Cre-assisted fine-mapping of neural circuits using orthogonal split inteins
Figures
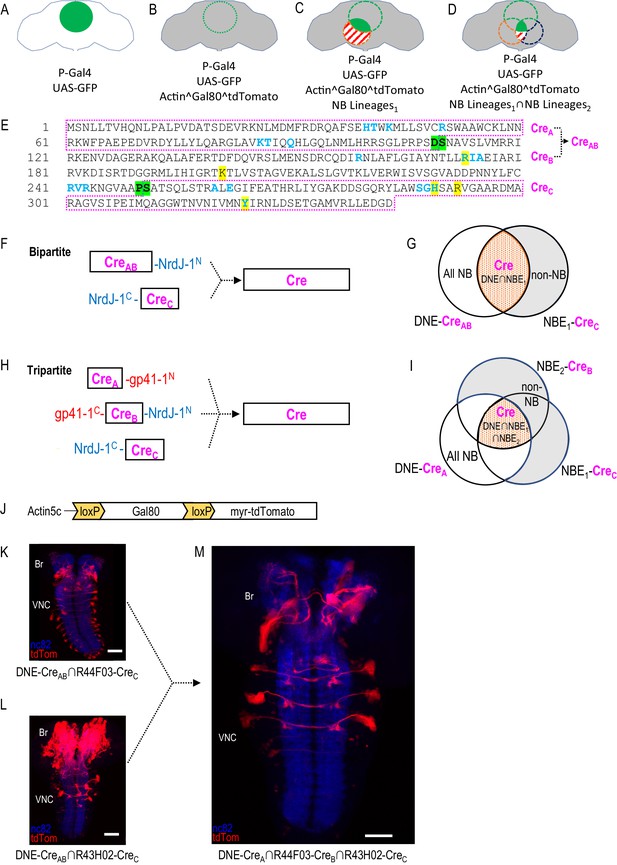
Restriction of NB targeting using split Cre components fused to split inteins.
(A–D) Components and genetic logic of the SpaRCLIn system. (A) A Gal4 driver that drives expression of UAS-transgenes, such as UAS-GFP, in a specific pattern of cells within the CNS (green filled circle). (B) Conditional expression of Gal80, a repressor of Gal4 activity, in all cells using an Actin5C promoter, subject to excision by Cre (gray shading indicates repression of Gal4 by Gal80). (C) Selective activation of Cre in specific NBs (red dotted circle) to excise Gal80 and permit expression of the marker tdTomato (red stripes) and activity of Gal4 (solid green) in neurons derived from those NBs. (D) Use of split Cre components to target NBs at the intersection of two NB expression patterns (red and blued dotted circles) to permit Gal4 activity selectively within cells derived from these NBs (solid green). Note that Gal80 excision results in persistent expression of tdTomato in the affected neurons, but that expression of UAS-reporters and effectors is determined by the temporal properties of the Gal4 line used to drive it. (E) Primary sequence of the Cre protein using the single letter amino acid code. Residues that participate in DNA-binding (blue) or catalysis (yellow highlight) are indicated as are the break-points (green highlight) chosen to generate the split Cre fragments for fusion to split inteins: CreA, CreB, CreAB, and CreC as indicated (magenta boxes). (F–G) The bipartite SpaRCLIn system. (F) Schematics of the Cre fragments fused to NrdJ-1 split inteins, indicating their ability to reconstitute full-length Cre, (G) CreAB expression is directed to all NBs (white plus red shading) using the NB-specific DNE enhancer (see text), and CreC expression is directed to a subset of NBs (red) by the NBE enhancer, which will also express in other cell types (gray). Only the NBs targeted by NBE will express both CreAB and CreC and reconstitute full-length Cre. (H–I) The tripartite SpaRCLIn system. (H) Similar to (F) except that the CreAB fragment has been further divided into CreA and CreB components which have been fused to gp41-1 split inteins at breakpoints. All three fragments are now required to reconstitute full-length Cre. (I) Venn diagram similar to (G) indicating the intersection of the three enhancers used to drive CreA (DNE), CreB (NBE2), and CreC (NBE1). (J) Schematic of the floxed Gal80 construct used in the SpaRCLIn system, the expression of which is driven by the ubiquitously active Actin5C promoter. Cre-mediated excision of Gal80 via the flanking loxP sites causes a myristoylated tdTomato (tdTom) red fluorescent protein to be expressed instead of Gal80. (K–M) Restriction of NB expression by SpaRCLIn. (K, L) tdTom expression (red) driven by the bipartite SpaRCLIn system using two different NBEs (44F03 and 43H02) to drive CreC expression. (M) tdTom expression driven by the tripartite SpaRCLIn system at the intersection of the two NBE expression patterns, which overlap in several NB pairs of the ventral nerve cord (VNC) and brain (Br). Neuropil labeling by the nc82 antibody is shown in blue. Scale bar: 50 μM. Note that the genotypes of the flies for panels of this and all subsequent figures are provided in Supplementary file 2.
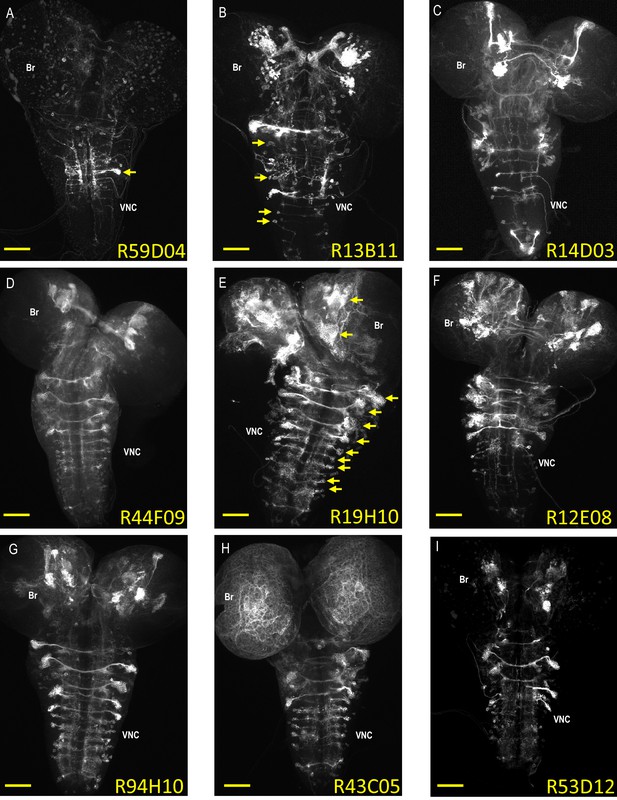
Expression patterns of neuroblast-active enhancers.
(A–I) Nine representative examples of CNS expression of the 134 neuroblast-active enhancers (NBEs) used in this study. Shown are volume-rendered confocal micrographs of larval CNS whole mounts taken from animals expressing tdTomato in NB lineages in which the indicated enhancers are active. The expression patterns were generated using the bipartite SpaRCLIn system described in Figure 1 to drive the tdTomato reporter in the labeled neuroblast lineages. Some of the NBEs show limited expression, as in (A), where only a single major neuroblast lineage with multiple, clustered progeny is labeled (arrow). Most, however, express in multiple NBs broadly distributed throughout the brain (Br) and ventral nerve cord (VNC), as in (E), where the arrows indicate some of the larger NB clones with many labeled progeny. The number of progeny in NB clones could be as small as one to two cells as in (B; arrows), indicating that NBEs could be active in some NBs only at later stages of neurogenesis where they would thus label sublineages. Scale bar: 50 μM.
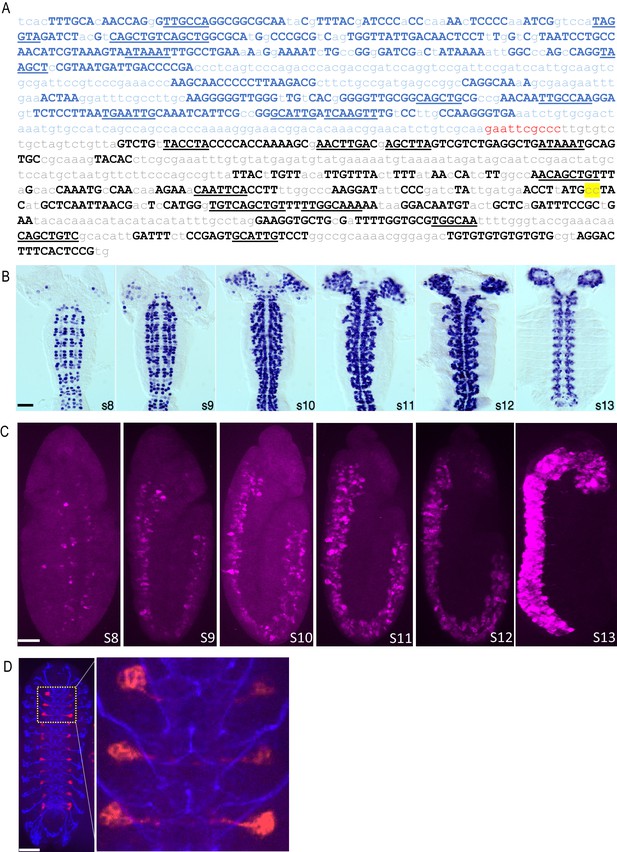
A neuroblast-specific deadpan-nerfin-1 enhancer, DNE.
(A) Sequence of the chimeric neuroblast enhancer used in this study, which is composed of an enhancer for the gene encoding the transcriptional repressor, deadpan (blue) fused via a 10 bp linker (red) to an enhancer for the gene encoding nerfin-1 (black). Upper case letters signify the highly conserved sequences used to identify the enhancers using the Evoprinter. Underlined are conserved sequence blocks shared by the two enhancers that represent putative transcription factor binding sites. Yellow highlight indicates two nucleotide substitutions that expand the range of the nerfin-1 enhancer expression in NBs. For further details see Materials and Methods. (B) Cis-regulatory activity of the DNE. The DNE was used to drive Gal4 expression, which was monitored during embryonic development by mRNA in situ hybridization. Shown are filleted wholemount embryos, stages 8–13 (anterior up). Most, if not all, CNS NBs are labeled during early to late stages of lineage development. By early embryonic stage 15, only a small subset of late neuroblasts have detectable signal and most, if not all, neurons and glia of the ventral cord and cephalic lobes have no detectable expression (data not shown). Scale bar: 50 μM. (C) DNE-mediated expression of CreAB and CreC fragments leads to reconstitution of Cre activity, excision of Gal80, and activation of tdTomato expression (magenta) in the embryonic CNS beginning at stage 8. Dorsal view, s8; lateral view, s9-13. (D) DNE-dependent Cre reconstitution and tdTomato expression in the NB 3–5 lineage of VNC hemisegments in a late-stage embryo. The previously described NB 3–5-specific Gal4 line, R59E09-Gal4 (Lacin and Truman, 2016), was used to drive expression of UAS-CreC in flies bearing the DNE-CreB, DNE-CreA and CreStop (i.e. actin^STOP^tdTomato) constructs. Excision of Gal80 specifically in NB 3–5 leads to tdTomato expression (red). Blue: 22C10 (anti- futsch) immunostaining. An enlarged image of the outlined region (left) is shown on the right. Scale bar: 50 μM.
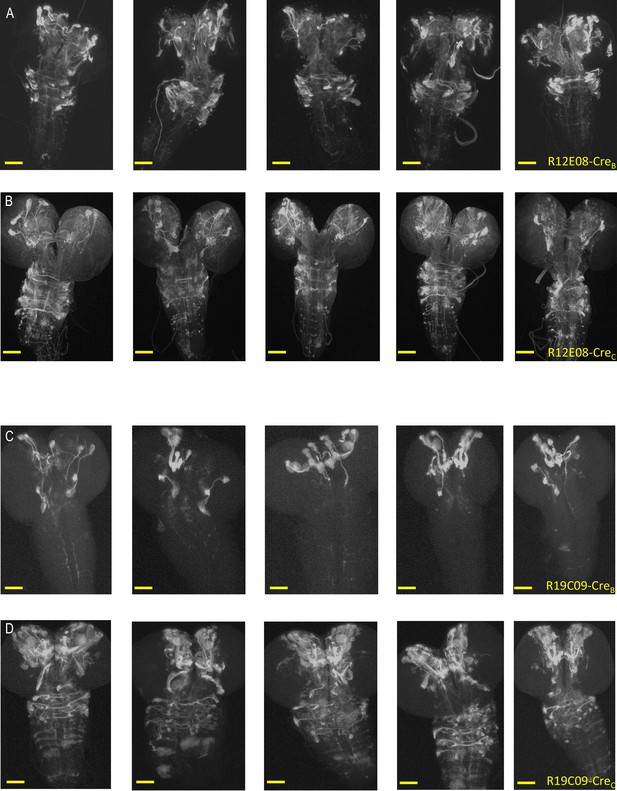
Reproducibility of expression patterns of NBE-CreB and -CreC lines.
(A–B) tdTomato expression patterns generated in 3rd instar larval CNS preparations by the R12E08 NBE driving: (A) CreB (insertion on chromosome II) or (B) CreC (insertion on Chromosome III). The expression patterns of five representative preparations are shown for each construct. In general, they appear similar for all preparations, which was the case for all but 24 of the 134 NBEs used to generate the NBE-CreB and NBE-CreC libraries. (Note that the DNE was used to drive CreA and CreC expression in (A) and CreAB expression in (B)). (C–D) tdTomato expression patterns generated as in (A–B) but using the R19C09 NBE to drive: (C) CreB (insertion on chromosome II) or (D) CreC (insertion on Chromosome III). R19C09 was one of the 24 NBEs that gave differing expression for the NBE-CreB and CreC constructs, with VNC lineages labeled when CreC, but not CreB, was used. Scale bars: 50 μM.
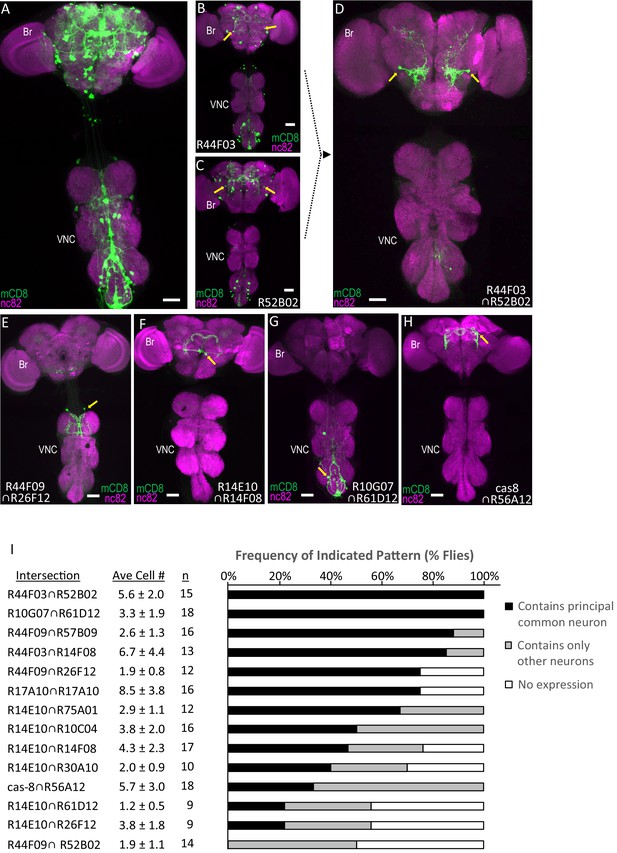
Parsing the TH-Gal4 expression pattern using SpaRCLIn.
(A) Expression pattern of the TH-Gal4 driver revealed by UAS-mCD8GFP (green). In all panels: Anti-nc82 labeled neuropil (magenta); ventral nerve cord; VNC; brain; Br. (B–D) Restriction of TH-Gal4 expression using SpaRCLIn. (B, C) mCD8GFP expression (green) in mature dopaminergic neurons isolated using the bipartite SpaRCLIn system and two different NBEs (R44F03 and R52B02) to drive CreC expression. A neuronal pair common to both patterns is indicated (yellow arrows). (D) mCD8GFP expression (green) driven by the tripartite SpaRCLIn system at the intersection of the two NBE expression patterns in B and C. (E–H) Examples of TH-Gal4 restriction to small numbers of neurons using the tripartite system and the indicated pairs of NBEs. Scale bar: 50 μM. (I) Size and stereotypy of the restricted expression patterns produced by the indicated Step two intersections. The average number of neurons per preparation (± standard deviation) observed for each intersection is shown together with the number of preparations examined. For each, intersection the neuron that was most frequently observed across preparations (i.e. the ‘principal common neuron’) was identified and the percentage of preparations containing this neuron is shown in the bar graph (black bars) together with the percentage of preparations showing expression only in other neurons (gray bars) or no expression (white bars). Examples of principal common neurons are indicated by yellow arrows in D-H.
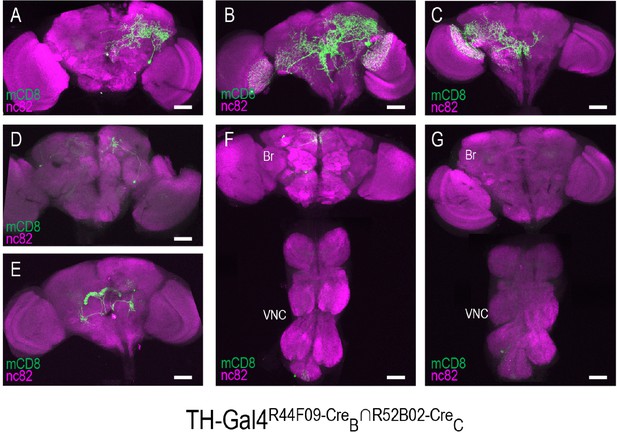
Stochastic expression within the TH-Gal4 pattern generated by SpaRCLIn.
(A–G) Seven distinct restrictions of the TH-Gal4 expression pattern produced by the same pair of NBEs: R44F09-CreB∩R52B02-CreC. For five of the CNS preparations (A–E), labeling of one or more neurons was observed only in the brain (Br), while in two preparations labeling was observed in cells of both the ventral nerve cord (VNC) and brain (F), or in the VNC alone (G). The identity of all neurons isolated in these preparations appeared to be unique. Anti-nc82 labeled neuropil (magenta); UAS-mCD8GFP (green). Scale bar: 50 μM.
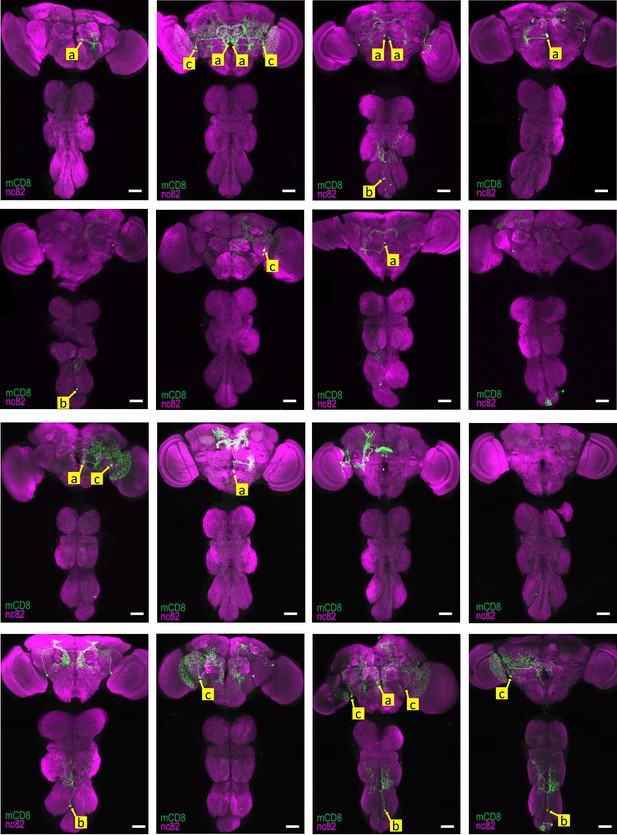
Reproducibility of labeling within the TH-Gal4 pattern generated by SpaRCLIn Expression patterns of all 16 CNS preparations for TH-Gal4R14E10-CreB∩R10C04-CreC.
Yellow labels (a-c) identify the somata of neurons identified to be the same in different preparations, based on position and morphology. The neuron labeled ‘a’ represents the primary expression pattern in that it occurs with the greatest frequency (8/16 preparations). Neurons ‘b’ and ‘c’ recur in 5 and 6 of the 16 preparations, respectively. In some cases, both neurons of these bilateral pairs are labeled, while in others only a single neuron is labeled. In all panels: Anti-nc82 labeled neuropil (magenta); UAS-mCD8GFP (green). Scale bar: 50 μM.
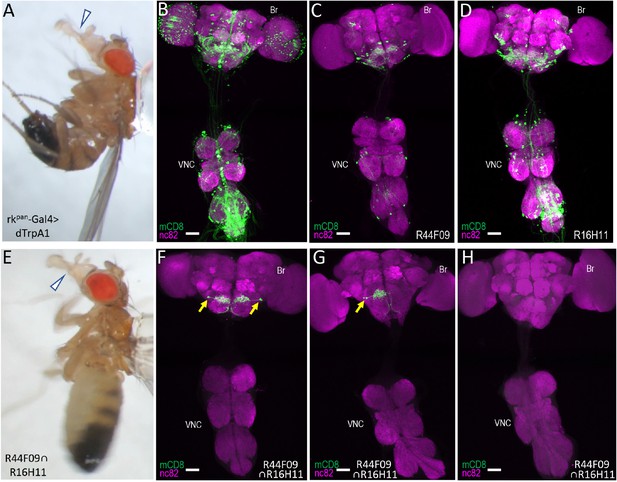
Identification of command neurons for PE within the rkpan- Gal4 pattern.
(A) Induced PE (arrowhead) in a fly expressing the heat-sensitive ion channel dTrpA1 under the control of the rkpan-Gal4 driver. Labels as described in the legend of Figure 2A. (B) Expression pattern of the rkpan-Gal4 driver revealed by UAS-mCD8GFP (green). In all panels: Anti-nc82 labeled neuropil (magenta); ventral nerve cord: VNC; brain: Br. (C–D) mCD8GFP expression (green) in mature subsets of RK-expressing neurons isolated using the bipartite SpaRCLIn system and NBEs R44F09 and R516H11 to drive CreC expression. (E) PE induced in a fly expressing dTrpA1 in the PErk neurons, isolated using the tripartite system with the R44F09 and R16H11 NBEs to parse the rkpan-Gal4 pattern. (F–H) Typical expression patterns in rkpan-Gal4R44F09 ∩ R16H11 flies, showing expression in both bilateral pairs of PErk neurons (F), one neuron of each of the two bilateral pairs of PErk neurons (G), or no neurons (H). All scale bars: 50 μM.
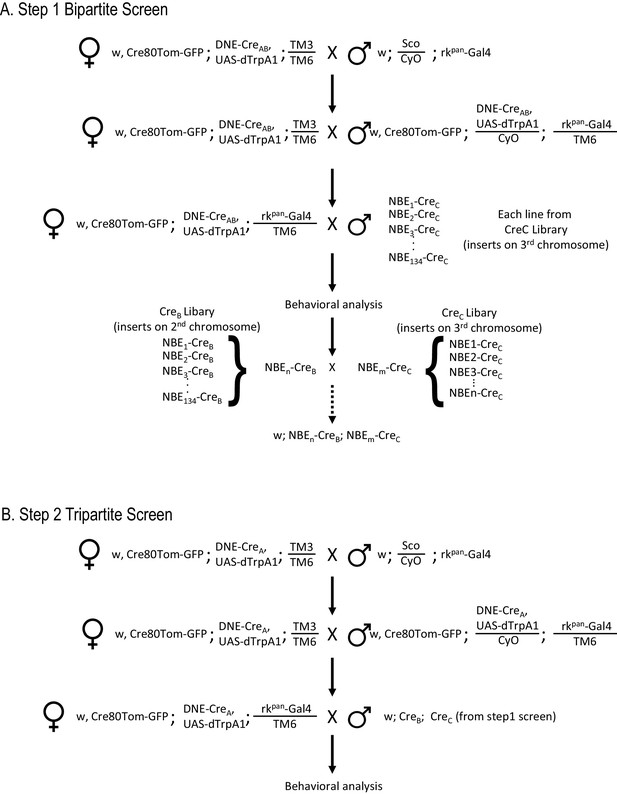
Workflow for SpaRCLIn Screens.
(A) Crossing scheme for implementing a Step one screen using the bipartite SpaRCLIn system. The example given illustrates the strategy to be used when the Gal4 driver is on chromosome III, but reagents for use when the driver is on chromosome II are also provided (see Key Resources Table). An initial set of crosses brings the Gal4 driver together with two essential components of SpaRCLIn: an X chromosome containing the combined Actin 5C-loxP-Gal80-loxP-tdTomato and UAS-mCD8GFP constructs (abbreviated here as Cre80Tom-GFP) and a 2nd chromosome containing the DNE-CreAB. If a functional screen is being performed, an effector—such as UAS-dTrpA1—can also be recombined with the DNE-CreAB or Gal4 driver on an autosome, as illustrated here. Alternatively, we have made variants of the Cre80Tom-GFP (on X) that contain UAS-dTrpA1 (Cre80-dTrpA1) or Kir2.1 (Cre80-Kir2.1) that can be used instead of Cre80Tom-GFP. Flies bearing the Gal4 driver and essential SpaRCLIn components, are then crossed in a final step to flies of the CreC library to generate the desired progeny for testing. The NBEs of those CreC library lines that test positive (e.g. NBEn-CreC and NBEm-CreC) can then be used for intersectional analysis in a Step two tripartite screen. This is done by combining one of the NBE-CreC components, say NBEn-CreC, with the NBEm-CreB component, which is readily accomplished by a series of genetic crosses (dotted arrow) because all NBE-CreC inserts are on chromosome III, and all NBE-CreB inserts are on II. (B) Crossing scheme for a tripartite SpaRCLIn screen. Initial crosses similar to those described for the bipartite screen are performed to combine essential SpaRCLIn components together with the Gal4 driver. Now, however, DNE-CreA is used instead of DNE-CreAB. Progeny with the final genotype for testing are generated using flies made as described in A that combine NBE-CreB and -CreC components.
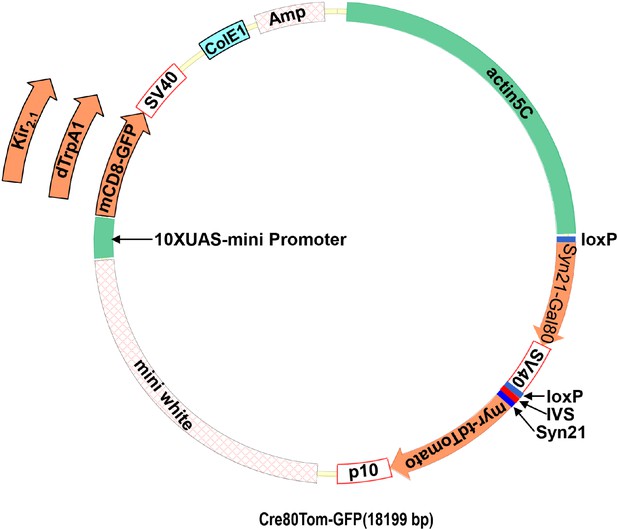
Dicistronic vector with floxed Gal80 and UAS constructs.
(A) Schematic of the plasmid used to make flies with the Cre80Tom construct and either the UAS-mCD8GFP, UAS-dTrpA1, or UAS-Kir2.1 construct. The latter are inserted into the plasmid via a unique NdeI restriction site in the plasmid. (B) Annotated sequence of the Cre80Tom-GFP expression construct.
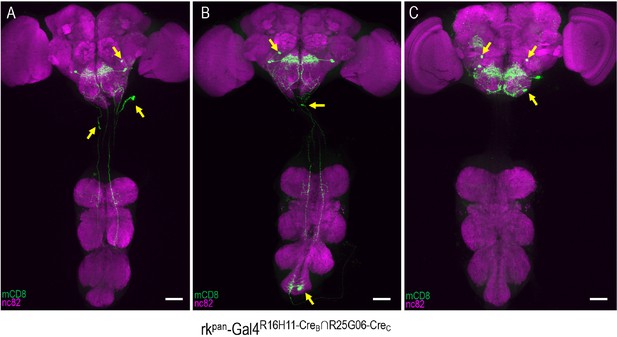
rkR16H11-CreB∩R25G06-CreC-Gal4 expression patterns include PErk and other neurons.
(A–C) Three representative examples of the labeling patterns obtained with the R16H11-CreB∩R25G06-CreC enhancer pair in the tripartite SpaRCLIn system. All animals whose expression was restricted in this way (n = 14) showed induced PE when expressing dTrpA1 and all had expression in the PErk neurons of the SEZ. In addition, however, each preparation also exhibited expression in a range of other neurons (arrows). Scale bar: 50 μM.
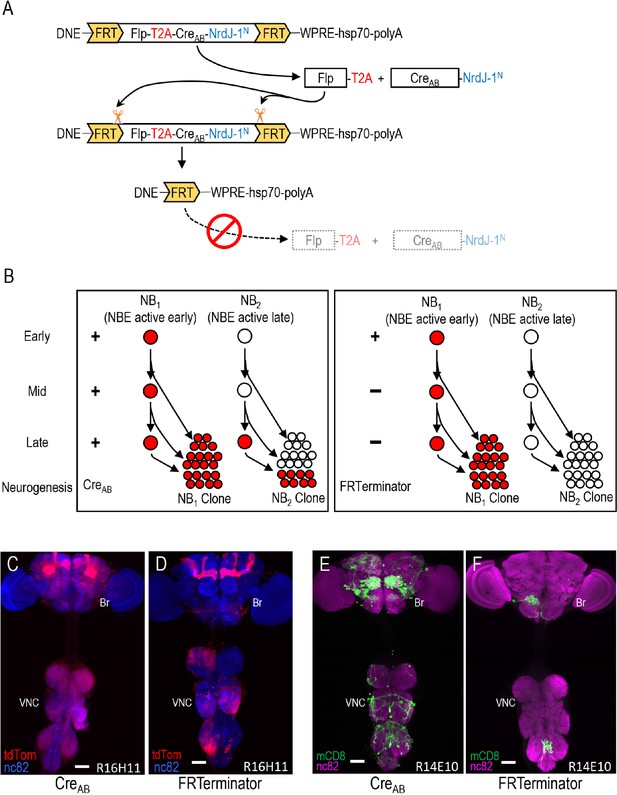
Limiting Cre activity to early NBs using FRTerminator.
(A) The FRTerminator construct: a DNE-CreAB that terminates its own expression. The FRTerminator expression cassette contains sequences for the Flp recombinase and CreAB-NrdJ-1N linked by a viral T2A sequence to ensure separate translation of the two gene products. The entire cassette is flanked by FRT sites. Upon expression of the cassette—which will occur in NBs at the onset of neurogenesis—Flp will excise the cassette, thus terminating any further expression of both Flp and CreAB-NrdJ-1N. (B) Schematic comparing the consequences of DNE-CreAB (left box) and FRTerminator (right box) action in two NB lineages (NB1 and NB2) in which an NBE (used to drive expression of CreC) is active. In NB1 the NBE is active early in neurogenesis and CreC will therefore be expressed in the young neuroblast. In contrast, the NBE becomes active only late in neurogenesis in NB2 and CreC is therefore only present in the older NB. Because DNE-CreAB is expressed throughout neurogenesis, it will be available to reconstitute full-length Cre whenever CreC is expressed. This means that Gal80 will be excised and tdTomato expression turned on (red) early in NB1—leading to the labeling of all progeny—and late in NB2—leading to labeling of only late-generated progeny. In contrast, FRTerminator is present only early in neurogenesis and Cre reconstitution (and tdTomato expression) will therefore occur only in NB1. No progeny of the NB2 clone will be labeled and the overall pattern of labeling will thus be diminished. (C–D) NB lineages targeted using NBER16H11-CreC and either the DNE-CreAB construct of the bipartite SpaRCLIn system (C), or FRTerminator (D). NB progeny are visualized with tdTomato (red) after excision of Gal80 by Cre. The breadth of tdTomato expression when using DNE-CreAB compared with FRTerminator reflects the loss of sublineages generated by NBs in which the R16H11 enhancer becomes active only later in neurogenesis, as illustrated in B. Anti-nc82 labeled neuropil (blue); ventral nerve cord; VNC; brain; Br. Scale bar: 50 μM. (E–F) Restriction of the rkpan-Gal4 expression pattern by SpaRCLIn using R14E10-CreC with DNE-CreAB (E) or FRTerminator (F). FRTerminator significantly reduces the expression pattern compared with the restriction obtained with DNE-CreAB, labeling principally the PErk neurons. Reporter: UAS-mCD8GFP (green); Anti-nc82 labeled neuropil (magenta); ventral nerve cord; VNC; brain; Br. Scale bar: 50 μM.
Videos
Activation of neurons in the rkpan-Gal4 pattern induces robust proboscis extension.
rkpan-Gal4 was used to drive expression of the heat-activated ion channel, UAS-dTrpA1. At 18°C the channel is inactive and animals expressing it throughout the rkpan-Gal4 pattern do not extend their proboscis. In contrast, at 31°C when the channel is activated, animals display prolonged proboscis extension.
Activation of the PErk neurons induces robust, rhythmic proboscis extension.
The tripartite SpaRCLIn system isolates a subset of neurons within the rkpan-Gal4DNE-CreA∩R16H11-CreB∩R44F09-CreC intersection called the PErk neurons. When activated using dTrpA1 and a temperature of 31°C repeated, rhythmic proboscis extension is induced.
Neuroanatomical location and projection pattern of the PErk neurons.
GFP-labeled PErk neurons (green) were imaged by confocal microscopy to show the location of their somata and their arborization. Neuropil labeled by nc82 antibody is shown in blue to identify brain regions.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (D. melanogaster) | w; Sco/Cyo; Rkpan-Gal4 | Diao and White, 2012 | N/A | |
| Genetic reagent (D. melanogaster) | w; UAS-TrpA1(attP16);+ | Bloomington Drosophila Stock Center | RRID:BDSC_26263 | |
| Genetic reagent (D. melanogaster) | w; +; TH-Gal4 | J Hirsh and S Birman | THGal4-1 | |
| Genetic reagent (D. melanogaster) | yw; mCD8-GFP/Cyo;+ | Gift of Liqun Luo | N/A | |
| Genetic reagent (D. melanogaster) | w; DNE-CreAB (JK22C);+ | This paper | HJ210 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | w, DNE-CreB[su(Hw)attP8]; Pin/CyO; TM3,Sb1/TM6B,Hu1,Tb1 | This paper | HJ201 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | w; +; DNE-CreA (VK00027) | This paper | HJ200 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | w; DNE-CreA (attP40); + | This paper | HJ200 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | w, Cre80Tom[su(Hw)attP8]; Pin/CyO; TM3,Sb1/TM6B,Hu1,Tb1 | This paper | HJ223 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | w, Cre80Tom-GFP[su(Hw)attP8]; Pin/CyO; TM3,Sb1/TM6B,Hu1,Tb1 | This paper | HJ224 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | w; DNE-Frterminator (attP40);+ | This paper | HJ473 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | w; +; DNE-CreBC (attP2) | This paper | HJ209 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | UAS-CreC (attP40) | This paper | HJ226 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | CreStop (attP2) | This paper | HJ225 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | w, Cre80-Kir2.1[su(Hw)attP8]; Pin/CyO; TM3,Sb1/TM6B,Hu1,Tb1 | This paper | HJ472-actin5C-FloxSyn21Gal80-pMUH-Kir2.1 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | w, Cre80-TrpA1[su(Hw)attP8]; Pin/CyO; TM3,Sb1/TM6B,Hu1,Tb1 | This paper | HJ382-actin5C-FloxSyn21Gal80-10XUAS-dTrpA1 | Supplementary file 4 |
| Genetic reagent (D. melanogaster) | R59E09-Gal4(attP2) | BloomingtonDrosophila Stock Center | RRID:BDSC_39220 | |
| Antibody | Rat monoclonal anti-Mouse CD8a | Invitrogen Life Technologies | RRID:AB_10392843 | 1:100 dilution |
| Antibody | Goat polyclonal Alexa Fluor 488 anti-rat IgG (H+L) | Invitrogen Life Technologies | RRID:AB_2534074 | 1:500 dilution |
| Antibody | Rabbit polyclonal Anti-RFP Antibody | Rockland, Imm. | RRID:AB_2209751 | 1:5000 dilution |
| Antibody | Mouse monoclonal Anti-futsch Antibody | Developmental Studies Hybridoma Bank | RRID:AB_528403 | 1:50 dilution |
| Antibody | Rabbit polyclonal Living Colors DsRed Polyclonal Antibody | CLONTECH Laboratories | RRID:AB_10013483 | 1:500 dilution |
| Antibody | Goat polyclonal Cy3 AffiniPure Goat Anti-Rabbit IgG (H+L) | Jackson Immuno-Research | RRID:AB_2338006 | 1:500 dilution |
| Antibody | Mouse monoclonal nc82 | Developmental Studies Hybridoma Bank | RRID:AB_2314866 | 1:50 dilution |
| Antibody | Goat polyclonal Alexa Fluor 488 goat anti-mouse IgG (H+L) | Invitrogen Life Technologies | RRID:AB_2534069 | 1:500 dilution |
| Antibody | Goat polyclonal Alexa Fluor 647-AffiniPure Goat Anti-Mouse IgG (H+L) | Jackson Immuno-Research | RRID:AB_2338914 | 1:500 dilution |
| Recombinant DNA reagent | CreA | This paper | HJP-176-IVS-Syn21-CreA-gp41-1N-WPREw | For making CreA line from promoter |
| Recombinant DNA reagent | CreB | This paper | HJP-180-IVS-Syn21-gp41-1C-CreB-NrdJ-1N-WPREw | For making CreB line from promoter |
| Recombinant DNA reagent | CreC | This paper | HJP-179-IVS-Syn21-NrdJ-1C-CreC-WPREw | For making CreC line from promoter |
| Recombinant DNA reagent | CreAB | This paper | HJP-178-IVS-Syn21-CreAB-NrdJ-1N-WPREw | For making CreAB line from promoter |
| Recombinant DNA reagent | CreBC | This paper | HJP-177-IVS-Syn21-gp41-1C-CreBC-WPREw | For making CreBC line from promoter |
| Recombinant DNA reagent | CreAU | This paper | HJP-194-IVS-Syn21-CreA-gp41-1N-WPREUw | For making CreA from enhancer |
| Recombinant DNA reagent | CreBU | This paper | HJP-195-IVS-Syn21-gp41-1C-CreB-NrdJ-1N-WPREUw | For making CreB line from enhancer |
| Recombinant DNA reagent | CreCU | This paper | HJP-196-IVS-Syn21-NrdJ-1C-CreC-WPREUw | For making CreC line from enhancer |
| Recombinant DNA reagent | CreABU | This paper | HJP-208-IVS-Syn21-CreAB-NrdJ-1N-WPREUw | For making CreAB line from enhancer |
| Recombinant DNA reagent | CreBCU | This paper | HJP-207-IVS-Syn21-gp41-1C-CreBC-WPREUw | For making CreBC line from enhancer |
| Recombinant DNA reagent | CreStop | This paper | HJP-225-actin5C-Flox-IVS-Syn21myr-tdTomato-p10w | |
| Recombinant DNA reagent | Cre80Tom | This paper | HJP-223-actin5C^Syn21Gal80^IVS-Syn21myr-tdTomato-p10w | |
| Recombinant DNA reagent | Cre80Tom-GFP | This paper | HJP-224-actin5C^Syn21Gal80^IVS-Syn21myr-tdTomato-10UAS-mCD8GFP-p10w | |
| Recombinant DNA reagent | UAS-CreC | This paper | HJP-226-10XUAS-NrdJ-1C-CreC | |
| Recombinant DNA reagent | DNE-FRterminator | This paper | HJP-473-DNE > -T2A-CreAB-NrdJ-1N | |
| Recombinant DNA reagent | Cre80-Kir2.1 | This paper | HJP-472-actin5C-FloxSyn21Gal80-10XUAS-EGFP-Kir2.1 | Genetic reagent (D. melanogaster) |
| Recombinant DNA reagent | Cre80-dTrpA1 | This paper | HJP-382-actin5C-FloxSyn21Gal80-10XUAS-dTrpA1 | Genetic reagent (D. melanogaster) |
| Chemical compound, drug | Gateway LR Clonase II Enzyme mix | Thermofisher Scientific | 11791100 | |
| Chemical compound, drug | In-Fusion HD Cloning Plus | Takara Bio USA, Inc | 638911 | |
| Chemical compound, drug | pCR8/GW/TOPO TA Cloning Kit with One Shot TOP10 Chemically Competent E. coli | Invitrogen Life Technologies | K2500-20 | |
| Chemical compound, drug | pENTR/D-TOPO Cloning Kit, with One Shot TOP10 Chemically Competent E. coli | Invitrogen Life Technologies | K240020 | |
| Chemical compound, drug | Q5 High-Fidelity 2X Master Mix | New England Biolabs | M0492S | |
| Chemical compound, drug | Quick Ligation Kit | New England Biolabs | M2200L |
Additional files
-
Supplementary file 1
List of Neuroblast Enhancers (NBEs).
The table lists all NBEs used in this study, their sources, and the names of the corresponding CreB and CreC constructs made with them.
- https://cdn.elifesciences.org/articles/53041/elife-53041-supp1-v2.xlsx
-
Supplementary file 2
Fly genotypes by Figure.
The table indicates the genetic crosses performed to generate the flies used in each figure of the paper (parental genotypes are shown).
- https://cdn.elifesciences.org/articles/53041/elife-53041-supp2-v2.xlsx
-
Supplementary file 3
Primer and gBlock sequences.
The table lists the primer and gBlock sequences used to generate constructs described in this paper.
- https://cdn.elifesciences.org/articles/53041/elife-53041-supp3-v2.xlsx
-
Supplementary file 4
Plasmid DNA sequences of all constructs made for this paper.
- https://cdn.elifesciences.org/articles/53041/elife-53041-supp4-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/53041/elife-53041-transrepform-v2.docx





