Pull-push neuromodulation of cortical plasticity enables rapid bi-directional shifts in ocular dominance
Figures

Pairing monocular stimulation with agonist for Gq- or Gs-receptors induce rapid and stable bidirectional changes of ocular dominance.
(A) Experimental schematic. Left: The binocular segment of V1 in one hemisphere was imaged on day 1 to determine responses to ipsi and contralateral eye stimulation. Middle: 24 hr later the contralateral eye was stimulated with high contrast drifting gratings of 16 orientations for 1 hr starting 30 min after the administration of neuromodulator agonist (isoproterenol, 15 mg/kg, i.p.; Ro 60–0175, 10 mg/kg, i.p). Right: after 24 hr in the dark V1 was imaged again. (B,C) Pairing with the 5HT2c agonist Ro 60–0175 specifically reduces the response to the stimulated eye and reduces the contralateral bias. (B) Example experiment. Left: vasculature pattern of the imaged region used for alignment. Scale bar, 1 mm. Middle: magnitude map of the visual response from the eye contralateral [C] or ipsilateral [I] to the imaged hemisphere. Gray scale (bottom): response magnitude as fractional change in reflection x104. Arrows: L, lateral, R, rostral. Right: histogram of ocular dominance index (ODI) in illustrated in the number of pixels (x-axis: ODI, y-axis: number of pixels) (C) Summary of changes in response amplitude of the conditioned contralateral eye (left) and the non-conditioned ipsilateral eye (middle) as well as the change of ODI (right) before (b) and after (a) conditioning. Thin line: individual experiments; thick line and symbols: average ± s.e.m. (D,E) Pairing with the β-adrenergic agonist isoproterenol enhances the response to the stimulated eye and the contralateral bias. Example experiment in D, summary results in E, conventions as in B, C. (E,F) Visual stimulation alone does not affect either the visual response or the contralateral bias. Example experiment in E, summary results in F, conventions as in B,C.
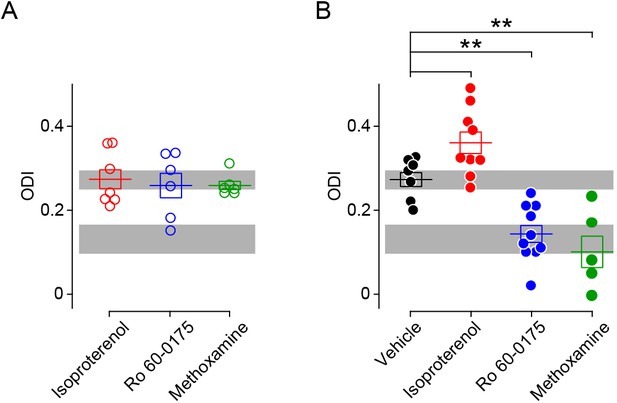
Comparison of ODIs in various conditioning conditions.
(A) In the absence on visual stimulation injection of neuromodulator agonists does not affect the ODI conditioning. (One-way ANOVA, F2,16 = 0.172, p>0.05). Gray box indicates 95% confidential interval of the ODI values from naive mice before conditioning (from Figure 1; n = 26). (B) Summary of ODI values after pairing visual stimulation with of neuromodulator agonists. Resulting ODI values were either higher (isoproterenol, red filled circle) or lower (Ro 60–0175, blue filled circle; methoxamine, green filled circle) than the ODI after visual conditioning paired with vehicle (black filled circle). (One-way ANOVA, F3,27 = 19.74, p<0.001; Post hoc Holm-Sidak t-test, *p<0.05, **p<0.01 compared to vehicle group). Shade of gray indicates 95% confidential interval of the ODI values from the juvenile mice (p31–p35) whose contralateral eye were closed by eyelid suture for three days (n = 7). Error bar: averages ± s.e.m.
-
Figure 1—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/54455/elife-54455-fig1-figsupp1-data1-v1.xlsx
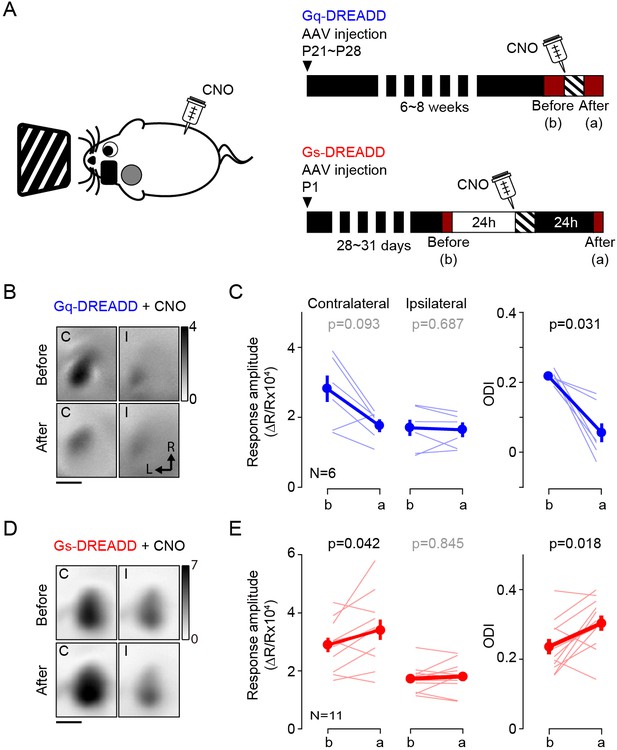
Activation of Gq or Gs-DREADDs enables rapid bidirectional changes of ocular dominance with visual stimulation.
(A) The schematics of the conditioning. One hour of monocular stimulation 30 min after CNO injection (10 mg/kg) is shown on the left; the experimental time schedule for the viral injections, conditioning and optical imaging is on the right. For Gq-DREADDs (upper right) AAVs encoding neuronal Gq-DREADDs was injected into V1 of C57BL/6J mice at P21-28, and 4–6 weeks later visual responses were imaged before and after conditioning. For Gs-DREADDs (lower right) AAVs encoding hSyn-Cre were injected at postnatal day one into the posterior region of left hemisphere of Cre-dependant Gs-DREADDs mice, and visual responses were imaged between P28 to P33, a day before and after the conditioning. (B,C) In Gq-DREADDs expressing mice pairing visual stimulation with the agonist CNO selectively depresses the responses to the stimulated eye and reduces the ODI. An example experiment showing visual responses imaged before (upper panels) and after (lower panels) conditioning with activation is presented in B). Each panel represents the magnitude map of the visual response from the eye contralateral (left) or ipsilateral (right) to the imaged hemisphere are illustrated. Gray scale (upper right) shows response magnitude as fractional change in reflection x104. Scale bar, 1 mm. Arrows: L, lateral, R, rostral. (C) Summary of changes in response amplitude of the conditioned contralateral eye (left) and the non-conditioned ipsilateral eye (middle) as well as the change of ODI (right) before (b) and after (a) conditioning. Thin line: individual experiments; thick line and symbols: average ± s.e.m. (D,E) In Gs-DREADDs expressing mice pairing visual stimulation with the agonist CNO selectively potentiates the responses the responses to the stimulated eye and increases the ODI. Example experiment in D, summary results in E, conventions in B,C.
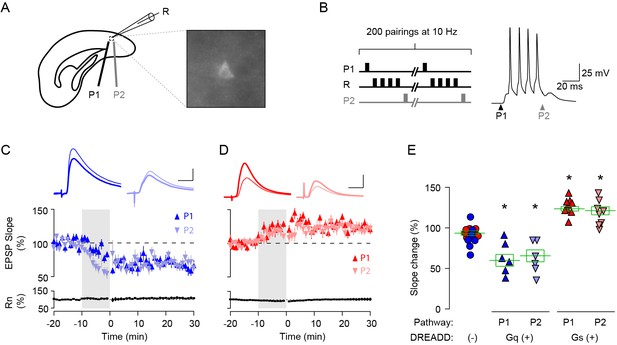
Pull-push regulation of LTP and LTD by activation of Gq- or Gs-coupled DREADD in L2/3 pyramidal neurons in V1.
(A) Experimental schematics. Whole-cell patch clamp in DREADD expressing L2/3 pyramidal neuron. Synaptic responses were recorded in labelled pyramidal cells (R) and evoked with two stimulating electrodes P1 and P2. (B) STDP conditioning paradigm. In one pathway, presynaptic stimulation preceded a burst of 4 postsynaptic spikes by 10 ms (P1: pre-post); in the other pathway, it occurred 10 ms after the burst (P2: post-pre). A burst of postsynaptic spikes was evoked by injecting four consecutive suprathreshold square-wave current pulse (2 ms, 100 Hz). (C,D) In L2/3 neurons expressing Gq- (C) or Gs- (D) coupled DREADD, STDP conditioning in the presence of CNO (10 µM) induced LTD (C) or LTP (D) in both pre-post (upward triangle) and post-pre pathways (downward triangle). Traces are averages of 30 EPSPs recorded before the perfusion of CNO (thin line) and at 20–30 min from the STDP conditioning (thick line). Plotted data: averages ± s.e.m. Scale, 10 ms, 2 mV in (C), 10 ms, 1 mV in (D). (F) Summary of STDP of Gq- or Gs-coupled DREADD expressing L2/3 neurons. Each data point indicates the mean of normalized slope after 20–30 min from the STDP conditioning. Data for the DREADD negative control group were pulled from P1 and P2 responses of non-fluorescence expressing neurons of Gq-(blue circles) and Gs-(red circles) DREADD virus injected animals. (One-way ANOVA, F4,40 = 29.11, p<0.001; Post hoc Holm-Sidak t-test, *p<0.05 compared to DREADD(-) group). Error bar: averages ± s.e.m.
-
Figure 2—figure supplement 1—source data 1
- https://cdn.elifesciences.org/articles/54455/elife-54455-fig2-figsupp1-data1-v1.xlsx
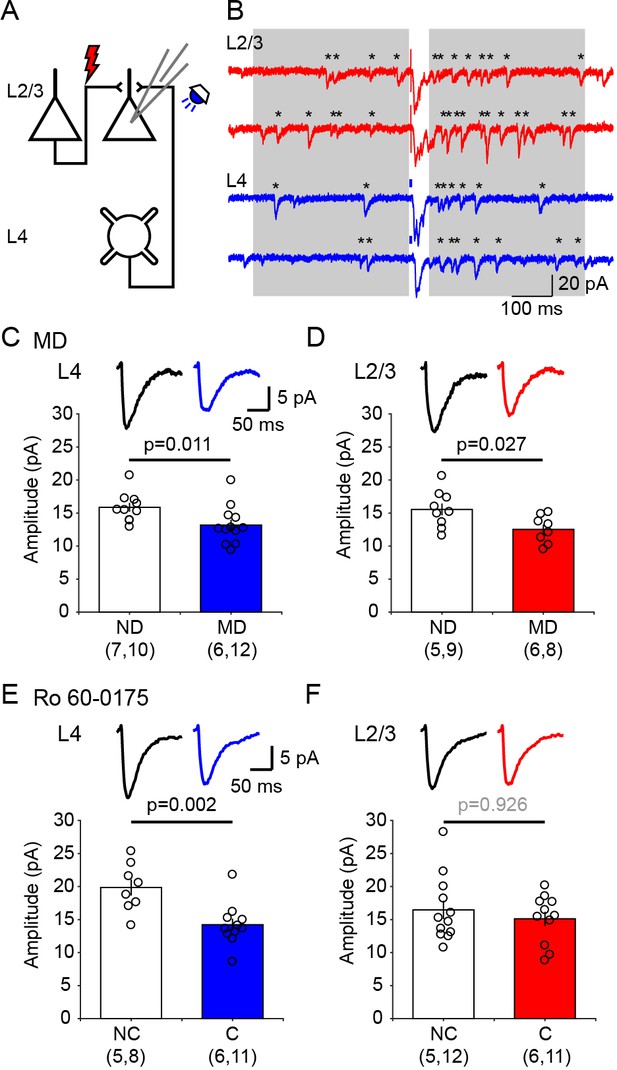
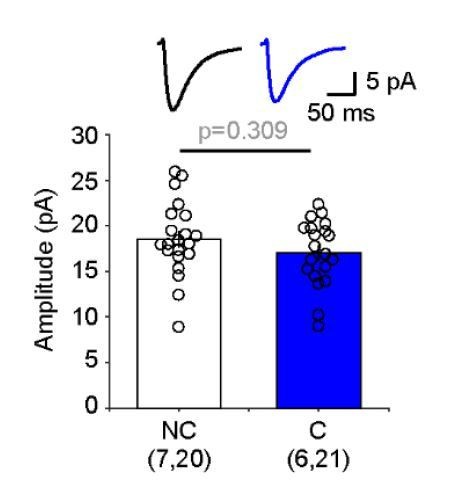
Ex vivo assessment of intracortical pathways modified by visual conditioning with Ro 60–0175 and by monocular deprivation (MD).
(A) Experimental schematics. Synaptic responses were recorded in L2/3 pyramidal cells and evoked electrically with an electrode placed 100–200 µm laterally to activate L2/3 inputs, and optogenetically with whole field flash stimulation (5 ms, 470 nm or white LED) to activate L4 inputs. (B) Example experiment showing Sr2+-induced desynchronization of responses to stimulation of L2/3 inputs (red traces) and L4 inputs (blue traces). Grey boxes indicate the time windows used to compute average evoked events. Individual events indicated with asterisks. (C, D) MD for 3 days reduced the average amplitude of quantal events evoked from both L4 (C) and L2/3 (D) inputs. The graphs show the computed average amplitude events in cells from MD mice and age-matched non-deprived mice cell. Traces on top are grand averages of events computed in all cells from all mice in the indicated condition. (E, F) Pairing monocular stimulation with Ro 60–0175 reduced the average amplitude of quantal events evoked from L4 (E) inputs, but not from L2/3 (F) inputs. Graphs and traces as in C, D. In parenthesis in (C–F) is the number of mice and cells. Experimenters were blind to the identity of the hemisphere recorded.
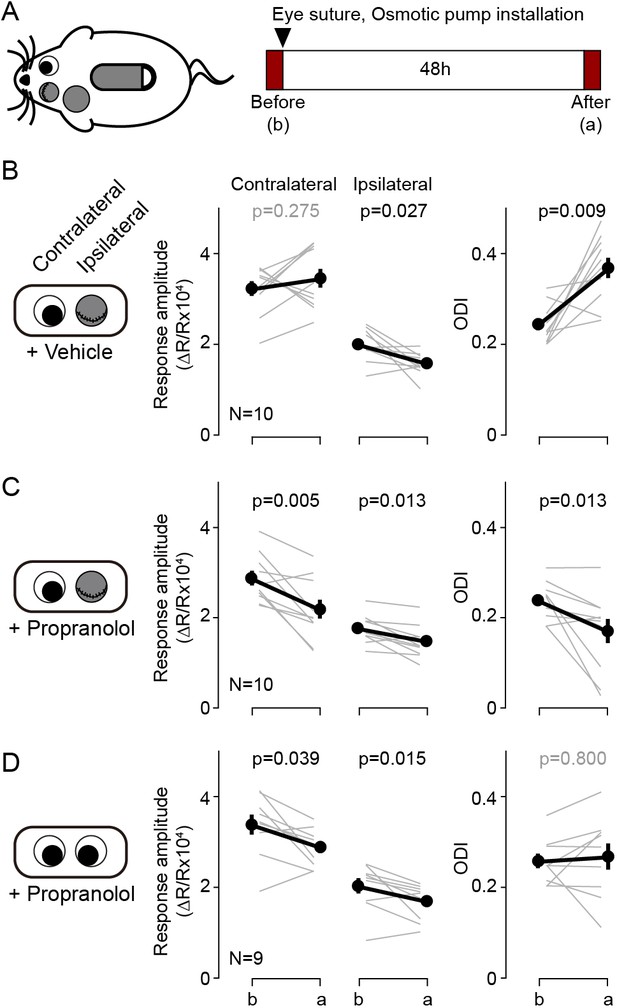
Blockade of β-adrenergic receptor during short-term monocular deprivation induces paradoxical depression of the open eye.
(A) Experimental schematics. At P28-31, the eye ipsilateral to the imaged hemisphere was closed by eyelid suture for two days (short-term MD). An osmotic pump filled with β-adrenergic receptor antagonist propranolol was installed subcutaneously shortly before the eye suture. Visual responses from each eye were imaged immediately before and after the short-term MD. Note that deprivation and imaging are done in the same side of mice, not in opposite sides as in all other figures. (B) In mice infused with vehicle, MD induced a selective depression of responses to the deprived ipsilateral eye, no changes in the responses to the open contralateral eye and a concomitant increase in the ODI. (C) In mice infused with the propranolol, MD depressed both responses and decreased the ODI. (D) Propranolol infusion in non-deprived mice causes a modest depression of both ipsi- and contralateral responses, but without altering the ODI. Thin lines in B-D represent results from individual experiments, thick lines and symbols represent averages ± s.e.m.
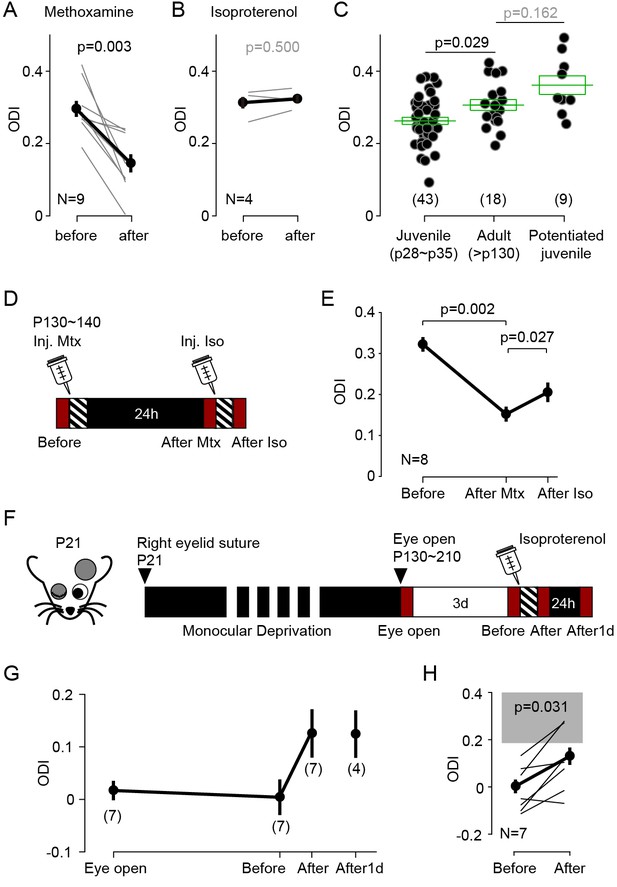
Neuromodulator agonists enable rapid bidirectional changes of ocular dominance after critical period.
(A) In adult mice (>P130) pairing monocular stimulation with systemic injection of the α-adrenergic receptor (Gq-coupled) agonist methoxamine induced a robust decrease of ODI. (B) The same conditioning, but paired with the β-adrenergic agonist isoproterenol, failed to change the ODI in adult mice. (C) In naïve adult mice ODI values are larger than ODI values of naïve juvenile mice and comparable to the ODI values in isoproterenol-enabled potentiation juvenile mice (One-way ANOVA, F2,67 = 7.043, p<0.01; Post hoc Holm-Sidak t-test). Filled circles represent data from individual mice; green boxes represent averages ± s.e.m. (D) Schematics of the experiments testing the effects of conditioning with isoproterenol (Iso) in adult mice preconditioned with methoxamine pairing (Mtx) the day before. (E) Changes in ODI one day after pairing with (After Mtx) and after pairing with Isoproterenol (After Iso). (F) Schematics of the experiments testing the effects of conditioning with isoproterenol in mice subjected to long-term MD (LTMD). The eyelids contralateral to the tested hemisphere were sutured at P21 and re-opened after P130 and the visual responses were imaged immediately (Eye open). Following 3 days of normal rearing (Before), after the visual conditioning to the deprived eye (After), and after a day in dark from the visual conditioning (After1d). (G) Changes in ODI in LTMD mice induced visual conditioning with isoproterenol. In parenthesis is the number of animals tested at each time point. (H) Expansion of panel E detailing the individual changes in ODI induced by the pairing conditioning. Gray box indicates 95% confidential interval of ODI values for the age matched naïve mice.
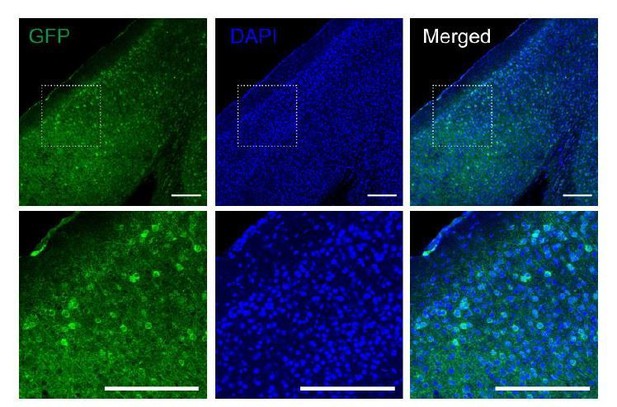
Fluorescence images of V1 area from the Gs-DREADDs mouse injected with rAAV-hSyn-Cre.
Slices were immunostained for GFP to visualize the Gs-DREADDs expressing neurons. Dotted lined squares indicate the region magnified at the bottom. Scale bar, 200 µm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | C57BL/6J | Jackson Laboratories | RRID:IMSR_JAX:000664 | |
| Strain, strain background (Mus musculus) | B6;C3-Tg(Scnn1a- cre)3Aibs/J | Jackson Laboratories | RRID:IMSR_JAX:009613 | |
| Strain, strain background (Mus musculus) | B6.Cg-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J | Jackson Laboratories | RRID:IMSR_JAX:012569 | |
| Strain, strain background (Mus musculus) | ROSA26-LSL-GsDREADD-CRE-luc | PMID:28167604 | ||
| Strain, strain background (AAV) | AAV2-CamKIIa-HA-rm3D(Gq)-IRES-mCitrine | UNC Vector Core | ||
| Strain, strain background (AAV) | AAV8-CamKIIa-HA-rm3D(Gs)-IRES-mCitrine | UNC Vector Core | ||
| Strain, strain background (AAV) | rAAV-hSyn-Cre | SignaGen Laboratories | Cat#: SL100888 | |
| Chemical compound, drug | chlorprothixene | Sigma-Aldrich | Cat#: C1671; CAS: 6469-93-8 | |
| Chemical compound, drug | isoflurane | Patterson Veterinary | Cat#: 07-890-8115 | |
| Antibody | anti-GFP antibody, (Chicken, polyclonal) | Aves Labs | Cat#: GFP-1020 RRID:AB_2307313 | 1:2000 |
| Antibody | Alexa 488 conjugated anti-chicken IgY (Goat, monoclonal) | Abcam | Cat#: ab150169 RRID:AB_2636803 | 1:400 |
| Chemical compound, drug | Isoproterenol | Tocris Bioscience | Cat#: 1747 | |
| Chemical compound, drug | Ro 60–0175 | Tocris Bioscience | Cat#: 1854 | |
| Chemical compound, drug | CNO | Enzo Life Sciences | Cat#: BML-NS105-0005 | |
| Chemical compound, drug | propranolol | Sigma-Aldrich | Cat#: P0884 | |
| Chemical compound, drug | nadolol | Sigma-Aldrich | Cat#: N1892 | |
| Chemical compound, drug | methoxamine | Sigma-Aldrich | Cat#: M6524 | |
| Chemical compound, drug | DAPI | Life technologies | Cat#: D-1306 | |
| Commercial assay or kit | Alzet 1007D | DURECT Corporation | Cat#: 0000290 | |
| Software, algorithm | MATLAB | Mathworks | RRID:SCR_001622 | |
| Software, algorithm | Prism | GraphPad Software | RRID:SCR_002798 | |
| Software, algorithm | Igor Pro | Wavemetrics | RRID:SCR_000325 | |
| Software, algorithm | Mini Analysis Software | Synaptosoft | RRID:SCR_002184 | |
| Other | CCD camera | Dalsa | Model#: DS-1A-01M30-12E | |
| Other | ProLong Gold antifade | Life Technologies | Cat#: P36930 |