Role of IL-4 in bone marrow driven dysregulated angiogenesis and age-related macular degeneration
Figures
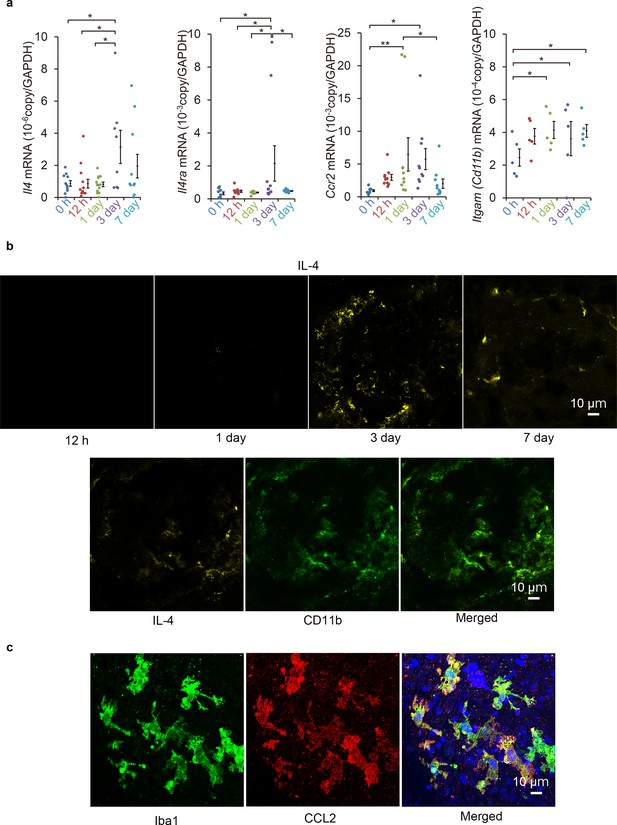
Induction of Il4 and Ccl2 in laser-exposed retinas and choroids of mice.
(a) Induction kinetics of the mRNAs of IL-4, IL-4Rα, CCR2, and CD11b. The induction of Ccl2 peaked at 1 day after the exposure followed by the peak induction of Il4 and Il4ra. (n = 4–14 eyes/group). (b) Kinetics of IL-4-expressing cells by immunohistochemical analyses. IL-4 expressing cells (yellow) accumulated along the margin of laser treated area at 3 days and then move inwards. The IL-4-expressing cells (yellow) were mainly CD11b positive (green) in laser treated areas at 3 days. (c) Localization of CCL2 expression in retinal tissue by immunohistochemistry. CCL2 induction is observed at 1 day after treatment, and CCL2-positive cells (red) are colocalized with the iba1-positive microglial cells (green). The nuclei were stained by TO-PRO-3 iodide (blue). Scale 10 μm. *p<0.05, **p<0.01. ANOVA with post hoc test and linear mixed-effects regression analysis.
-
Figure 1—source data 1
Induction kinetics of the mRNAs of IL-4, IL-4Rα, CCR2, and CD11b.
- https://cdn.elifesciences.org/articles/54257/elife-54257-fig1-data1-v1.xlsx
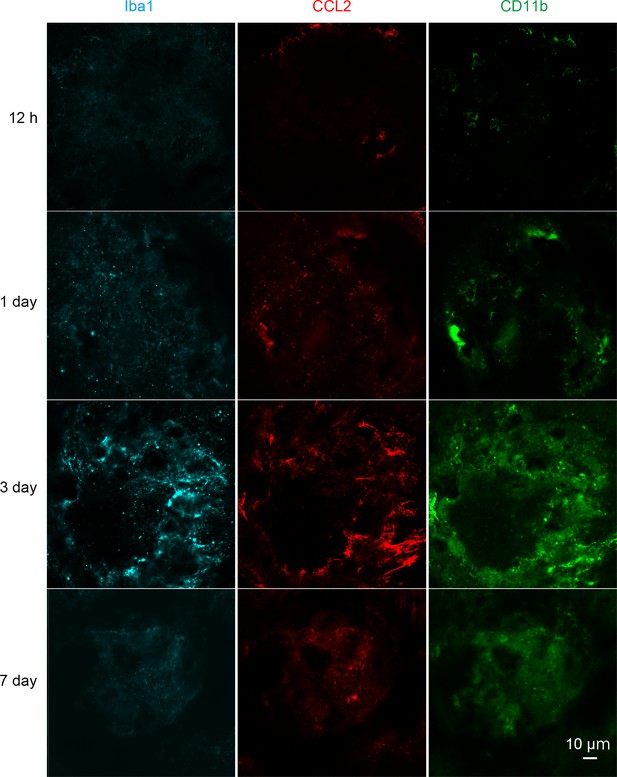
Kinetics of IL-4-expressing cells by immunohistochemical analyses in retinal flat mount.
Iba1 (cyan), CCL2 (red) and CD11b (green) expressing cells accumulated in the laser treated area at 1 to 3 days. The CCL2 and CD11b expression peaked at 1 to 3 days and decreased gradually thereafter. Scale 10 μm.
Localization of bone marrow-derived cells and microglial cells in the retinal tissue of GFP bone marrow chimeric mouse in 3D rendering at 3 days after laser irradiation.
Bone marrow-derived cells (green) are located in the subretinal layer around the laser irradiation lesion 3 days after laser irradiation. Bone marrow-derived cells (green) expressing CD11b (cyan) were spatially and morphologically distinct from the accumulated microglial cells (arrows). CD11b (cyan) positive microglial cells migrated on CD31 (red) positive retinal and choroidal vessels and are observed on the surface of the choroidal neovascularization. Scale 50 μm.
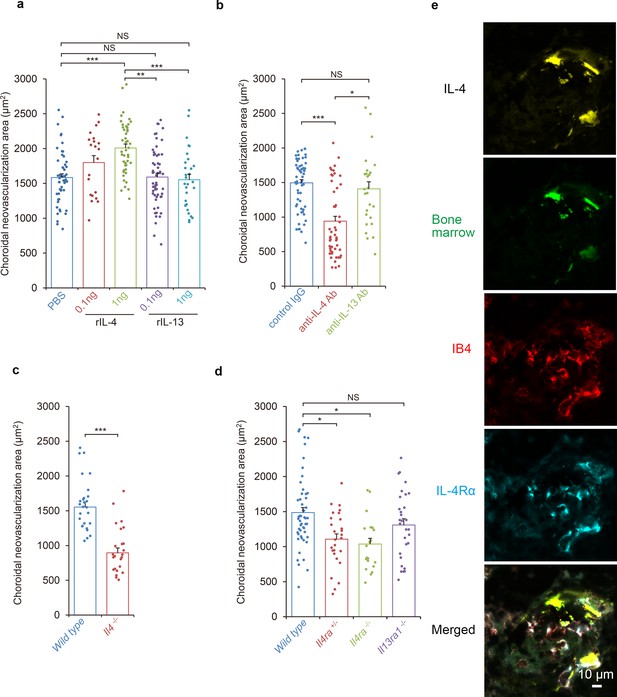
Requirements of IL-4/IL-4Rα in the inductive phase of choroidal neovascularization (CNV).
(a) Effect of systemic administration of recombinant murine IL-4 (rIL-4) or recombinant IL-13 (rIL-13) in the inductive phase in a CNV model. (n = 7–19 eyes/group). (b) Inhibitory effect of systemic administration of anti-IL-4 antibody in the inductive phase of CNV formation. (n = 7–20 eyes/group). (c) Impaired CNV development in Il4 deficient mice. CNV development is significantly impaired in Il4-/- mice compared to wild type. (n = 8–9 eyes/group). (d) Impaired CNV development by IL-4 receptors deficiency. CNV development is significantly impaired in Il4ra-/- and Il4ra+/- mice compared to wild type. This impairment is more marked in the homozygotes. CNV development is not impaired for Il13ra1-/- mice. (n = 7–17 eyes/group) (e) Bone marrow chimeric mice reconstituted with GFP transgenic bone marrow cells that were exposed to laser to induce CNVs. The CNV lesions after 14 days were analyzed for lineage cell markers by immunohistochemistry. CNVs are formed as clusters of isolectin IB4-positive vascular endothelial cells (red). Bone marrow-derived cells (green) were co-localized with isolectin-positive vascular endothelial cells. IL-4 positive cells (yellow) are distributed at the margins of the CNVs and precisely match the location of the bone marrow-derived cells (green). IL-4Rα-positive cells (cyan) partly overlapped the bone marrow-derived cells, and precisely match the location of the vascular endothelial cells in the CNV lesion. *p<0.005, **p<0.001, ***p<0.0005. Nested ANOVA with post hoc test. Scale 10 μm.
-
Figure 2—source data 1
Requirements of IL-4/IL-4Rα in the inductive phase of CNV.
- https://cdn.elifesciences.org/articles/54257/elife-54257-fig2-data1-v1.xlsx

Kinetics of IL-4, IL-4Rα, CCR2 and CD11b-expressing cells and GFP-positive bone marrow derived cells determined by immunohistochemical analyses.
The distribution of GFP transgenic bone marrow cells (green) shows dynamic changes after laser irradiation. GFP transgenic bone marrow cells remain around the choroidal scar at 1 day after the laser irradiation. Then GFP transgenic bone marrow cells spread out in the subretinal space at 3 days after laser irradiation and some GFP transgenic bone marrow cells return to the center area of CNV lesion. IL-4 (yellow), IL-4Rα-, CCR2-, and CD11b-positive cells (cyan) partly overlap the distribution of the GFP transgenic bone marrow cells. Scale 50 μm.
Localization of bone marrow-derived cells and endothelial cells in the retinal tissue of GFP bone marrow chimeric mouse in a 3D rendering 7 days after laser irradiation.
CCR2 (cyan) expressing bone marrow-derived cells (green) are incorporated into CD31 (red) expressing vascular endothelial morphology (arrows) and were spatially distinct from monocytes lineage cells. Scale 50 μm.
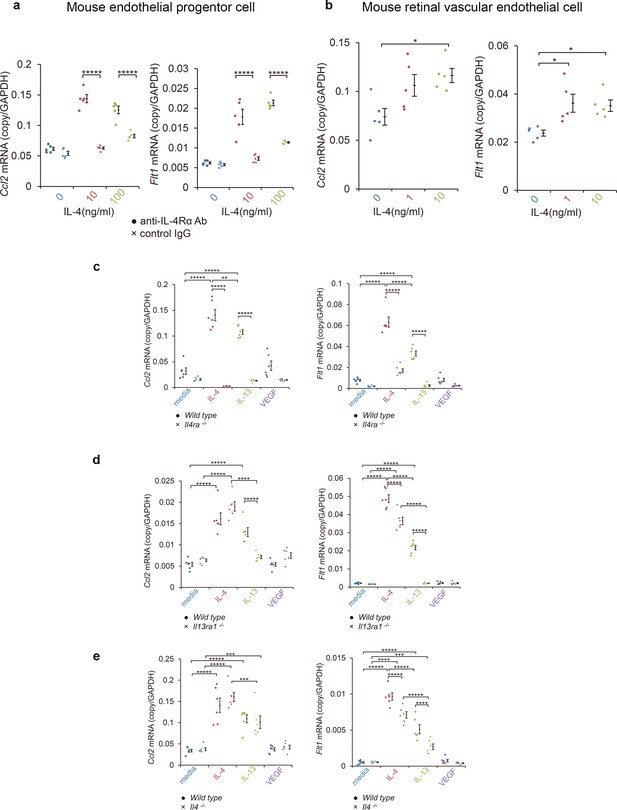
Induction of Ccl2 and Flt1 in bone marrow-derived endothelial progenitor cells (EPC) and retinal vascular endothelial cells by IL-4.
(a) Induction of Ccl2 and Flt1 in bone marrow-derived endothelial progenitor cells by murine IL-4. IL-4 stimulated bone marrow-derived EPCs induced Ccl2 and Flt1 in a dose dependent manner. This induction is abolished by anti-IL-4Rα antibody. (n = 5/group). (b) Induction of Ccl2 and Flt1 in retinal vascular endothelial cells by IL-4. IL-4 stimulated vascular endothelial cells to express Ccl2 and Flt1 in a dose dependent manner. (n = 5/group). (c) Inhibition of IL-4/IL-13-mediated Ccl2 and Flt1 induction in EPCs by Il4ra deficiency (n = 6/group). IL-4 and IL-13 exposure induced Ccl2 and Flt1 in EPCs. This induction is not present in the EPCs of Il4ra-/- mice. (d) Inhibition of IL-13-mediated Ccl2 and Flt1 induction in EPCs by Il13ra1 deficiency (n = 6/group). The IL-13-induced the expression of Ccl2 and Flt1 is significantly reduced in Il13ra1-/- EPCs of mice. IL-4-induced Ccl2 and Flt1 mRNA is not affected in Il13ra1-/- EPCs of mice. (e) EPCs of Il4 -/- mice respond to induce Ccl2/Flt1 mRNA by IL-4/IL-13 exposure. (n = 6/group). *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001, *****p<0.0005. ANOVA with post hoc test.
-
Figure 3—source data 1
Induction of Ccl2 and Flt1 in bone marrow-derived EPC and retinal vascular endothelial cells by IL-4.
- https://cdn.elifesciences.org/articles/54257/elife-54257-fig3-data1-v1.xlsx
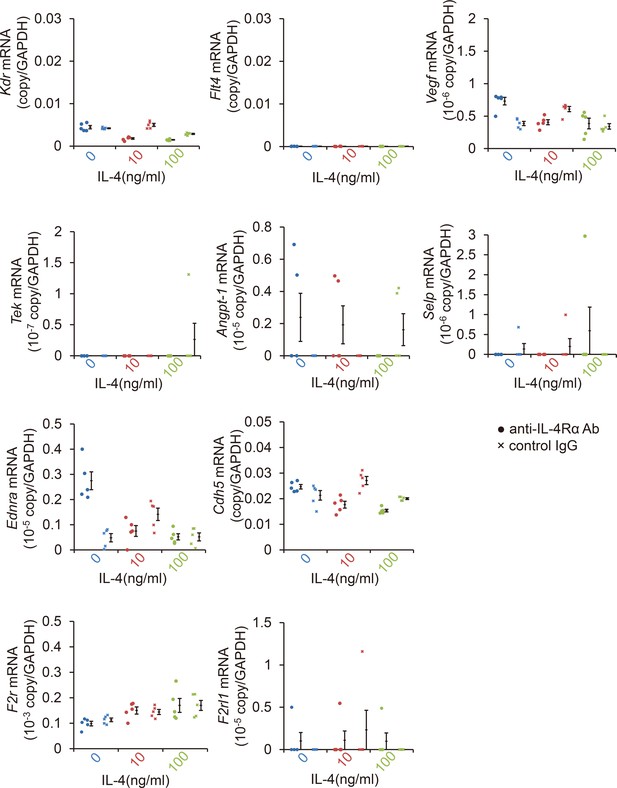
Profile of angiogenic mRNAs of bone marrow-derived endothelial progenitor cells (EPCs) after IL-4 exposure.
IL-4 exposure did not affect the induction of angiogenesis-related mRNAs of EPCs including Vegf, Kdr, Flt4, Tek, Angpt1, Selp, Ednra, Cdh5, F2r, F2rl1 after 24 hr. (RT-PCR, n = 5–8/group).
-
Figure 3—figure supplement 1—source data 1
Profile of angiogenic mRNAs of bone marrow-derived EPCs after IL-4 exposure.
- https://cdn.elifesciences.org/articles/54257/elife-54257-fig3-figsupp1-data1-v1.xlsx
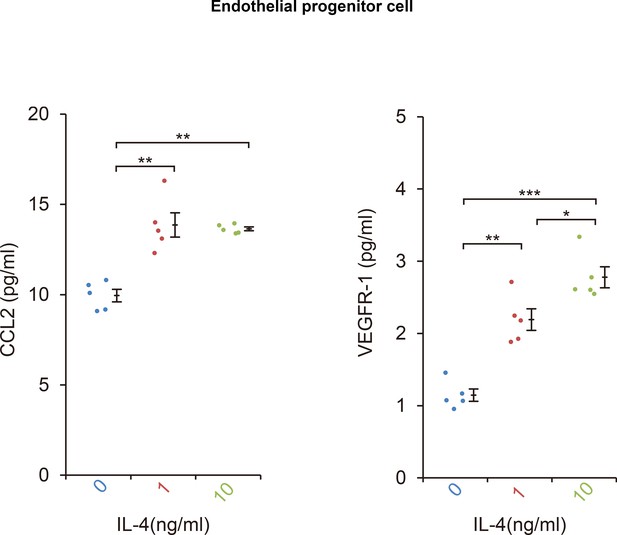
The CCL2 and VEGFR-1 protein levels in bone marrow-derived EPCs after IL-4 exposure.
IL-4 exposure significantly stimulated CCL2 and VEGFR-1 protein secretion from EPC after 24 hr. (ELISA, n = 5/group). *p<0.05, **p<0.0005, ***p<0.0001. ANOVA with post hoc test.
-
Figure 3—figure supplement 2—source data 1
The CCL2 and VEGFR-1 protein levels in bone marrow-derived EPCs after IL-4 exposure.
- https://cdn.elifesciences.org/articles/54257/elife-54257-fig3-figsupp2-data1-v1.xlsx
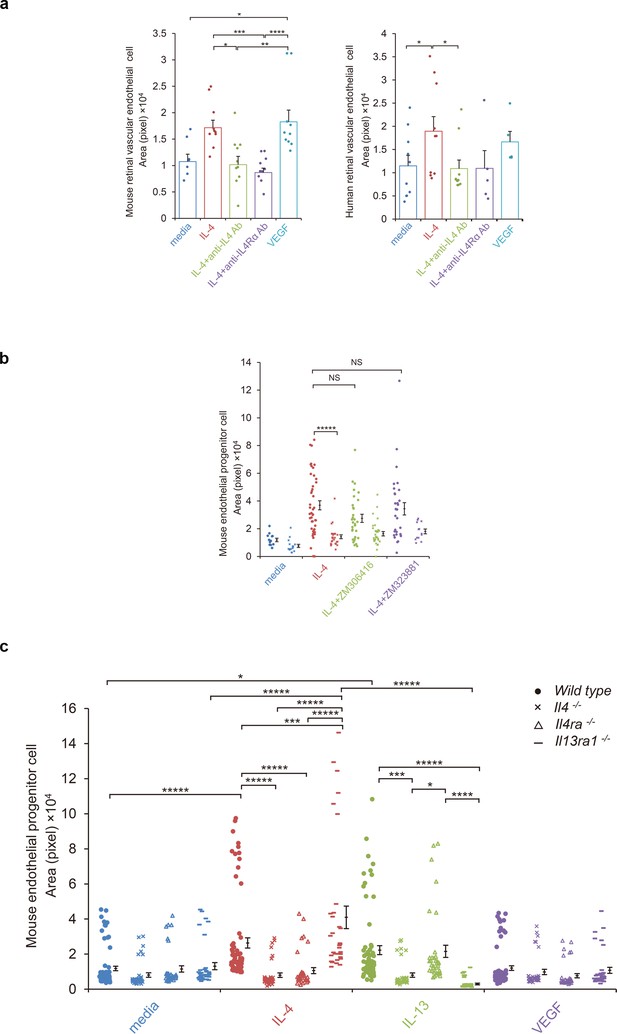
IL-4-induced tube formation in endothelial progenitor cells (EPCs) and retinal vascular endothelial cells.
(a) IL-4-induced tube formation of retinal vascular endothelial cells. Human and murine IL-4 exposure (10 ng/ml) significantly stimulated tube formation of human and murine retinal microvascular endothelial cells in vitro, respectively. Anti-IL-4 or IL-4Rα antibodies abolished the IL-4 induced-tube formation. VEGF exposure (10 ng/ml) also stimulated tube formation. (n = 7–10/group). (b) IL-4-induced tube formation in bone marrow-derived EPCs. IL-4 exposure (10 ng/ml) significantly stimulated tube formation of EPCs. The IL-4-induced tube formation was significantly reduced in EPCs from Il4ra-/- mice but was not affected by inhibition of VEGF receptor tyrosine kinase (ZM 306416) or VEGFR-2 (ZM 323881) (n = 13–45/group). (c) Requirements of IL-4 for tube formation response of EPCs. IL-4 (10 ng/ml) and IL-13 (10 ng/ml) induced tube formation of bone marrow-derived EPCs. These actions were abolished in the EPCs from Il4-/- bone marrow cells. EPCs from Il4ra-/- mice did not respond to IL-4, however they responded to IL-13 by tube formation. EPCs from Il13ra1-/- mice did not respond to IL-13 but responded to IL-4 by tube formation. (n = 35–72/group). *p<0.05, **p<0.01, ***p<0.005, ****p<0.001, *****p<0.0005. ANOVA with post hoc test and linear mixed-effects regression analysis.
-
Figure 4—source data 1
IL-4-induced tube formation inEPCs and retinal vascular endothelial cells.
- https://cdn.elifesciences.org/articles/54257/elife-54257-fig4-data1-v1.xlsx
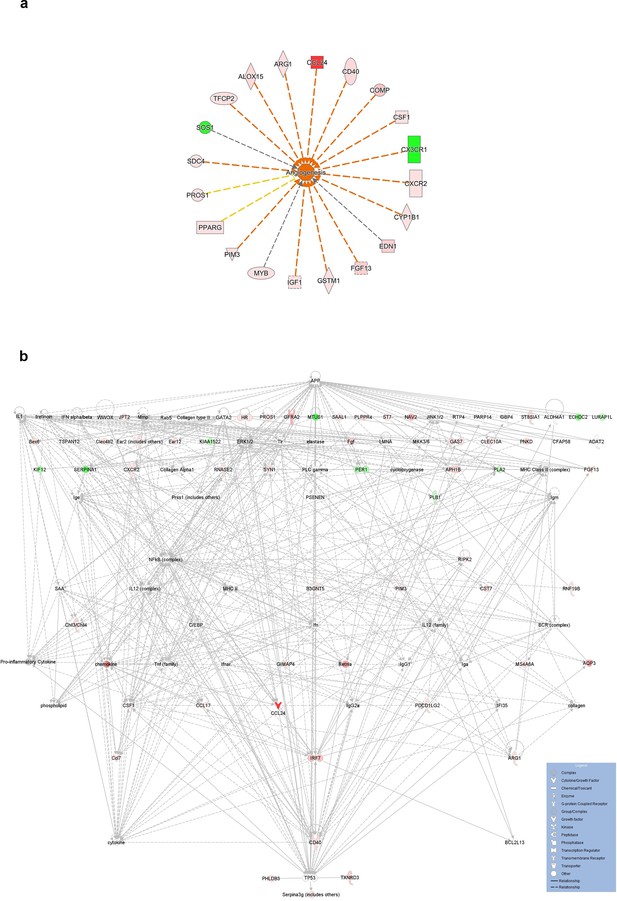
IL-4Rα-mediated transcriptional networks of bone marrow-derived EPCs.
(a) IL-4Rα-mediated transcriptional networks associated with angiogenesis in bone marrow-derived EPCs after IL-4 treatment. Z score = 2.781, p=1.8 × 10−4. (b) IL-4Rα-mediated transcriptional networks of bone marrow-derived EPCs. Top three highest significant networks (p<1 × 10−27) were merged and shown in hierarchical layout. Amyloid-beta A4 protein (App) and cellular tumor antigen p53 (Tp53) are shown in the top and bottom position. Red and green indicates up and down regulation, respectively. IPA was accessed on 2020/4/5.
-
Figure 4—figure supplement 1—source data 1
IL-4Rα-mediated transcriptional networks of bone marrow-derived EPCs.
- https://cdn.elifesciences.org/articles/54257/elife-54257-fig4-figsupp1-data1-v1.txt
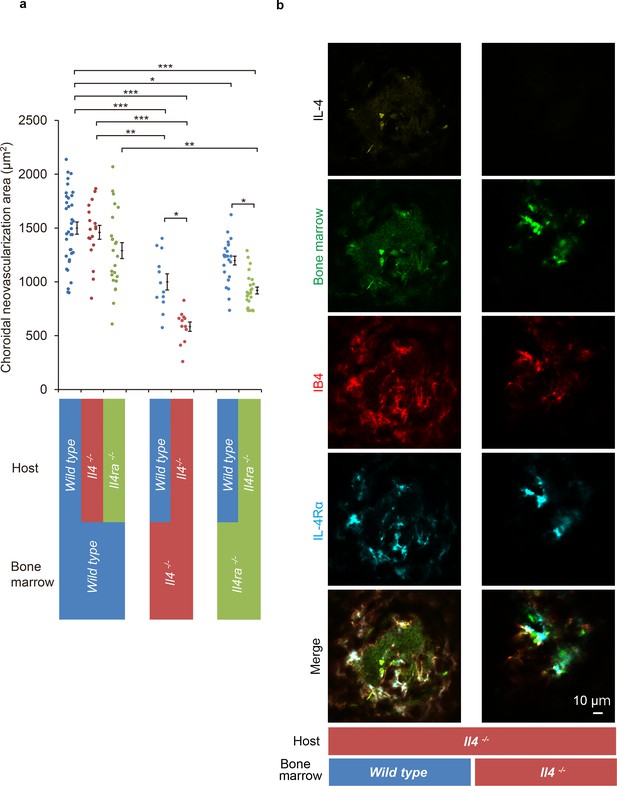
Role of IL-4/IL-4Rα on bone marrow-mediated choroidal neovascularization in bone marrow chimeric mice.
(a) Requirement of IL-4 in bone marrow for choroidal neovascularization (CNV). Bone marrow chimeric mice were constructed on backgrounds of wild type, Il4-/-, or Il4ra-/- mice by transfer of wild type Il4-/-, or Il4ra-/- bone marrow cells. Il4-/- mice with Il4-/- bone marrow are most significantly impaired in CNV formation. This impairment of CNV formation is restored when bone marrow reconstituted with wild type bone marrow. Il4ra-/- mice with Il4ra-/- bone marrow cells are impaired in CNV formation. This impairment is partially restored when reconstituted with wild type bone marrow cells. (n = 4–12 eyes/group). Six of 10 IL-4-/- bone marrow chimeric mice in each group did not survive through procedures and/or were euthanized. *p<0.05, **p<0.005, ***p<0.0005. Nested ANOVA with post hoc test. (b) Immunohistochemical analysis of CNV of the bone marrow chimeric mice on Il4-/- background 14 days after laser treatment. Endothelial cells in the CNV were labeled with isolectin IB4 (red). In the Il4-/- mice reconstituted with wild type bone marrow, the CNV lesion contained IL-4 (yellow) secreting bone marrow cells (green). The IL-4Rα-positive cells (cyan) partly overlapped with bone marrow-derived cells. Scale 10 μm.
-
Figure 5—source data 1
Requirement of IL-4 in bone marrow for CNV.
- https://cdn.elifesciences.org/articles/54257/elife-54257-fig5-data1-v1.xlsx

Immunohistochemical analysis of CNV of the bone marrow chimeric mice on Il4ra-/- and wild type background 14 days after laser treatment.
Bone marrow-derived cells (green) and endothelial cells (red) were localized in the retinal tissue of wild type and Il4ra-/- bone marrow chimeric mice on Il4ra-/- background and wild type, Il4-/- and Il4ra-/- bone marrow chimeric mice on wild type background.
Endothelial cells in the CNV were labeled with isolectin IB4 (red). Reduced sized CNV contained wild type bone-derived cells. Scale 10 μm.
Tables
Increase of IL-4 concentration in aqueous humor of eyes with age-related macular degeneration.
| Cytokines (pg/ml) | Control (n = 104) | age-related macular degeneration (n = 234) | P value |
|---|---|---|---|
| IL-4 | 0.3 ± 0.1 | 0.9 ± 0.1 | p=0.0000 |
| IL-13 | 3.8 ± 0.7 | 5.2 ± 0.7 | NS |
-
Two-tailed t test; Mean ± standard error of the means (SEMs).
Association of IL-4 concentration in aqueous humor with subtype of age-related macular degeneration.
| Relative risk ratio | Relative risk ratio | ||||
|---|---|---|---|---|---|
| N | IL-4 (quintile) | P value | IL-13 (quintile) | P value | |
| Control | 104 | - | - | - | - |
| Typical AMD | 33 | 2.11 ± 0.33 | 0.000 | 2.09 ± 0.39 | 0.000 |
| Polypoidal choroidal vasculopathy (PCV) | 78 | 1.70 ± 0.17 | 0.000 | 1.39 ± 0.15 | 0.002 |
| Retinal angiomatous proliferation (RAP) | 11 | 2.46 ± 0.69 | 0.001 | 1.48 ± 0.37 | 0.11 |
-
Multinomial logistic regression analysis after age adjustment; Mean ± standard error of the means (SEMs).
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (M. musculus) | C57BL/6J (wt) | PMID:15729571 | RRID:IMSR_JAX:000664 | |
| Genetic reagent (M. musculus) | C57BL/6-Tg(CAG-EGFP) | PMID:9175875 | RRID:IMSR_JAX:003291 | |
| Genetic reagent (M. musculus) | C57BL/6-Il4tm1Nnt/J | PMID:8906833 | RRID:IMSR_JAX:002518 | |
| Genetic reagent (M. musculus) | BALB/c-Il4ratm1Sz/J | PMID:9380721 | RRID:IMSR_JAX:003514 | |
| Genetic reagent (M. musculus) | Il13ra1tm1Twy | PMID:18066066 | RRID:MGI:3772446 | Dr. Marc E Rothenberg, Cincinnati Children's Hospital Medical Center University of Cincinnati College of Medicine |
| Cell line (M. musculus) | C57BL/6 Mouse Primary Retinal Microvascular Endothelial Cells | Cell Biologics | C57-6065 | |
| Cell line (H. sapiens) | Primary Human Retinal Microvascular Endothelial Cells | Cell Systems | ACBRI 181 | |
| Antibody | anti IL-4 (rat monoclonal) | Biolegend | Cat. #: 504108, RRID:AB_315322 | IHC(1:200) |
| Antibody | anti IL-4 (rabbit polyclonal) | abcam | Cat. #: ab9622, RRID:AB_308736 | IHC(1:200) |
| Antibody | anti Phospho-Tyr497 IL-4R/CD124 (rabbit polyclonal) | Assay Biotechnology Company | Cat. #: A1064, RRID:AB_10683571 | IHC(1:200) |
| anti CD124 (rat monoclonal) | BD Pharmingen | Cat. #: 552288, RRID:AB_394356 | 0.1–10 ng/ mL TVI | |
| Antibody | anti IL-13 receptor alpha 1 (rabbit polyclonal) | abcam | Cat. #: ab-79277, RRID:AB_1640587 | IHC(1:200) |
| anti IL13 antibody (rabbit polyclonal) | GeneTex, Inc | Cat. #: GTX59763, | 0.1–10 ng/ mL TVI | |
| Antibody | anti CD11b (rat monoclonal) | eBioscience | Cat. #: 14-0112-82, RRID:AB_467108 | IHC(1:200) |
| Antibody | anti CD11b (rat monoclonal) Alexa Fluor 594 | Biolegend | Cat. #: 101254, RRID:AB_2563231 | IHC(1:100) |
| Antibody | CD11b (M1/70) (rat monoclonal) FITC | eBioscience | Cat. #: 11-0112-41, RRID:AB_11042156 | IHC(1:100) |
| Antibody | anti CCR2 (rabbit polyclonal) DyLight 550 | Novus Biologicals | Cat. #: NBP1-48338R | IHC(1:100) |
| Antibody | anti MCP-1 (hamster monoclonal) | Biolegend | Cat. #: 505906, RRID:AB_2071552 | IHC(1:200) |
| Antibody | anti Iba1 (rabbit polyclonal) | FUJIFILM Wako Pure Chemical Corporation | Cat. # 019–19741, RRID:AB_839504 | IHC(1:200) |
| Antibody | anti CD31 (rabbit polyclomal) | abcam | Cat. #: ab28364, RRID:AB_726362 | IHC(1:200) |
| Antibody | anti-mouse CD31(rat monoclonal) Alexa Fluor 647 | Biolegend | Cat. #: 102516, RRID:AB_2161029 | IHC(1:100) |
| Antibody | anti rat IgG (goat polyclonal) Brilliant Violet 421 | Biolegend | Cat. #: 405414, RRID:AB_10900808 | IHC(1:100) |
| Antibody | anti rabbit IgG (goat polyclonal) DyLight 488 | Vector Laboratories | Cat. #: DI-1488, RRID:AB_2336402 | IHC(1:100) |
| Antibody | anti rabbit IgG (donkey polyclonal) Alexa Fluor 555 | Biolegend | Cat. #: 406412, RRID:AB_2563181 | IHC(1:100) |
| Antibody | anti rabbit IgG (goat polyclonal) HiLyte Fluor 555 | AnaSpec | Cat. #: AS-61056–05 H555 | IHC(1:100) |
| Antibody | anti hamster IgG (goat polyclonal) DyLight 594 | Biolegend | Cat. #: 405504, RRID:AB_1575119 | |
| Antibody | anti rabbit IgG (goat polyclonal) PE | Santa Cruz Biotechnology | Cat. #: sc-3739, RRID:AB_649004 | IHC(1:100) |
| Antibody | anti rabbit IgG (donkey polyclonal) Alexa Fluor 647 | abcam | Cat. #: ab150075, RRID:AB_2752244 | IHC(1:100) |
| Antibody | anti rabbit IgG (donkey polyclonal) DyLight 649 | Biolegend | Cat. #: 406406, RRID:AB_1575135 | IHC(1:100) |
| Antibody | anti mouse IgG2A (rat monoclonal) | R and D | Cat. #: mab006, RRID:AB_357349 | 0.1–10 ng/ mL TVI |
| Antibody | anti mouse Fc gamma RII/RIII (CD32/CD16)(goat polyclonal) | R and D | Cat. #: AF1460-SP, Accession # P08101 | IHC (0.2 μg/mL) |
| Peptide, recombinant protein | recombinant murine IL-4 | R and D | Cat. #: 404 ML | |
| Peptide, recombinant protein | recombinant human IL-4 | Peprotec | Cat. #: AF-200–04 | |
| Peptide, recombinant protein | recombinant murine IL-13 | Peprotec | Cat. #: 210–13 | |
| Chemical compound, drug | ZM306416 hydrochloride | abcam | Cat. #: ab144576 | |
| Chemical compound, drug | ZM323881 hydrochloride | R and D | Cat. #: 2475/1 | |
| Chemical compound, drug | bovine serum albumin | Sigma-Aldrich | Cat. #: A2153 | |
| Chemical compound, drug | fetal bovine serum | Sigma-Aldrich | Cat. #: 12103C | |
| Chemical compound, drug | Medetomidine | Chemscene LLC | Cat. #: 86347-14-0 | |
| Chemical compound, drug | Butorphanol tartrate | FUJIFILM Wako Pure Chemical Corporation | Cat. #: 58786-99-5 | |
| Chemical compound, drug | Midazolam | FUJIFILM Wako Pure Chemical Corporation | Cat. #: 59467-70-8 | |
| Chemical compound, drug | Tropicamide, Phenylephrine Hydrochloride | Santen Pharmaceuitical Co., Ltd. | Cat. #: 1319810Q1053 | |
| Chemical compound, drug | Hydroxy methyl cellulose | SENJU Pharmaceutical Co.,Ltd | Cat.#: 131980AQ1038 | |
| Chemical compound, drug | RNAlater solution | Ambion | Cat. #: AM7021 | |
| Chemical compound, drug | Triton X-100 | Sigma-Aldrich | Cat. #: X100 100ml | |
| Chemical compound, drug | Tween 20 | Sigma-Aldrich | Cat. #: P1379-100ml | |
| Chemical compound, drug | Paraformadehyde | Electron Microscopy Science | Cat. #: 15710 | |
| Commercial assay or kit | ISOLECTIN B4 Fluorescein | Vector Laboratories | Cat. #: FL-1201, RRID:AB_2314663 | IHC(1:100) |
| Commercial assay or kit | ISOLECTIN B4 DyLight 594 | Vector Laboratories | Cat. #: FL-1207 | IHC(1:100) |
| Commercial assay or kit | DAPI | Roche Diagnostics | Cat. #: 10 236 276 001 | 1 μg/ml |
| Commercial assay or kit | TO-PRO-3 iodide | Molecular Probes, Inc | Cat. #: T-3605 | IHC(1:100) |
| Commercial assay or kit | VECTASHIELDAntifade Mounting Medium | Vector Laboratories | Cat. #: H-1000, RRID:AB_2336789 | |
| Commercial assay or kit | Fluorescence Mounting Medium | DAKO | Cat. #: 15710 | |
| Commercial assay or kit | SurePrint G3 Mouse GE 8 × 60K Microarray | Agilent Technologies | Cat. #: AGLMO002 | |
| Commercial assay or kit | QuantiTect Reverse Transcription Kit | Qiagen | Cat. #: 205311 | |
| Commercial assay or kit | QuantiTect SYBR Green PCR kit | Qiagen | Cat. #: 204143 | |
| Commercial assay or kit | ELISA kits | ThermoFisher Scientific | Cat. #: BMS6005, EMFLT1 | |
| Commercial assay or kit | PKH26 Red Fluorescent Cell Linker Kit for General Cell Membrane Labeling | Sigma-Aldrich | Cat. #: PKH26GL-1KT | |
| Commercial assay or kit | RNeasy Mini Kit | Qiagen | Cat. #: 74104 | |
| Commercial assay or kit | DMEM:F12 | ThermoFisher Scientific | Cat. #: 11330057 | |
| Commercial assay or kit | recombinant human GMCSF | Peprotec | Cat. #: AF-300–03 | |
| Commercial assay or kit | recombinant murine GMCSF | Peprotec | Cat. #: 315–03 | |
| Software, algorithm | Ingenuity Pathway Analysis, March 2020 | Qiagen | RRID:SCR_008653 | |
| Software, algorithm | Adobe Photoshop CS5, Version 12.0.5 | Adobe | RRID:SCR_014199 | |
| Software, algorithm | STATA 16.1 | StataCorp LLC | RRID:SCR_012763 | |
| Software, algorithm | GeneSpring Software | Agilent Technologies | RRID:SCR_009196 |
Additional files
-
Supplementary file 1
Sequences of primer pairs used in quantitative reverse-transcription polymerase chain reaction.
- https://cdn.elifesciences.org/articles/54257/elife-54257-supp1-v1.xls
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/54257/elife-54257-transrepform-v1.docx





