Distinct roles for innexin gap junctions and hemichannels in mechanosensation
Figures
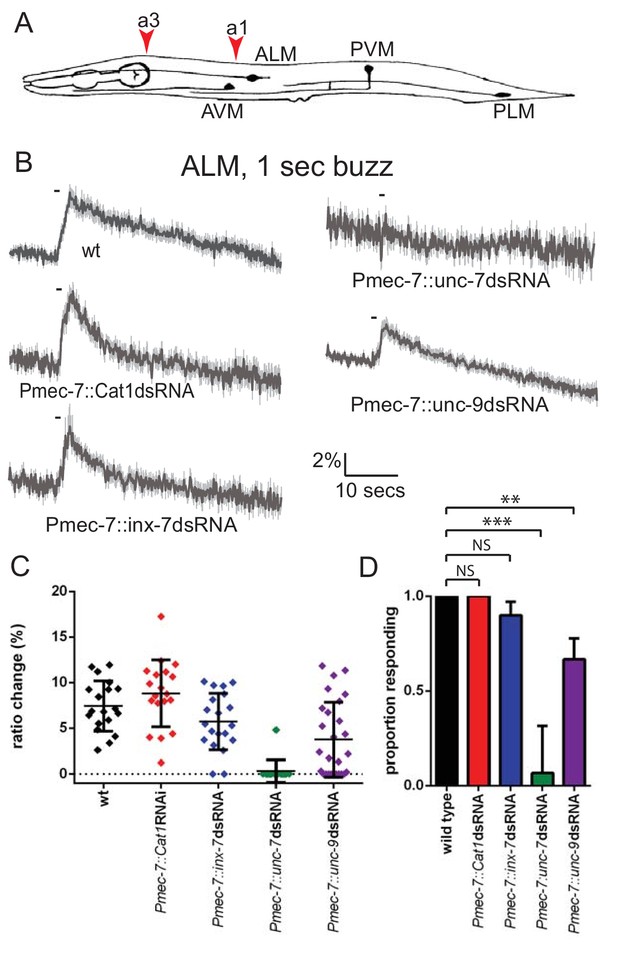
unc-7 and unc-9 function in touch responses in ALM.
(A) Schematic showing positions of cell bodies and processes of the C. elegans touch receptor neurons. ALM and PLM are lateral pairs (left and right), of which only one of each is shown. Red arrowheads show stimulation sites. Except where stated, animals were stimulated at a3. As in later figures, we present average traces of % ratio change, a scatter plot showing individual ratio changes and a graph showing proportion exhibiting a Ca2+ response. (B,C,D) Gentle touch responses recorded in ALM for wild type animals and animals expressing dsRNA under control of Pmec-7. (B) Average traces of % ratio change. Gray indicates SEM. (C) Scatter plot showing individual ratio changes (diamonds). Bars indicate mean ± SEM. (D) Graph showing proportion exhibiting a Ca2+ response. Error bars indicate SE. unc-7 (<0.0001) and unc-9 (p=0.0062) RNAi are significantly different from wild type, while E. coli Cat1 (p=0.10.0) and inx-7 (p=0.4872) RNAi are not, Fisher’s exact test (N = 19, 19, 20, 15, 27, in the order shown in the graphs).
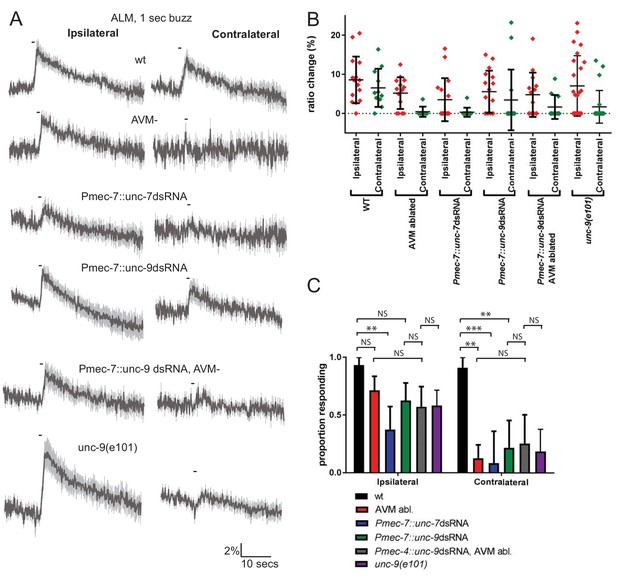
Innexins are required for mechanosensation and electrical coupling of touch neurons.
(A, B, C) Gentle touch responses recorded in ALM for wild type worms, worms in which AVM has been laser ablated and worms expressing dsRNA under control of Pmec-7. Neurons have been classified as ‘ipsilateral’ or ‘contralateral’, according to the position of the cell body relative to the stimulation site and the hypothetical midline of the animal. (A) Average traces of % ratio change. Gray indicates SEM. (B) Scatter plot showing individual ratio changes (diamonds). Bars indicate mean ± SEM. (C) Graph showing proportion exhibiting a Ca2+ response. Error bars indicate SE. In ipsilateral neurons, the proportion of AVM ablated animals (p=0.1686) or unc-9 RNAi animals (p=0.1686) responding is not significantly different to wild type, while unc-7 RNAi animals respond at a significantly reduced rate (p=0.0021). Combining unc-9 RNAi with AVM ablation is not significantly different from either unc-9 RNAi alone (p=0.7104), AVM ablation alone (p=0.6946) or unc-9(e101) (p=1). In contralateral neurons, the proportions of AVM ablated, unc-7 RNAi and unc-9 RNAi animals responding are all significantly lower than wild type (p=0.0012; p=0.0001; p=0.001). Combining unc-9 RNAi with AVM ablation is not significantly different from either unc-9 RNAi alone (p=1.0), AVM ablation alone (p=0.6027) or unc-9(e101) (p=0.661), Fisher’s exact test (N = 15, 14, 16, 16, 14, 24, 11, 8, 12, 14, 11, 22, in the order shown in C).
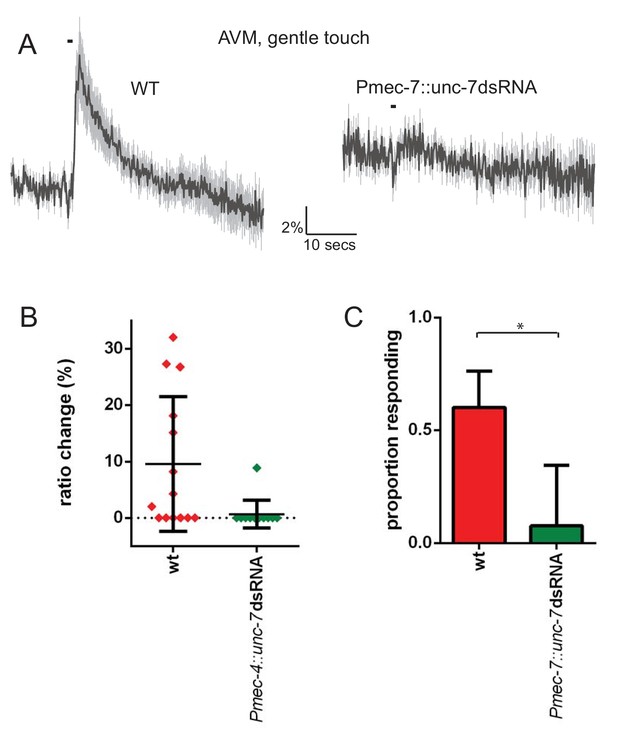
unc-7 is required for gentle touch response in AVM.
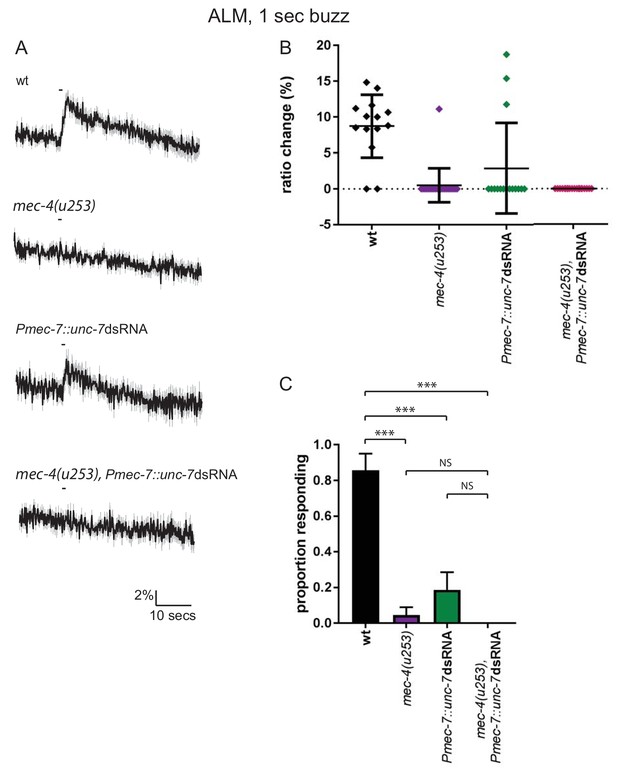
(A, B, C) Ca2+ response to 1 s buzz in AVM, for wild type and TRN-specific unc-7 RNAi animals. All AVM neurons assayed were located in the ‘near’ half of the animal, with respect to the stimulation site, as determined by the position of the cell body. (A) Average traces of % ratio change. Gray indicates SEM. (B) Scatter plot showing individual ratio changes (diamonds). Bars indicate mean ± SEM. (C) Graph showing proportion exhibiting a Ca2+ response. Error bars indicate SE. The proportion of AVM ‘near’ neurons responding is significantly lower in unc-7 RNAi animals compared to wild type (p=0.0128, Fisher’s exact test, N = 14, 13).
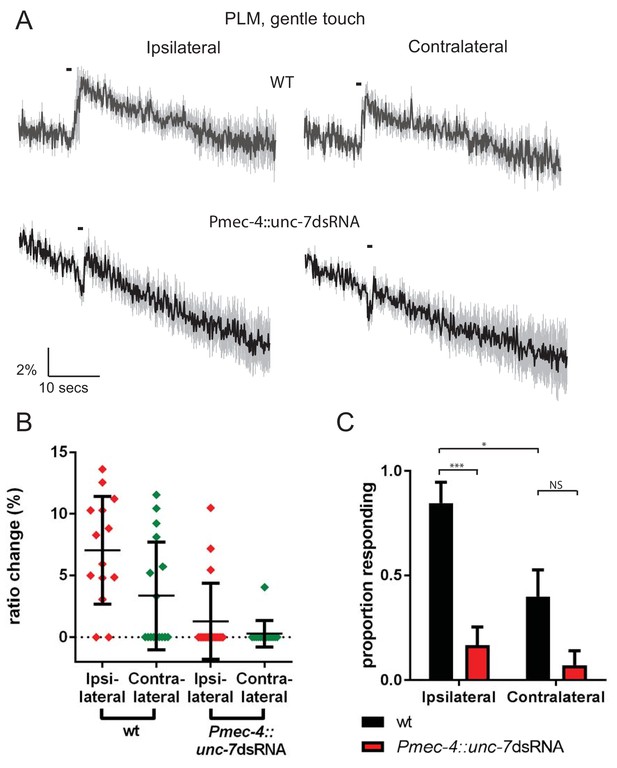
PLML and PLMR do not cooperate via gap junctions.
(A, B, C) Ca2+ response to 1 s buzz in PLM, for wild type and TRN-specific unc-7 RNAi animals. (A) Average traces of % ratio change. Gray indicates SEM. (B) Scatter plot showing individual ratio changes (diamonds). Bars indicate mean ± SEM. (C) Graph showing proportion exhibiting a Ca2+ response. Error bars indicate SEM. The proportion responding is significantly lower in ‘contralateral’ neurons compared to those ipsilateral to the stimulation site (p<0.05, Fisher’s exact test, N = 14, 18, 16, 14, in the order shown in C).
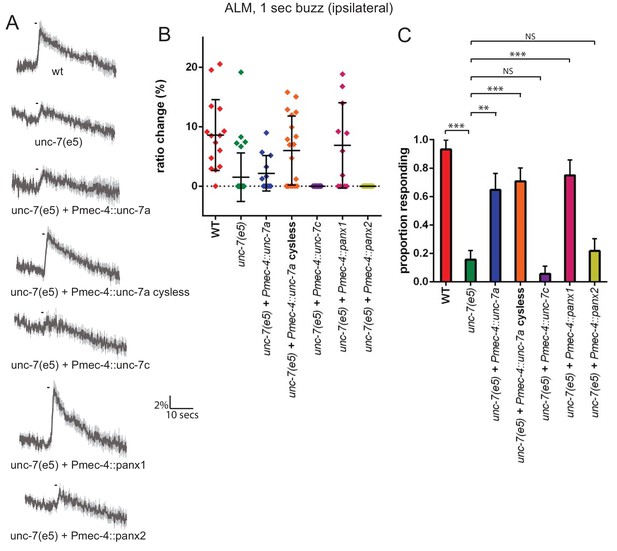
The mechanosensory function of UNC-7 is gap junction-independent.
(A, B, C) Gentle touch responses recorded in ALM for wild type, unc-7(e5), and unc-7(e5) animals expressing unc-7 isoforms or pannexins under the control of Pmec-4. ‘Cysless’ indicates C173A, C191A, C377A, C394A. All neurons recorded were ipsilateral, according to the position of the cell body relative to the stimulation site and the hypothetical midline of the animal. (A) Average traces of % ratio change. Gray indicates SEM. (B) Scatter plot showing individual ratio changes (diamonds). Bars indicate mean ± SEM. (C) Graph showing proportion exhibiting a Ca2+ response. Error bars indicate SE. The response frequency is significantly reduced in unc-7(e5) compared to wildtype (p<0.0001). This is significantly rescued by TRN expression of wild type (p=0.001) or cysless unc-7a (p<0.0001). Cysless unc-7 still significantly rescued the mutant when AVM was ablated (p<0.0001), and there was no significant difference between AVM ablated and unablated cysless unc-7-expressing animals (p=0.4701). While unc-7c (p=0.3991) and mouse panx2 (p=0.7257) did not significantly rescue, panx1 did (p<0.0001), Fisher’s exact test (N = 15, 32, 12, 19, 13, 11, 18, in the order shown in the graphs).
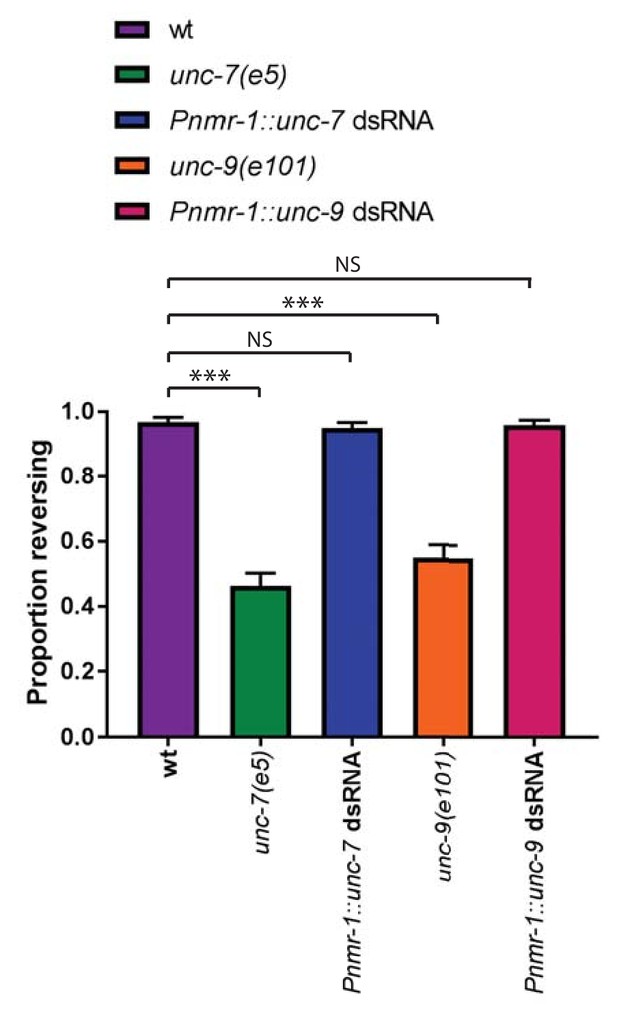
Expression of unc-7 or unc-9 dsRNA in other neurons does not disrupt the gentle touch response.
Behavioural response to anterior gentle touch, for the genotypes indicated. Error bars are SEM. Whereas both unc-7 and unc-9 null animals exhibit a significant defect (p<0.0001 in each case), Pnmr-1::unc-7 dsRNA (p=0.573) and Pnmr-unc-9 dsRNA (p=0.7701) animals do not, Fisher’s exact test (N = 160 for each genotype).
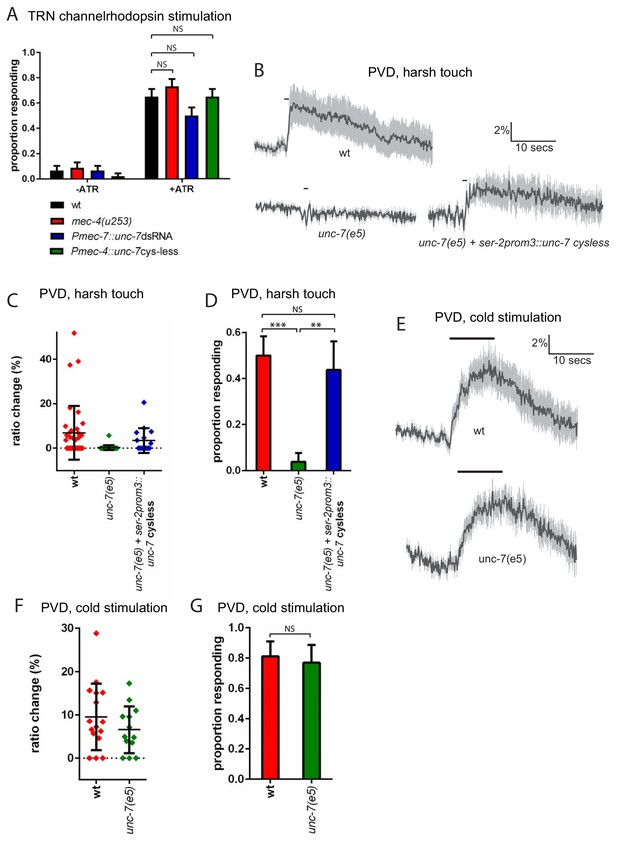
unc-7 is specifically required for mechanosensation.
(A) Behavioural response to light stimulation of animals expressing channelrhodopsin in the TRNs. The proportion of wild type animals responding was not significantly different to that for Pmec-7::unc-7dsRNA (p=0.1393), Pmec-4::unc-7 cysless (p=1) or mec-4(u253) (p=0.4294) animals (N = 45, 45, 45, 45, 60, 60, 60, 60). All these experiments were carried out in a lite-1 mutant background to eliminate effects of endogenous blue light responses (see strain list, Supplementary file 1). (B,C,D) Harsh touch responses recorded in PVD for wild type and unc-7(e5) animals. (B) Average traces of % ratio change. Gray indicates SEM. (C) Scatter plot showing individual ratio changes (diamonds). Bars indicate mean ± SEM. (D) Graph showing proportion exhibiting a Ca2+ response. Error bars indicate SE. The proportion responding is significantly reduced in unc-7(e5) animals (p<0.0001); and this is significantly rescued (p=0.0026) to a response rate not significantly different (p=0.7683) from wild type (N = 36, 26, 16). (E,F,G) Cold responses recorded in PVD for wild type and unc-7(e5) animals. (E) Average traces of % ratio change. Black bar indicates shift from 22°C to 15°C. Gray indicates SEM. (F) Scatter plot showing individual ratio changes (diamonds). Bars indicate mean ± SEM. (G) Graph showing proportion exhibiting a Ca2+ response. Error bars indicate SE. The proportion responding is not significantly different (p=1), Fisher’s exact test, N = 16, 13).
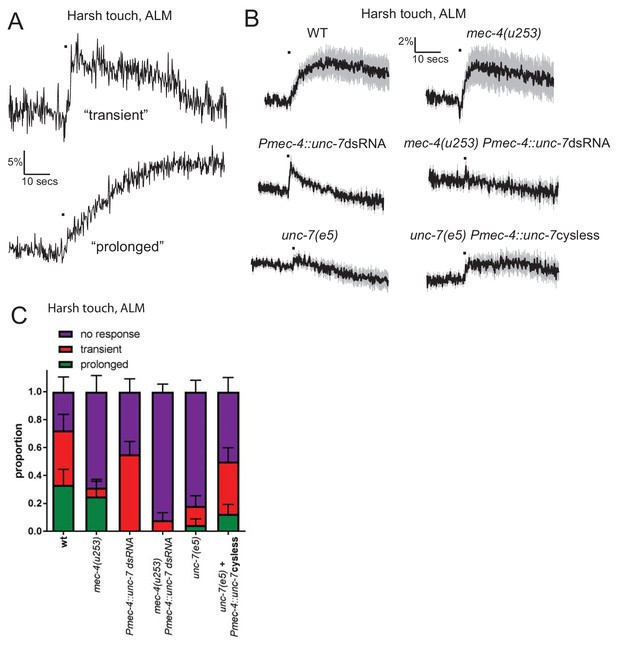
UNC-7 and MEC-4 have distinct roles in harsh touch.
(A, B, C) Ca2+ responses recorded in ALM in response to harsh touch. (A) Representative examples of the two types of Ca2+ responses to harsh touch stimulation in ALM. (B) Average traces of % ratio change. Gray indicates SEM. (C) Proportion of animals displaying the indicated types of Ca2+ response to harsh touch in ALM. Error bars are SE. The transient responses (characterized by a rapid onset and a decay to baseline beginning immediately after the stimulus ends) resemble typical gentle touch responses in the TRNs; prolonged responses (characterized by a slow onset that continues for several seconds following the end of the stimulus) is only seen in response to harsh touch. The transient responses are significantly disrupted in the absence of mec-4 (p=0.0425), while the prolonged responses are significantly disrupted by unc-7 knockdown (p=0.0328). unc-7 RNAi, mec-4 null combined completely abolishes both types of response (p=0.4898 when compared to zero responses; p=0.0001 when total response rate is compared to unc-7 RNAi; p=0.0891 when compared to mec-4 null). unc-7(e5) significantly disrupts the total response rate (p=0.0011) and this is significantly rescued by TRN expression of unc-7 cysless (p=0.0324), Fisher’s exact test (N = 18, 16, 29, 25, 22, 24 in the order shown on graphs).
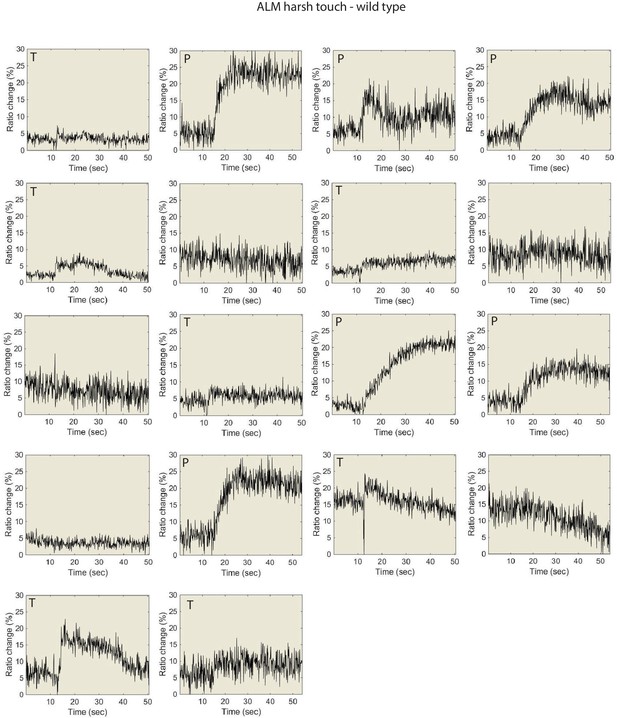
Individual traces for the data shown in Figure 5, for wild type animals.
ALM Ca2+ responses to harsh touch. P denotes ‘prolonged’ type, T denotes “transient type responses.
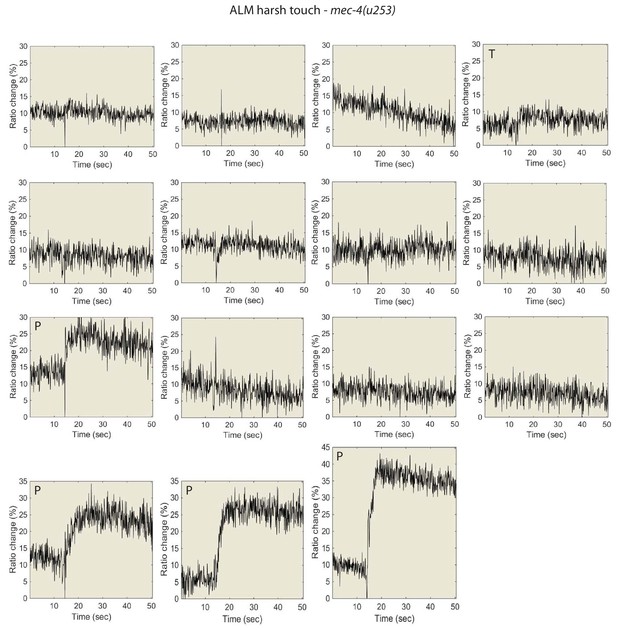
Individual traces for the data shown in Figure 5, for mec-4(u253) animals.
ALM Ca2+ responses to harsh touch. P denotes ‘prolonged’ type, T denotes “transient types responses.
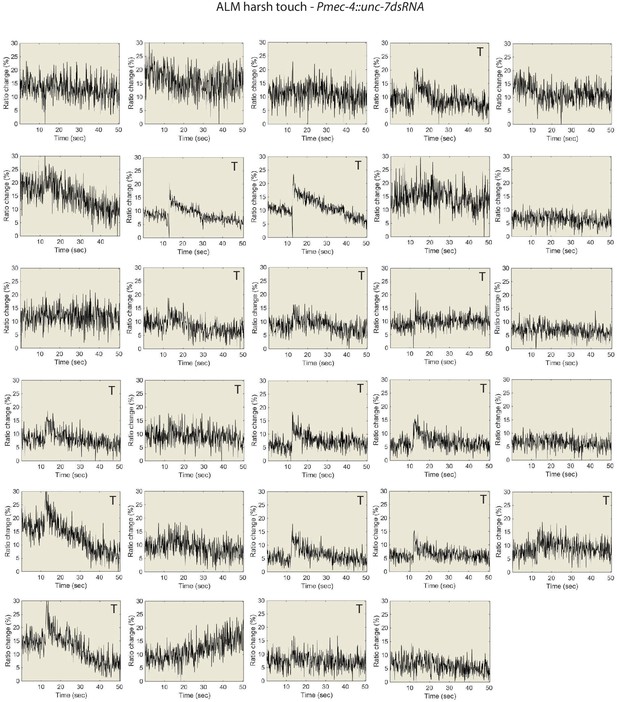
Individual traces for the data shown in Figure 5, Pmec-7::unc-7 dsRNA animals.
ALM Ca2+ responses to harsh touch. P denotes ‘prolonged’ type, T denotes “transient type responses.
mec-4 mutation and unc-7 RNAi both severely disrupt the ALM response to gentle touch.
(A, B, C) Ca2+ responses to gentle touch recorded in ALM. (A) Average traces of % ratio change. Gray indicates SEM. (B) Scatter plot showing individual ratio changes (diamonds). Bars indicate mean ± SEM. (C) Proportion of animals displaying the indicated types of Ca2+ response to gentle touch in ALM. Error bars are SE. mec-4(253) or unc-7 RNAi, or combined mutation of mec-4 and unc-7 RNAi severely disrupt the response to gentle touch (p<0.0001, p=0.0007, p<0.0001, respectively). The proportion of responses for mec-4(u253), unc-7 RNAi combined was zero, but this is not significantly different from either mec-4(u253) (p=1) or unc-7 RNAi alone p=0.0784), Fisher’s exact test. N = 14, 22, 16, 20, in the order shown in the graphs.
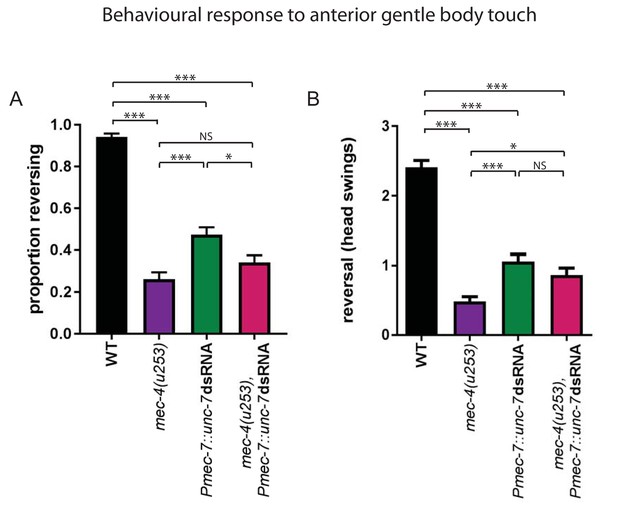
unc-7 RNAi disrupts the behavioural response to gentle touch.
(A, B) Behavioural response to anterior gentle touch, for genotypes indicated. Animals were stimulated at the back of the terminal bulb. Error bars are SEM, N = 190 for each genotype. (A) Proportion of animals exhibiting a reversal response. mec-4(u253) or unc-7 RNAi, or the combination, all significantly disrupt the response (p<0.0001 in each case). Both mec-4(u253) and the combination were significantly more disrupted than unc-7 RNAi alone (p<0.0001, p=0.0121); mec-4(u253) alone was not significantly different from the combination (p=0.1178), Fisher’s exact test. (B) Duration of the reversal response, measured as number of head swings. mec-4(u253) or unc-7 RNAi, or the combination, all significantly reduced the length of the response (p<0.0001 in each case). mec-4(u253) showed significantly shorter responses than unc-7 RNAi (p<0.0001) or the combination (p=0.024); unc-7 RNAi alone was not significantly different from the combination (p=0.18), Student’s t-test.
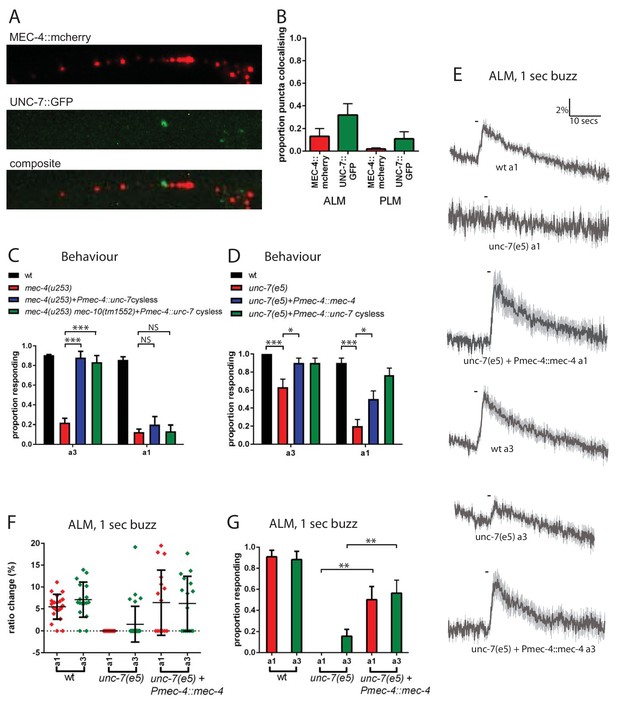
UNC-7 and MEC-4 act independently in touch neuron mechanosensation.
(A, B) Confocal microscopy of TRN neurons expressing mec-4::mcherry and unc-7a::gfp. (A) Example images of PLM, and composite of the two channels, showing colocalisation in white. (B) Percentage of particles colocalising with particles of the other colour, based on centres of mass coincidence (N = 11, 11, 8, 8; total number of puncta = 161, 141, 156, 92). (C, D) Behavioural response to anterior gentle touch, for genotypes indicated. Animals were stimulated either at the back of the terminal bulb (a3) or approximately 50 µm anterior of the cell body of ALM (a1). Error bars are SE. TRN expression of unc-7 cysless significantly rescued the behavioural defect of mec-4(u253), including when mec-10 was also defective, when stimulated at a3 (p<0.0001 for both); but not when stimulated at a1 (p=0.3423; p=1.0) (N = 40 for each genotype). unc-7(e5) animals are significantly defective in the behavioural response to gentle touch at a3 (p=0.0003) and a1 (p<0.0001), and TRN expression of mec-4 significantly rescued this, at a3 (p=0.0303) and a1 (p=0.0292) (N = 30 for each genotype). (E,F,G) Ca2+ responses to gentle touch recorded in ALM, for wild type, unc-7(e5), and unc-7(e5) animals expressing Pmec-4::mec-4. (E) Average traces of % ratio change. Light gray indicates SEM. (F) Scatter plot showing individual ratio changes (diamonds). Bars indicate mean ± SEM. (G) Graph showing proportion exhibiting a Ca2+ response. Error bars indicate SE. Expression of mec-4 significantly rescued the Ca2+ response defect of unc-7(e5), whether stimulated at a3 (p=0.0064) or a1 (p=0.0033), Fisher’s exact test (N = 21, 17, 13, 32, 16, 16 in the order shown on graphs).
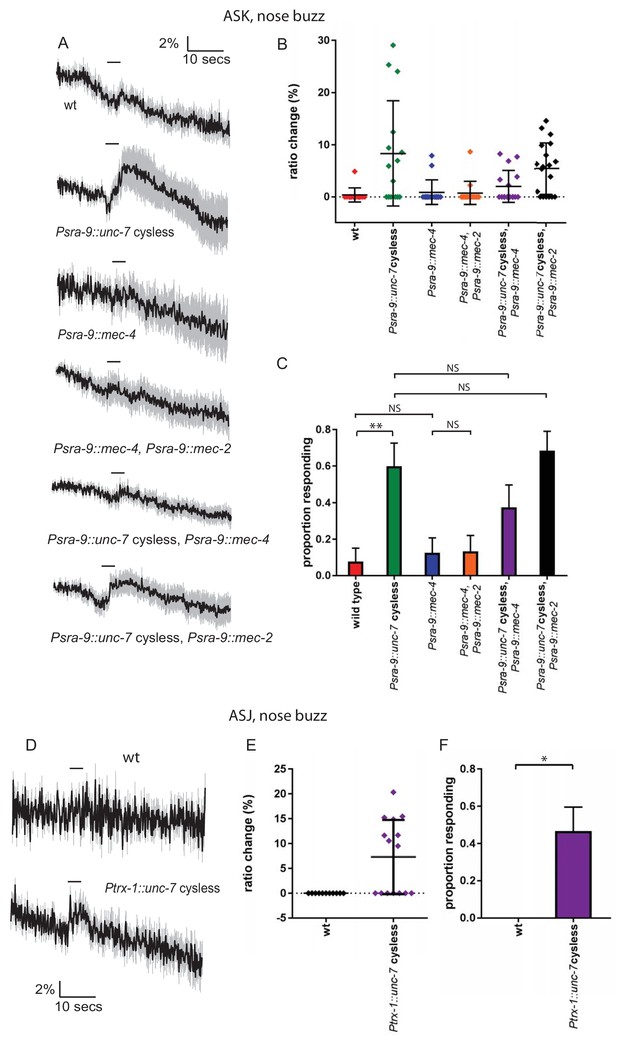
Heterologous expression of UNC-7 hemichannels in olfactory neurons confers touch sensitivity.
(A, B, C) Nose touch responses recorded in ASK of wild type animals and animals expressing unc-7 cysless or mec-4 in ASK. (A) Average traces of % ratio change. Light gray indicates SEM. (B) Scatter plot showing individual ratio changes. Bars indicate mean ± SEM. (C) Graph showing proportion exhibiting a Ca2+ response. Error bars indicate SE. Wild type ASK neurons do not significantly respond to nose touch (p=1.0), but expression of unc-7 cysless significantly increases the response rate (p=0.006). Expression of mec-4 does not significantly increase the response rate (p=1.0), and coexpression of mec-4 does not significantly alter the response rate for unc-7 cysless expressing animals (p=0.2890). Coexpression of mec-2 does not significantly increase the response rate for mec-4 or unc-7, Fisher’s exact test (N = 13, 15, 16, 15, 16, 19 in the order shown on graphs). (D, E, F) Nose touch responses recorded in ASJ of wild type animals and animals expressing unc-7 cysless in ASJ. (D) Average traces of % ratio change. Light gray indicates SEM. (E) Scatter plot showing individual ratio changes. Bars indicate mean ± SEM. (F) Graph showing proportion exhibiting a Ca2+ response. Error bars indicate SE. Wild type ASJ neurons do not significantly respond to nose touch (p=1), but expression of unc-7 cysless significantly increases the response rate (p=0.0103), Fisher’s exact test (N = 11, 15).
Additional files
-
Source code 1
SpikeFinder 4.4.
- https://cdn.elifesciences.org/articles/50597/elife-50597-code1-v3.zip
-
Source code 2
NeuronTracker 3.1.
- https://cdn.elifesciences.org/articles/50597/elife-50597-code2-v3.rar
-
Supplementary file 1
Strains used in this study.
- https://cdn.elifesciences.org/articles/50597/elife-50597-supp1-v3.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/50597/elife-50597-transrepform-v3.docx





