected with a control siRNA or with siRNA targeting either BAK, BAX or BOK and treated with zVAD (20 µM) when analyzed next for cytochrome C release. Twenty four hours later for MCF10A, (a) one part of the cells was lysed and extracts were analyzed by western blotting with antibodies against BAK, BAX, BOK and GAPDH. (b, c) In parallel, immunofluorescence staining was performed with an anti-cleaved caspase 3 or anti-cytochrome C antibodies and nuclei were labeled with Hoechst. The percentage of cytochrome C release or of cleaved-caspase-3-positive cells was determined with respect to the number of GFP-positive cells. At least 100 cells were counted per well (n = 3;± S.D.). ns, nonsignificant; **, p<0.01; ***, p<0.001 as determined by Student’s t test.
-
Figure 2—figure supplement 1—source data 1
Source data of Figure 2—figure supplement 1b–c reporting counting of GFP, active caspase 3, and cytochrome C release positive cells, calculation of the percentage, mean and SD, diagram conception and statistical analyses.
- https://cdn.elifesciences.org/articles/50041/elife-50041-fig2-figsupp1-data1-v1.xlsx
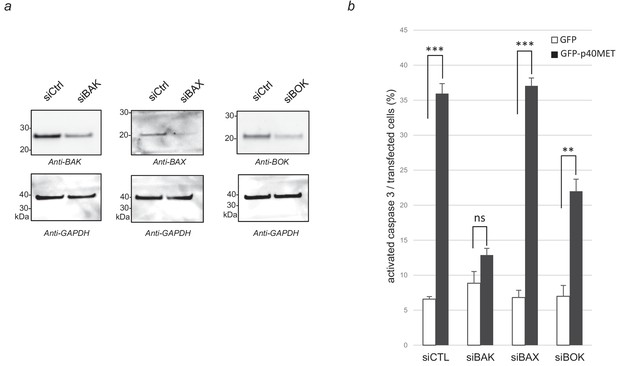
Effect of BAK, BAX and BOK silencing on p40MET-induced apoptosis in IHH cells.
One day before transfection with a vector expressing either GFP or GFP-p40MET, IHH cells cultured on 12 well plates were transfected with a control siRNA or with siRNA targeting either BAK, BAX or BOK. Forty eight hours later, (a) one part of the cells was lysed and extracts were analyzed by western blotting with antibodies against BAK, BAX, BOK and GAPDH. (b) In parallel, immunofluorescence staining was performed with an anti-cleaved caspase 3 antibody and nuclei were labeled with Hoechst. The percentage cleaved-caspase-3-positive cells was determined with respect to the number of GFP-positive cells. At least 100 cells were counted per well (n = 3;± S.D.). ns, nonsignificant; **, p<0.01; ***, p<0.001 as determined by Student’s t test.
-
Figure 2—figure supplement 2—source data 1
Source data of Figure 2—figure supplement 2b reporting counting of GFP and active caspase 3 positive cells, calculation of the percentage, mean and SD, diagram conception and statistical analyses.
- https://cdn.elifesciences.org/articles/50041/elife-50041-fig2-figsupp2-data1-v1.xlsx
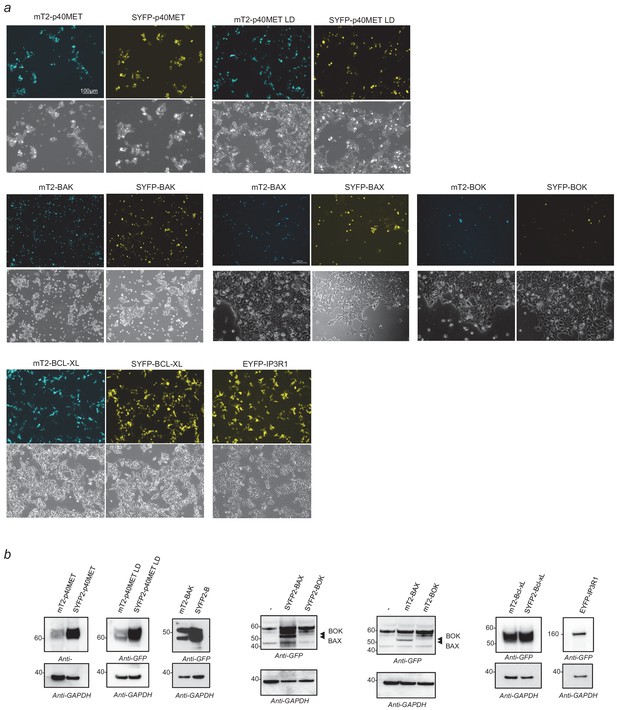
Validation of the vectors expressing m-Turquoise2- or SYFP2-tagged proteins in FRET experiments.
(a–b) HEK293 cells were transiently transfected with a vector expressing mTurquoise2- or SYFP2-tagged p40MET, BAK, BAX, BOK, BCL-XL, or EYFP-IP3R1. Twenty-four hours after transfection, cells were (a) analyzed by fluorescence microscopy to check for expression of fluorescent proteins and (b) lysed. Extracts were resolved by 10% SDS-PAGE and analyzed by western blotting with anti-GFP and anti-GAPDH antibodies.
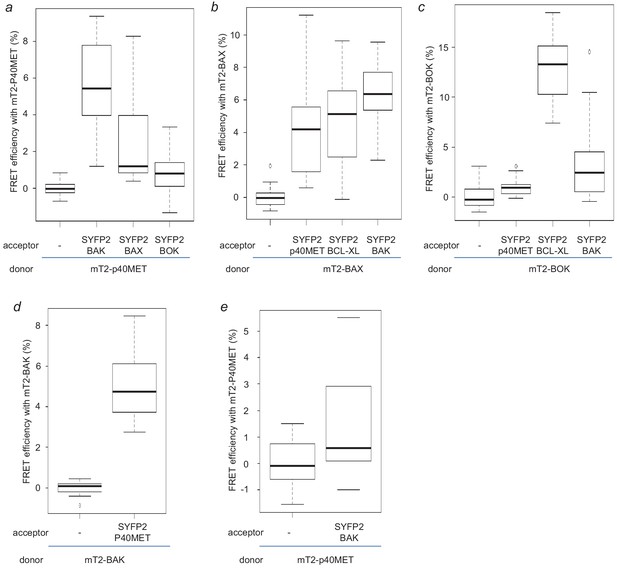
FRET experiments between p40MET and BCL2 family members.
(a–c) HEK293 cells were co-transfected with a vector expressing mT2-fused with either p40MET (a), BAX (b) or BOK (c) as a FRET donor and with a vector expressing the indicated SYFP2-tagged acceptors. The fluorescence lifetime of mT2-fused protein was measured by Time-Correlated Single Photon Counting (TCSPC) and the FRET efficiency was calculated with respect to the donor-alone condition. (d–e) IHH cells were co-transfected with a vector expressing mT2 fused with BAK (d), or p40MET (e) as a FRET donor and with a vector expressing SYFP2-tagged p40MET (d) or BAK (e). The fluorescence lifetime of mT2-fused protein was measured by Time-Correlated Single Photon Counting (TCSPC) and the FRET efficiency was calculated with respect to the donor-alone condition.
-
Figure 2—figure supplement 4—source data 1
Source data of Figure 2—figure supplement 4a–e including FRET source data and diagram conception.
- https://cdn.elifesciences.org/articles/50041/elife-50041-fig2-figsupp4-data1-v1.xlsx
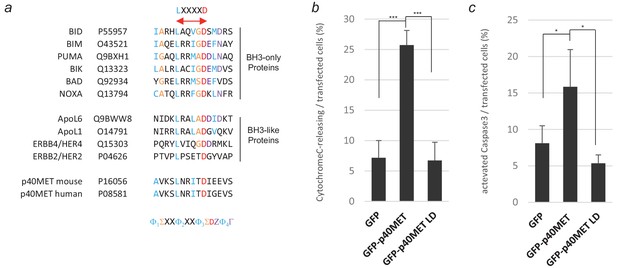
Consequence of putative BH3 domain mutation on p40MET-induced apoptosis.
(a) Sequences of BH3-only proteins and BH3-like proteins were aligned with those of murine and human p40MET. The consensus sequence for the BH3 domain is shown below, with the most conserved residues highlighted in colors. (b, c) MCF10A epithelial cells were transiently transfected with a vector expressing GFP, GFP-p40MET, or GFP-p40MET LD (in which residues L1110 and D1115 were mutated). Twenty-four hours after transfection, the cells were fixed and labeled with an appropriate antibody: anti-cytochrome C (d) or anti-cleaved caspase 3 antibody (e) to evaluate apoptosis. The percentage of cytochrome C release or of caspase-3-positive cells was determined with respect to the number of GFP-positive cells. At least 100 cells were counted per well (n = 3;± S.D). *, p<0.05; ***, p<0.001 as determined by Student’s t test.
-
Figure 2—figure supplement 5—source data 1
Source data of Figure 2—figure supplement 5b–c reporting counting of GFP, active caspase 3 and cytochrome C release positive cells, computation of the percentage, mean and SD, diagram conception and statistical analyses.
- https://cdn.elifesciences.org/articles/50041/elife-50041-fig2-figsupp5-data1-v1.xlsx
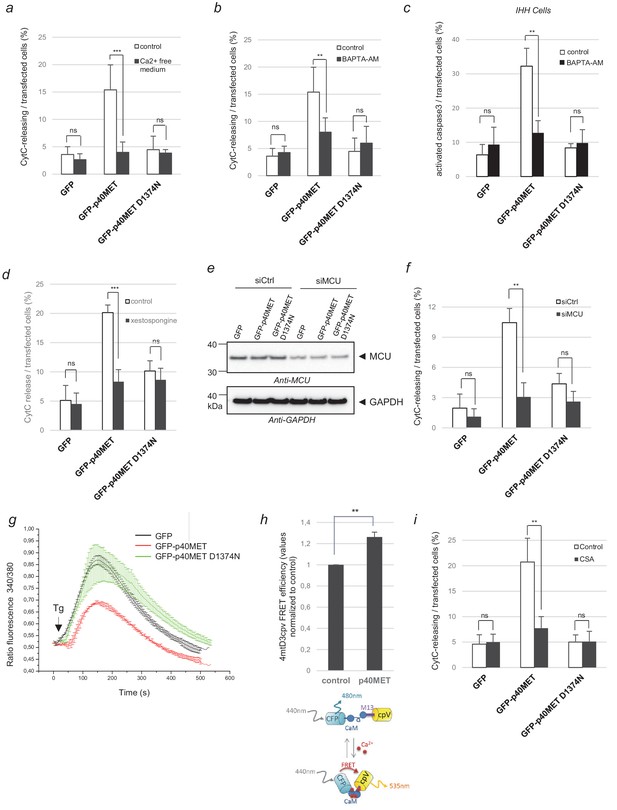
Evaluation of p40MET-induced apoptosis after inhibition of calcium exchanges between the ER and mitochondria.
(a, b) MCF10A epithelial cells were starved overnight in a calcium-free medium or treated with the calcium chelator BAPTA-AM (10 µM). The next day cells were transfected with a vector expressing a GFP-tagged fragment. (c) IHH cells were transfected with a vector expressing a GFP-tagged fragment and treated the next day with the calcium chelator BAPTA-AM (10 µM) for 24 hr. (d) MCF10A cells were transiently transfected with a vector expressing GFP, GFP-p40MET, or GFP-p40MET D1374N and treated or not with the IP3R inhibitor xestospongin-B (5 µM). (a–d) After 24 hr transfection for MCF10A and 48 hr for IHH, cells were fixed and processed for immunostaining with an anti-cytochrome C or anti-cleaved caspase 3 antibody to evaluate apoptosis. The percentage of cytochrome C release or of cleaved caspase-3-positive cells was determined with respect to the number of GFP positive cells. At least 100 cells were counted per well (n = 6;± S.D.) for MCF10A and at least 60 cells (n = 4;± S.D.) for IHH. (e–f) One day before transfection with a vector expressing a GFP-tagged fragment, MCF10A cells were transfected with a control siRNA or with a mixture of three siRNAs targeting the mitochondrial calcium channel MCU. Twenty-four hours later, (e) one part of the cells was lysed and extracts were analyzed by western blotting with an anti-MCU and an anti-GAPDH antibody. (f) For immunofluorescence, staining was performed with an anti-cytochrome-c antibody and nuclei were labeled with Hoechst. The percentage of transfected cells displaying cytochrome-c release was determined. At least 60 cells were counted per well (n = 3;± S.D.). (g) HEK293 cells were transfected with a vector expressing a GFP-tagged fragment. The next day, the cells were incubated in Ca2+-free HBS solution and treated with 1 µM Thapsigargin (Tg). The calcium concentration was determined by estimating the uncorrected 340 nm/380 nm fluorescence ratio of fura-2AM. At least 20 cells were measured per condition (n = 3;± S.D.). The presented results are representative of three independent experiments. Black arrows indicate Tg injection. (h) HEK293 cells were co-transfected with the 4mtD3cpv biosensor and a plasmid encoding either GFP or GFP-p40MET. The CFP fluorescence lifetime was recorded and the FRET efficiency, indicative of the calcium level in the mitochondria, was calculated with respect to the level observed for the control, set as reference. At least 30 cells were counted for each condition (n = 3;± S.D.). Below, schematic representation of the 4mtD3cpv biosensor constituted by two fluorescent probes linked by a calmodulin binding site, allowing FRET measurement. (i) MCF-10A epithelial cells were transiently transfected with a vector expressing GFP, GFP-p40MET, or GFP-p40MET D1374N and were treated or not with 2.5 μM cyclosporinA (CSA). Twenty-four hours after transfection, the nuclei were stained with Hoechst and immunofluorescence staining was performed with an anti-Flag antibody and an anti-cytochrome C antibody. The percentage of MET-transfected cells displaying cytochrome C release was determined. At least 200 cells were counted per well (n = 3;± S.D.). ns, non significant; *, p<0.05; **, p<0.01 as determined by Student’s t test.
-
Figure 3—source data 1
Source data of Figure 3a–b–c–d–f–i reporting counting of GFP, active caspase 3 and cytochrome C release positive cells, computation of the percentage, mean and SD, diagram conception and statistical analyses; source data of Figure 3h including FRET source data and diagram conception.
- https://cdn.elifesciences.org/articles/50041/elife-50041-fig3-data1-v1.xlsx
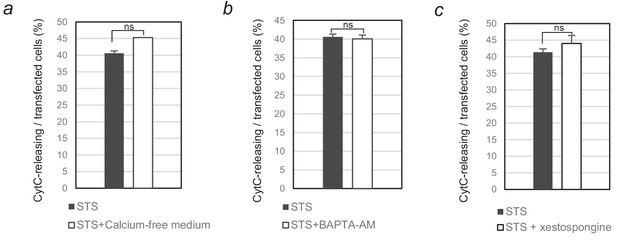
Involvement of calcium flux in staurosporine-induced apoptosis.
(a–c) MCF10A epithelial cells were starved overnight in classical or calcium-free medium (a) or in the presence or absence of 10 µM calcium chelator BAPTA-AM (b). The next day, they were treated for 6 hr with 1 µM staurosporine to induce apoptosis in presence or absence of IP3R inhibitor xestospongin-B (c). Immunofluorescence staining was performed with an anti-cytochrome c antibody to detect apoptotic cells. Nuclei were detected with Hoechst. At least 100 cells were counted per well (n = 3;± S.D). ns, non-significant; determined by Student’s t test.
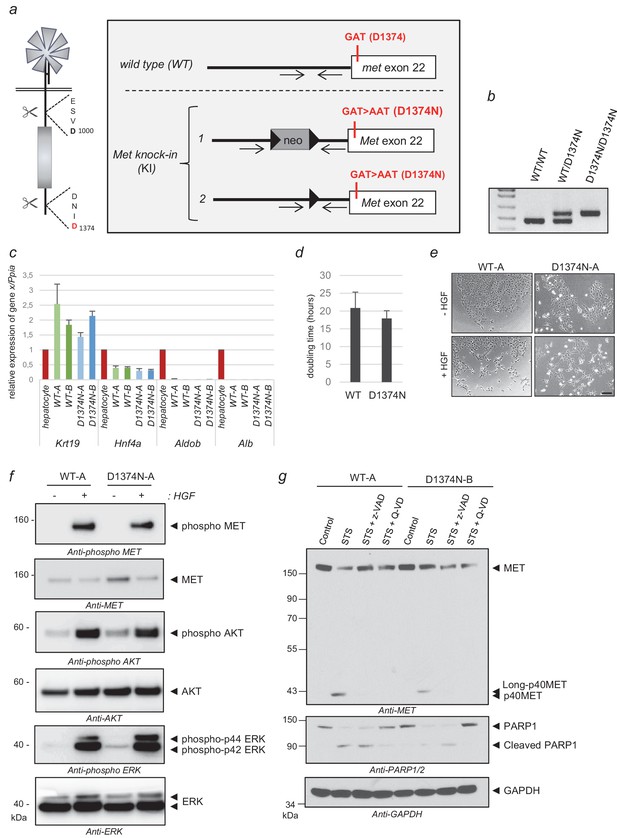
Generation of MET knock-in mice mutated at the C-terminal caspase cleavage site and isolation of hepatic progenitors.
(a) Strategy for generating knock-in mice. The targeted Met allele is depicted. White boxes represent Met exon 22 with, in red, the generated knock-in (KI) mutation GAT >AAT (D1374N). Bold black arrowheads indicate LoxP sites. The positions of the genotyping primers are marked with thin black arrows. (b) To confirm the presence of the KI mutation in Met exon 22, PCR genotyping was performed with primers flanking the loxP sites and amplifying a 476 bp of the wild-type allele (WT) and a 563 bp fragment of the Met KI allele (D1374N). (c) Levels of Krt19, Hnf4a, Aldob, and Alb transcripts were measured by RT-qPCR in Bipotential Mouse Embryonic Liver cell (BMEL) clones derived from WT and MET D1374N mice, and murine hepatocytes as a control. Analyses of two WT BMEL clones (Clones A and B) and two D1374N clones (clones A and B) are shown. The results presented are averages of three independent experiments, with errors bars showing standard deviations. (d) BMEL cells were cultured under routine conditions and were counted after 24, 48, or 72 hr and the doubling times of the WT and MET D1374N BMEL cells were established by averaging the values obtained for the two corresponding clones. (e) Cells were seeded at low confluence. The next day the cells were starved for 30 min in the presence or absence of 10 ng/ml HGF/SF. Representative pictures were taken after 24 hr; scale bar = 100 µm (f) BMEL cells were starved overnight in RPMI-0% FCS and stimulated or not for 10 min with 20 ng/ml HGF/SF. For each condition, the same amount of whole cell lysate was analyzed by western blotting with antibodies against mouse MET, ERK, AKT, and their phosphorylated forms. (g) BMEL cells were treated for 7 hr with 1 µM staurosporine (STS) with or without the pan-caspase inhibitor zVAD-FMK or Q-VD (20 µM). The same amount of protein was resolved by SDS-PAGE and analyzed by immunoblotting with antibodies against the MET kinase domain and cleaved PARP, to assess apoptosis induction, and GAPDH, to assess loading.
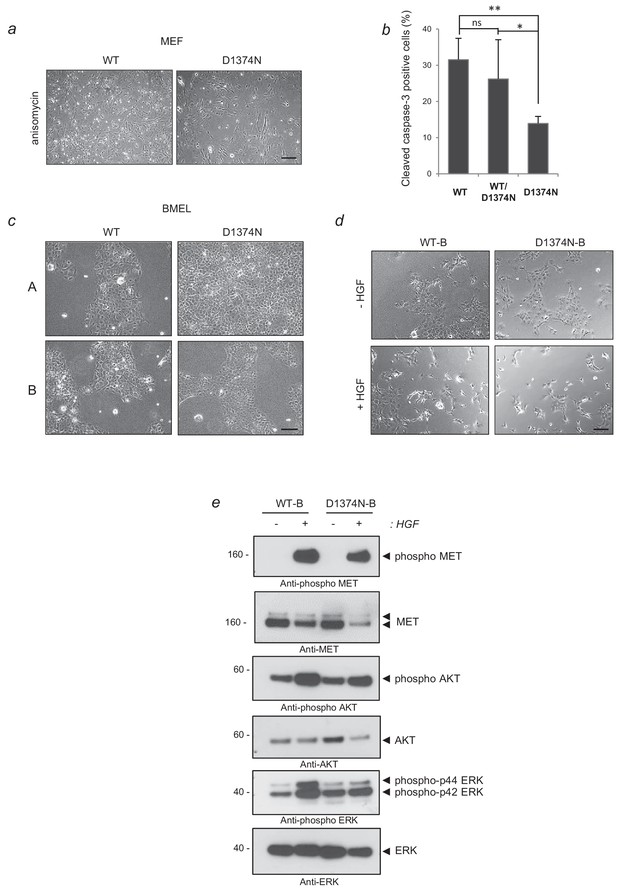
Culture phenotype of MEF and BMEL cells and responses of WT-B and D1374N-B clones to survival or apoptosis induction.
(a) MEF cells were seeded in complete medium in gelatin-coated plates. The next day cells were exposed to anisomycin 50 µM for 5 hr. Pictures were then taken and evidenced the presence of a greater proportion of rounded apoptotic cells in WT vs D1374N MEF cells. (b) Immunofluorescence against active caspase-3 was carried out under the same apoptotic induction conditions and quantified. At least 100 cells were counted per well (n = 4;± S.D.). ns, non significant; *, p<0.05; **, p<0.01 as determined by Student’s t test. (c) Representative pictures of BMEL cells belonging to two WT clones (A and B) and two D1374N clones (A and B). (d) Cells were seeded at low confluence. The next day the cells were starved and treated or not with 10 ng/ml HGF/SF. Representative pictures of WT-clone B and D1374N-clone B cells were taken after 24 hr. (e) BMEL cells (WT-B and D1374N-B) were starved over-night in RPMI-0% FCS and stimulated or not for 10 min with 20 ng/ml HGF/SF. For each condition, the same amount of whole cell lysate was resolved by 12% SDS-PAGE and analyzed by western blotting with antibodies against mouse MET, ERK, AKT and their phosphorylated forms.
-
Figure 4—figure supplement 1—source data 1
Source data of Figure 4—figure supplement 1b reporting counting of active caspase 3 positive cells according to the number of cells per field, computation of the mean, percentage, SD, diagram conception and statistical analyses.
- https://cdn.elifesciences.org/articles/50041/elife-50041-fig4-figsupp1-data1-v1.xlsx
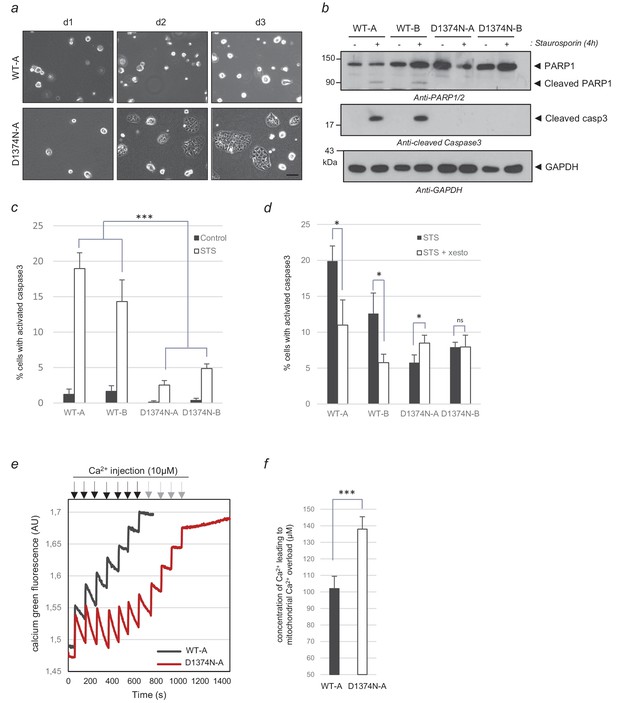
Comparison of WT and MET D1374N BMEL cell death.
(a) Representative pictures of BMEL cells (clones WT-A and D1374N-A) cultured for 1, 2, and 3 days (d1, d2 and d3) without a collagen coating; scale bar = 100 µm. (b–c) BMEL cells were cultured for 24 hr on a (b) Petri dish or (c) an ibidi slide, both coated with poly-L-lysine, and treated for 4 hr with 1 µM staurosporine. (b) For each condition, the same amount of whole-cell lysate was analyzed by western blotting with antibodies against cleaved caspase 3, PARP1, and GAPDH. (c) Cells were fixed and immunofluorescence staining was performed with anti-cleaved caspase 3 antibody. Nuclei were stained with Hoechst. At least 200 cells per well were counted and the percentages of cleaved-caspase 3 positive cells, averaged over three independent experiments, are represented (n = 6;± S.D.). (d) BMEL cells were cultured for 24 hr on an ibidi slide coated with poly-L-lysine and treated for 4 hr with 5 µM xestospongin-B and 1 µM staurosporine. Immunofluorescence staining was performed with an anti-cleaved caspase-3 antibody. The nuclei were stained with Hoechst. At least 80 cells per well were counted and percentages of cells displaying cleaved caspase 3 are shown (n = 3;± S.D.) (e) BMEL cells (WT-A and D1374N-A) were treated for 4 hr with 1 µM staurosporine to induce apoptosis. The mitochondrial Ca2+ uptake capacity of digitonin-permeabilized BMEL cells (250000/ml) was measured with a cytosolic calcium green Ca2+ probe upon addition of sequential Ca2+ pulses (black and gray arrows) to the medium in an O2K-oxygraph apparatus (Oroboros). (f) The measurements of three independent experiments were averaged. ns, nonsignificant; *, p<0.05; **, p<0.01; ***, p<0.001 as determined by Student’s t test. Black arrows = Ca2+ injection for WT and D1374N cells; gray arrows = Ca2+ injection for D1374N cells only.
-
Figure 5—source data 1
Source data of Figure 5c–d reporting counting of GFP and active caspase 3 positive cells, computation of the percentage, mean and SD, diagram conception and statistical analyses; source data of Figure 5f reporting concentration of Ca++ uptake, computation of the mean and statistical analysis.
- https://cdn.elifesciences.org/articles/50041/elife-50041-fig5-data1-v1.xlsx
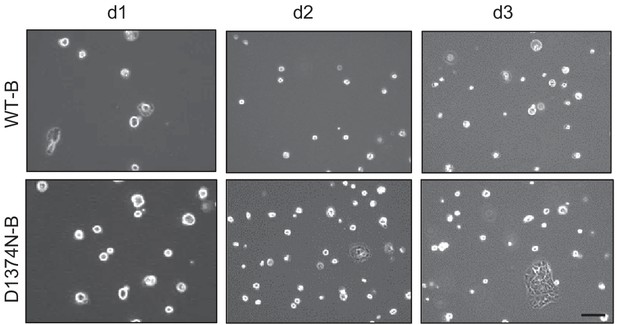
Culture of BMEL WT-B and D1374N-B clones without collagen coating.
Representative pictures of BMEL cells (WT-B and D1374N-B) cultured cultured for 1, 2, and 3 days (d1, d2 and d3) in RPMI-10% FCS without collagen coating; scale bar = 100 µm.
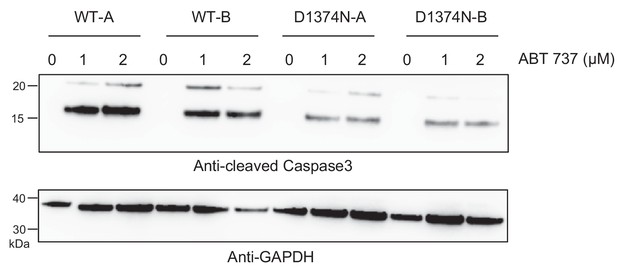
Induction of BMEL apoptosis by ABT 737.
BMEL cells were cultured for 24 hr on a 6-well plate coated with poly-L-lysine, and treated with BH3 mimetic ABT 737 at 1 μM for 4 hr or 2 μM for 2 hr. For each condition, the same amount of whole-cell lysate was analyzed by western blotting with antibodies against cleaved caspase 3 and GAPDH.
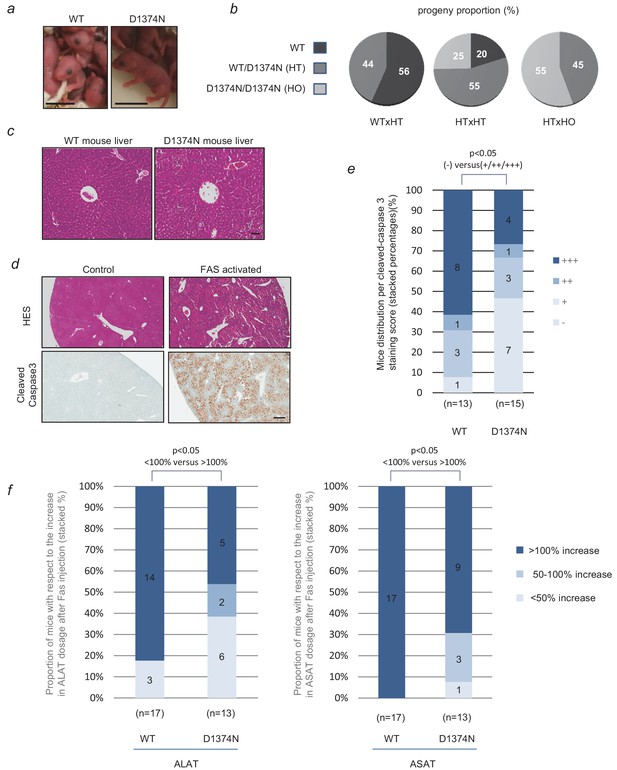
Evaluation of hepatocytes apoptosis in WT and D1374N mice.
(a) Pictures of newborn wild-type (WT) and MET D1374N mice; scale bar = 1 cm. (b) Pie chart representation of the proportions of WT, heterozygous (HT) and homozygous (HO) progeny obtained by crossing mice with different genotypes. (c) WT and MET D1374N mouse liver sections stained with hematoxylin/eosin, showing a hepatic lobule with a centro-lobular vein at the center; scale bar = 50 µm. (d) Example of hematoxylin/eosin and cleaved-caspase 3 staining (brown) in the livers of WT mice pretreated before the experiment with 100 µl of 5 mg/ml Crizotinib and then treated for 4 hr with Jo-2 antibody against FAS (4 µg/20 g mouse weight); scale bar = 300 µm. (e) WT and D1374N mice were treated as reported in (d). The total liver area and cleaved caspase-3 staining area were quantified for each slide. The data represent the distribution of cleaved-caspase 3 staining scores (-: 0% to 2%; +: 2% to 5%; ++: 5% to 10%; +++:>10%). In each column, the number of mice obtaining each score is indicated. Statistical analysis applied to differences in negative (-) and positive (+, ++ and +++) staining between WT and D1374N. p<0.05 was determined by Fisher test (WT: n = 13; D1374N: n = 15). (f) WT and D1374N mice were treated as reported in (d). Blood samples were collected just before and 4 hr after Jo-2 antibody treatment. The graphs represent the distribution of the evolution of plasma ALAT (left) or ASAT (right) levels between these 2 time points (up to 50% increase, 50–100% increase or more than 100% increase). Statistical analysis applied to differences in <100% increase and >100% increase between WT and D1374N. p<0.05 was determined by Fisher test (WT: n = 17; D1374N: n = 13).
-
Figure 6—source data 1
Source data of Figure 6e reporting percentage of active caspase 3 in liver IHC, repartition in staining score (-;+;++;+++), computation of the percentages, diagram conception and statistical analyses; source data of Figure 6f reporting ALAT and ASAT concentration in mouse blood, relative increase, diagram conception and statistical analyses.
- https://cdn.elifesciences.org/articles/50041/elife-50041-fig6-data1-v1.xlsx
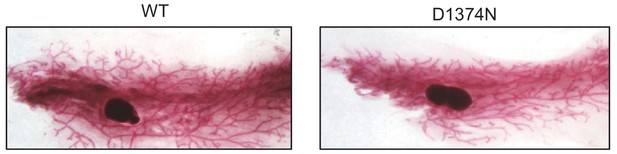
Mammary gland organization in WT and MET D1374N mice.
Whole-mount morphology of WT and MET D1374N mammary glands. Pairs #5 of 3-month-old mice were harvested, laid over microscopy slides, and incubated overnight with Carnoy’s fixative. Glands were subsequently stained with Carmine Alum stain, cleared with xylene, and mounted with Permount mounting medium. WT and MET D1374N mammary glands display similar organization, with comparable levels of branching morphogenesis in the whole gland volume.
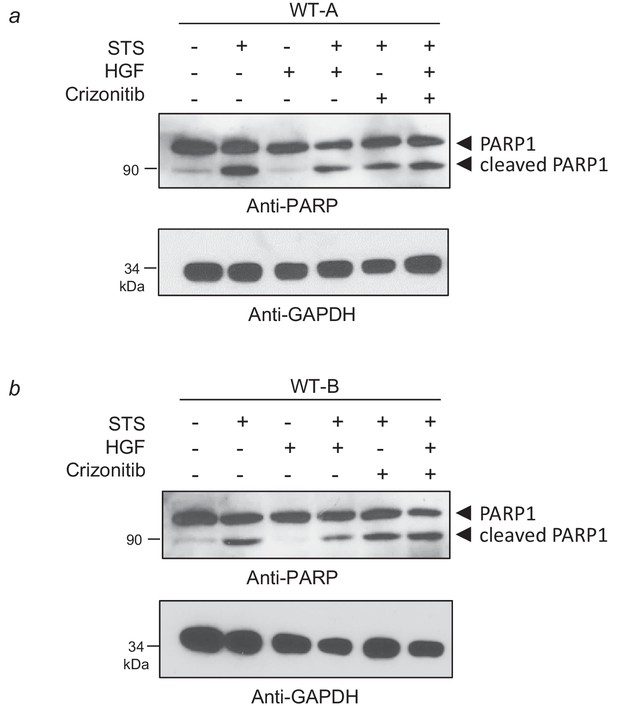
Evidence of Crizotinib efficacy against HGF-induced survival in BMEL cells.
(a–b) WT BMEL cells were cultured on a 6-well plate coated with poly-L-lysine. The next day, they were treated or not for 4 hr with 1 µM staurosporine, 20 ng/ml HGF/SF, or 0.1 µM Crizotinib. For each condition, the same amount of whole cell lysate was resolved by SDS-PAGE and analyzed by western blotting with antibodies against PARP1 and GAPDH.
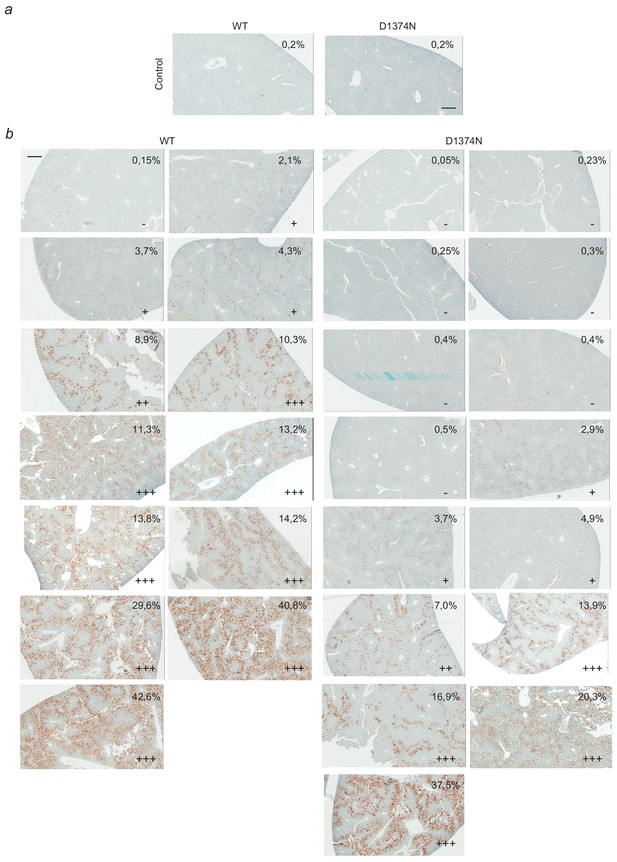
Complete-panel illustration of cleaved-caspase 3 staining of liver slices.
(a–b) Photographs of all mouse livers (WT and D1374N) stained with antibody against cleaved caspase 3. Mice were pretreated with Crizotinib 5 days, 2 days, and 1 day before the experiment and fulminant hepatitis was induced (b) or not (a) by Jo-2 injection (4 hr). Scores determined according to the proportion of stained area over the liver slice area (from negative - to highly positive +++) are indicated on each picture; scale bar = 300 µm.
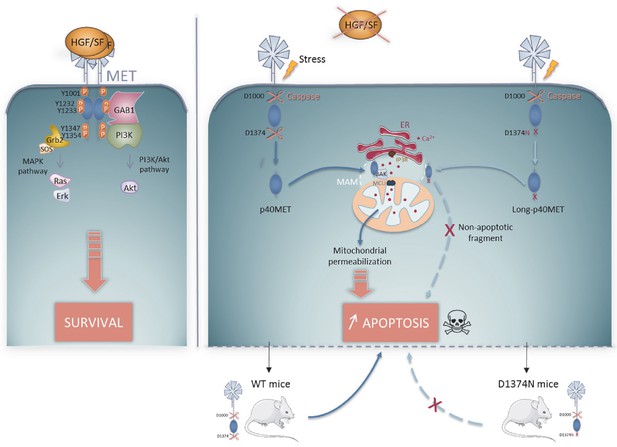
Schematic representation of the MET pro-survival and pro-apoptotic pathways in the presence of HGF/SF, MET receptor dimerizes and activates pro-survival signaling by activating the MAPK and PI3K/AKT pathways.
In the absence of ligand, MET is cleaved by caspase 3 at a juxtamembrane and a C-terminal site to generate the p40MET fragment, which translocates to the MAM microdomain. p40MET may interact with BAK and promote deregulation of the Ca2+ flux between the ER and the mitochondria, causing mitochondrial permeabilization involved in amplification of apoptosis. This dual role of MET classifies it as a dependence receptor. D1374N MET, mutated at the C-terminal caspase site, generates a slightly longer fragment that can no longer promote apoptosis. Upon stress induction by FAS activation in WT and D1374N mice, D1374N hepatocytes show less apoptosis. This suggests that C-terminal caspase cleavage of MET is important for optimal apoptosis in vivo.
Additional files
-
Supplementary file 1
Key Resources Table.
- https://cdn.elifesciences.org/articles/50041/elife-50041-supp1-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/50041/elife-50041-transrepform-v1.docx





