MARCH5 mediates NOXA-dependent MCL1 degradation driven by kinase inhibitors and integrated stress response activation
Figures
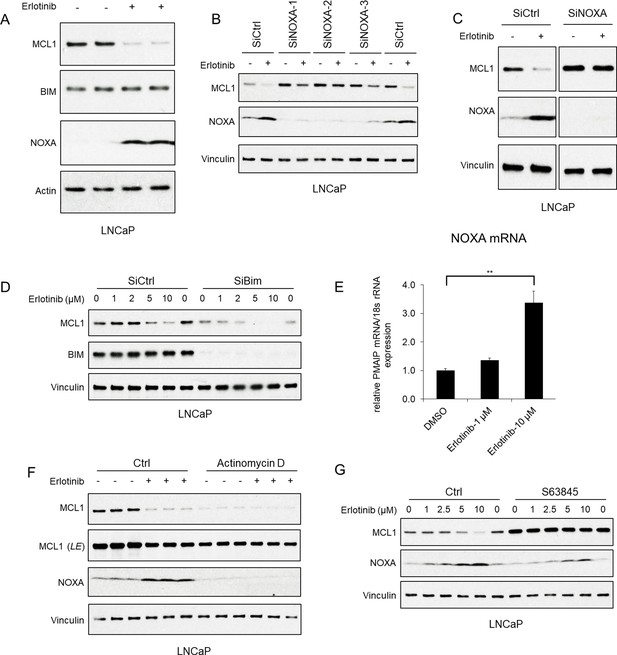
EGFR inhibition decreases MCL1 via NOXA-dependent mechanism.
(A) LNCaP cells were treated with EGFR inhibitor erlotinib (10 μM) for 3 hr, followed by immunoblotting. (B) LNCaP cells were transfected with three distinct NOXA siRNA or non-target control siRNA for 3 days, then were treated with erlotinib (10 μM) for 3 hr. (C) LNCaP cells were transfected with pooled NOXA siRNAs or non-target control siRNA for 3 days, then were treated with erlotinib (10 μM) for 5 hr. (D) LNCaP cells transfected with pooled BIM siRNAs or non-target control siRNA were treated with erlotinib (0–10 μM) for 3 hr. (E) LNCaP cells were treated with erlotinib (0–10 μM) for 2 hr, followed by NOXA (PMAIP) mRNA measurement by qRT-PCR. Data reflect biological triplicates with each mRNA sample assayed in duplicate (technical replicate). 18 s rRNA was used as an internal control. (**, p<0.01). (F) LNCaP cells were pretreated with RNA synthesis inhibitor actinomycin D (10 μg/ml) for 30 min, followed by treatment with erlotinib (10 μM) for 3 hr. LE, long exposure. (G) LNCaP cells were pretreated with MCL1 inhibitor S63845 (500 nM) for 3 hr, followed by treatment with erlotinib (0–10 μM) for 3 hr. Immunoblots are representative of results obtained in at least three independent experiments.
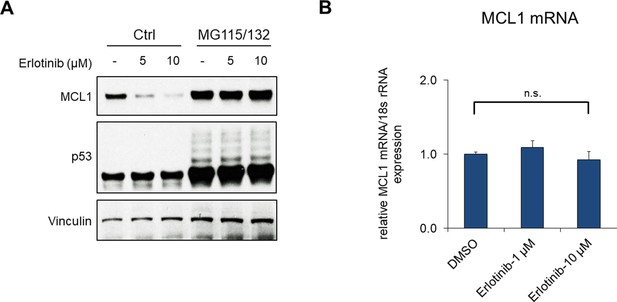
EGFR inhibition increases proteasome-dependent MCL1 degradation.
(A) LNCaP cells were pretreated with MG115 (10 μM) and MG132 (10 μM) for 30 min, followed by treatment with erlotinib for 4 hr. (B) LNCaP cells were treated with DMSO or erlotinib for 2 hr, followed by MCL1 mRNA measurement by qRT-PCR. 18 s rRNA was used as an internal control (n.s., not significant).
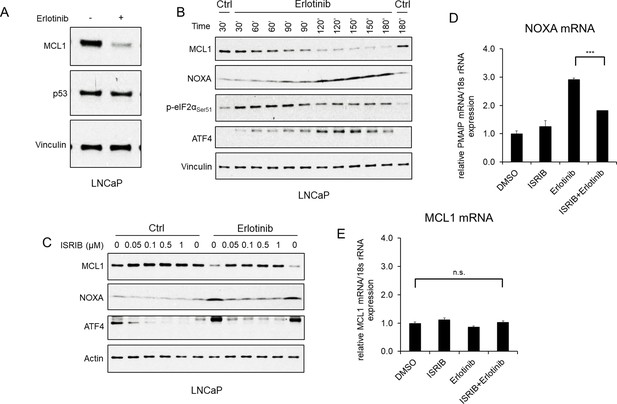
EGFR inhibition upregulates NOXA through ISR activation.
(A) LNCaP cells were treated with erlotinib (10 μM) for 3 hr, followed by immunoblotting. (B) LNCaP cells were treated with erlotinib (10 μM) at time 0 and were harvested over a time course from 30 to 180 min. (C) LNCaP cells were treated with ISR inhibitor ISRIB trans-isomer (0–1 μM) for 1 hr, followed by treatment with erlotinib (10 μM) for 3 hr. The weak band migrating just above the major ATF4 band was proportional to the major band and may reflect a posttranslational modification. (D and E) LNCaP cells were pretreated with ISRIB trans-isomer (100 nM) or DMSO for 1 hr, followed by erlotinib (10 μM) or DMSO for 2 hr. NOXA (PMAIP) mRNA (D) and MCL1 mRNA (E) were measured by qRT-PCR. Data reflect biological triplicates with each mRNA sample assayed in duplicate (technical replicate). 18 s rRNA was used as an internal control. (n.s., not significant; ***, p<0.001). Immunoblots in (A) and (C) are representative of results obtained in three independent experiments, and (B) is representative of two independent experiments.
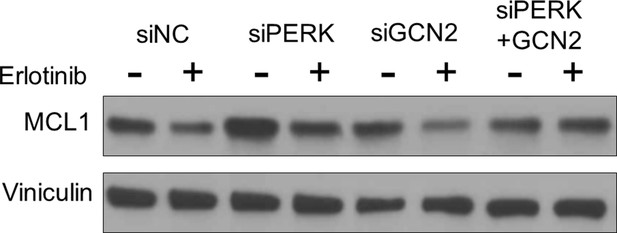
LNCaP cells were transfected with siRNA pools targeting PERK, GCN2, the combined PERK and GCN2 pools, or a nontarget control siRNA (siNC).
At 72 hr after transfection, cells were treated for 4 hr with Erlotinib (5 mM) or vehicle, followed by immunoblotting.
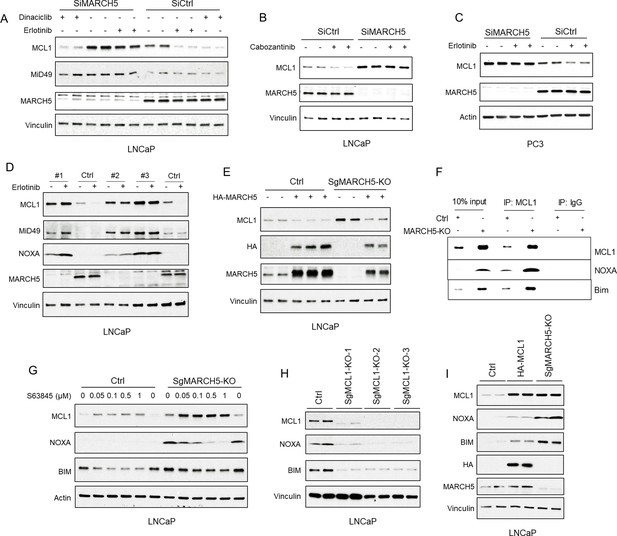
Tyrosine kinase inhibitors decrease MCL1 via mitochondria-associated E3 ligase MARCH5.
(A) LNCaP cells transfected with pooled MARCH5 siRNAs or non-target control siRNA were treated with DMSO, erlotinib (10 μM), or dinaciclib (200 nM) for 4 hr. (B) LNCaP cells transfected with pooled MARCH5 siRNAs or non-target control siRNA were treated with multi-kinase inhibitor cabozantinib (5 μM) for 5 hr. (C) PC3 cells transfected with pooled MARCH5 siRNAs or non-target control siRNA were treated with erlotinib (10 μM) for 4 hr. (D) Three MARCH5-deficient LNCaP subclones generated with CRISPR/CAS9 and guide RNAs (SgMARCH5-KO #1–3), and one negative control clone (Ctrl), were treated with erlotinib (10 μM) for 4 hr. (E) SgMARCH5-KO or control LNCaP cells were transiently transfected with HA-tagged MARCH5, followed by immunoblotting. (F) Cell lysates of SgMARCH5-KO or control LNCaP with same protein amounts were subject to immunoprecipitation using anti-MCL1 rabbit antibody or control rabbit IgG with protein A agarose, followed by immunoblotting with mouse antibodies targeting for indicated proteins. (G) SgMARCH5-KO or control LNCaP cells were treated with S63845 (0–1 μM) for 12 hr. (H) Three MCL1-deficient LNCaP subclones generated with CRISPR/CAS9 and guide RNAs (MCL1-KO-1–3) or one negative control clone (Ctrl) were lysed and were immunoblotted for indicated proteins. (I) SgMARCH5-KO, HA-tagged MCL1 overexpressing (HA-MCL1), or control LNCaP cells were lysed and immunoblotted for indicated proteins. Immunoblot in B is representative of results obtained in two independent experiments, and the remainder are representative of at least three independent experiments.
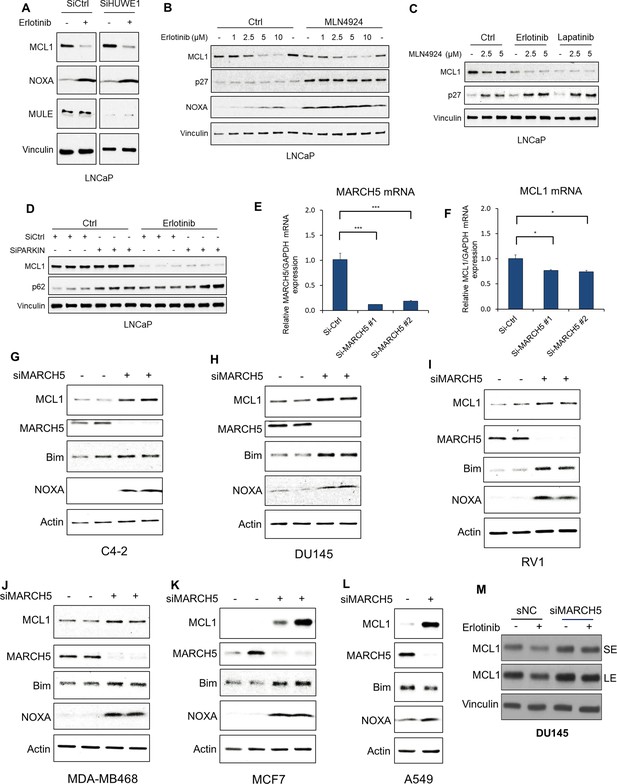
MARCH5 knockdown increases MCL1 in additional PCa, breast, and lung cancer cell lines.
(A) LNCaP cells transfected with pooled HUWE1 (MULE) siRNAs or non-target control siRNA were treated with erlotinib (10 μM) for 5 hr, followed by immunoblotting. (B) LNCaP cells were pretreated with NEDD8 inhibitor MLN4924 (2.5 μM) for 1 hr, followed by treatment with erlotinib (0–10 μM) for 4 hr. Efficacy of NEDD8 block by MLN4924 was confirmed by blotting for p27. (C) LNCaP cells were pretreated with MLN4924 (0–5 μM) for 1 hr, followed by treatment with DMSO, erlotinib (10 μM), or EGFR/ERBB2 inhibitor lapatinib (10 μM) for 3 hr. (D) LNCaP cells transfected with pooled PARKIN siRNAs or non-target control siRNA were treated with erlotinib (10 μM) for 4 hr.(E and F) LNCaP cells were transfected with MARCH5 pooled siRNAs (#1, Dharmacon), an individual siRNA (#2, Fisher) or non-target control. MARCH5 mRNA (E) and MCL1 mRNA (F) were measured by qRT-PCR. GAPDH was used as an internal control. (*, p<0.05; ***, p<0.001). (G–L) C4-2 (G), DU145 (H) and RV1 (I) prostate cancer cells, MDA-MB468 (J) and MCF7 (K) breast cancer cells, and A549 lung cancer cells (L) were transfected with MARCH5 siRNA, followed by immunoblotting. (M) DU145 cells were transfected with siMARCH5 or noncoding siRNA (siNC) for 3 days, and then stimulated with erlotinib (5 mM) for 4 hr. SE, short exposure; LE, long exposure.
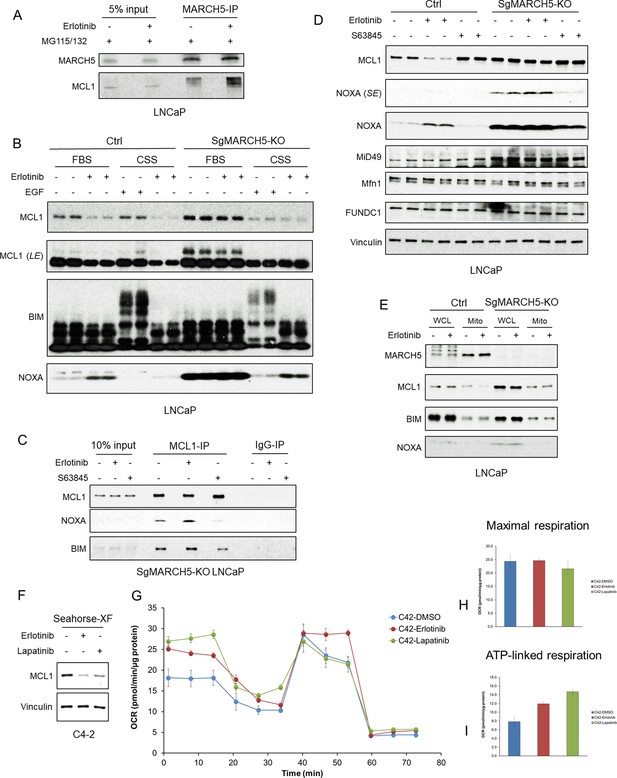
EGFR inhibition enhances MARCH5-MCL1 interaction without altering MARCH5 activity.
(A) LNCaP cells were pretreated with proteasome inhibitors MG115 (10 μM) and MG132 (10 μM) for 1 hr, followed by treatment with erlotinib (10 μM) or DMSO for 3 hr. The cell lysates were subject to immunoprecipitation using anti-MARCH5 rabbit antibody with protein A agarose, followed by immunoblotting with anti-MARCH5 rabbit antibody or anti-MCL1 mouse antibody. The decreased mobility of MCL1 in the IP lanes may reflect impaired migration due to large amounts of Ig in the sample, but phosphorylation or other posttranslational modification is possible. (B) SgMARCH5-KO or control LNCaP cells were pre-incubated in normal serum medium (FBS) or charcoal-stripped serum medium (CSS) for 1 day, followed by treatment with erlotinib (10 μM) for 3 hr or EGF (100 ng/ml) for 30 min. LE, long exposure. (C) SgMARCH5-KO LNCaP cells were treated with erlotinib (10 μM), S63845 (0.5 μM), or DMSO for 3 hr. The cell lysates were immunopurified with anti-MCL1 rabbit antibody or control rabbit IgG and protein A agarose, followed by immunoblotting with mouse antibodies targeting for indicated proteins. (D) SgMARCH5-KO or control LNCaP cells were treated with erlotinib (10 μM), S63845 (500 nM), or DMSO for 3 hr. SE, short exposure. (E) SgMARCH5-KO or control LNCaP cells were treated with erlotinib (10 μM) for 2 hr. Proteins extracted from whole cell lysates (WCL) or isolated mitochondria (Mito) were analyzed by western blot. WCL, whole cell lysate. Mito, mitochondrial fraction. (F) LNCaP-derived C4-2 cells were incubated in Seahorse XF medium and treated with erlotinib (10 μM) or lapatinib (10 μM) for 4 hr. (G–I) C4-2 cells were treated with erlotinib (10 μM), lapatinib (10 μM), or DMSO for 3 hr, and maximal oxygen consumption rate (H) and ATP-linked oxygen consumption rate (I) were analyzed by a mitochondria stress test (G). Data in G-I are mean and standard deviation from three independent experiments. Immunoblot in (B) is representative of results obtained in two independent experiments, and the remainder are representative of at least three independent experiments.
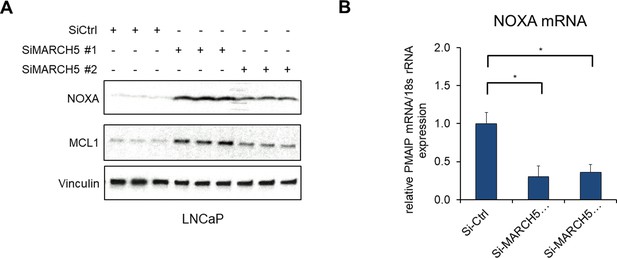
MARCH5 depletion increases NOXA protein.
(A and B) LNCaP cells were transfected with MARCH5 pooled siRNAs (#1, Dharmacon), an individual siRNA (#2, Fisher), or non-target control, followed by western blot (A) or qRT-PCR (B). (*, p<0.05).
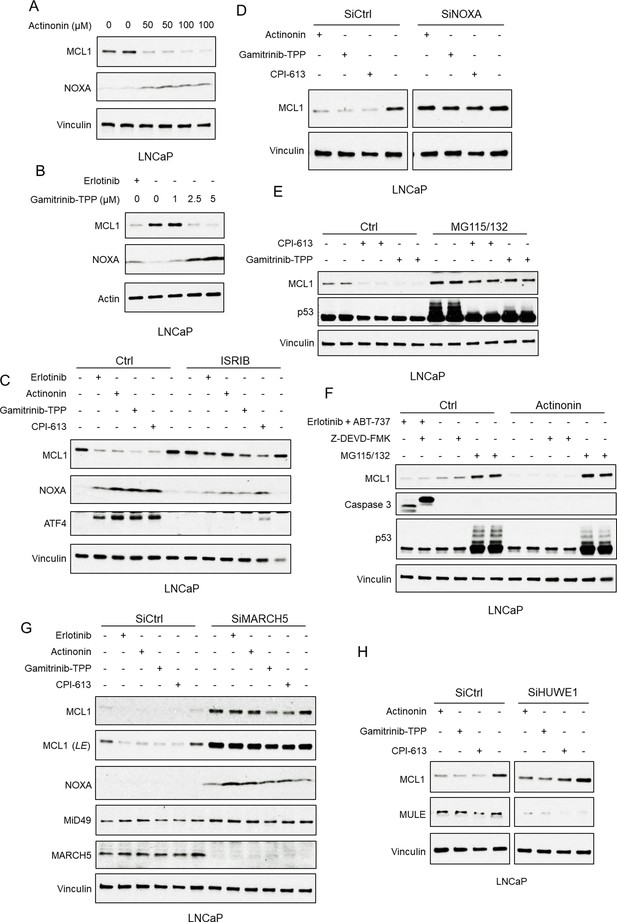
Mitochondria-targeted agents upregulate NOXA and induce MARCH5-dependent MCL1 degradation.
(A) LNCaP cells were treated with human mitochondrial translation inhibitor actinonin (0–100 μM) for 5 hr, and whole cell lysates were then assessed by immunoblotting. (B) LNCaP cells were treated with mitochondrial HSP90 inhibitor gamitrinib-TPP (0–5 μM) or erlotinib (10 μM) for 9 hr. (C) LNCaP cells were pretreated with ISRIB trans-isomer (1 μM) for 1 hr, followed by treatment with erlotinib (10 μM), actinonin (100 μM), gamitrinib-TPP (5 μM), or pyruvate dehydrogenase/α-ketoglutarate dehydrogenase inhibitor CPI-613 (200 μM) for 5 hr. (D) LNCaP cells transfected with pooled NOXA siRNAs or non-target control siRNA were treated with actinonin (100 μM), gamitrinib-TPP (5 μM), CPI-613 (200 μM), or DMSO for 5 hr. (E) LNCaP cells were pretreated with MG115 (10 μM) and MG132 (10 μM) for 1 hr, followed by treatment with CPI-613 (200 μM), gamitrinib-TPP (5 μM), or DMSO for 4 hr. Efficacy of proteasome block by MG115/MG132 was confirmed by blotting for p53. (F) LNCaP cells were pretreated with MG115/MG132 (10 μM each), caspase inhibitor Z-DEVD-FMK (20 μM), or DMSO for 1 hr, followed by treatment with actinonin (75 μM), combination of erlotinib (10 μM) and BCLXL/BCL2 inhibitor ABT-737 (5 μM), or DMSO for 5 hr. Efficacy of caspase block by Z-DEVD-FMK and proteasome block by MG115/MG132 were confirmed by blotting for cleaved caspase three and p53, respectively. (G) LNCaP cells transfected with pooled MARCH5 siRNAs or non-target control siRNA were treated with erlotinib (10 μM), actinonin (100 μM), gamitrinib-TPP (5 μM), CPI-613 (200 μM), or DMSO for 5 hr. LE, long exposure. (H) LNCaP cells transfected with pooled HUWE1 (MULE) siRNAs or non-target control siRNA were treated with actinonin (100 μM), gamitrinib-TPP (5 μM), CPI-613 (200 μM), or DMSO for 5 hr. Immunoblots in (A and F) are representative of results obtained in two independent experiments, and the remainder are representative of at least three independent experiments.
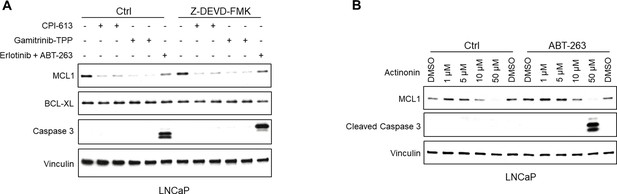
Mitochondria-targeted agents increase caspase-independent MCL1 degradation and synergize with BCLXL/BCL2 inhibitor to induce apoptosis.
(A) LNCaP cells were pretreated with caspase inhibitor Z-DEVD-FMK (20 μM) for 1 hr, followed by treatment with CPI-613 (200 μM), gamitrinib-TPP (5 μM), or erlotinib (10 μM) and BCLXL/BCL2 inhibitor ABT-737 (5 μM) for 5 hr. Efficacy of caspase block by Z-DEVD-FMK was confirmed by blotting for caspase 3. (B) LNCaP were treated with actinonin with or without BCLXL/BCL2 inhibitor ABT-263 (500 nM) for 5 hr.
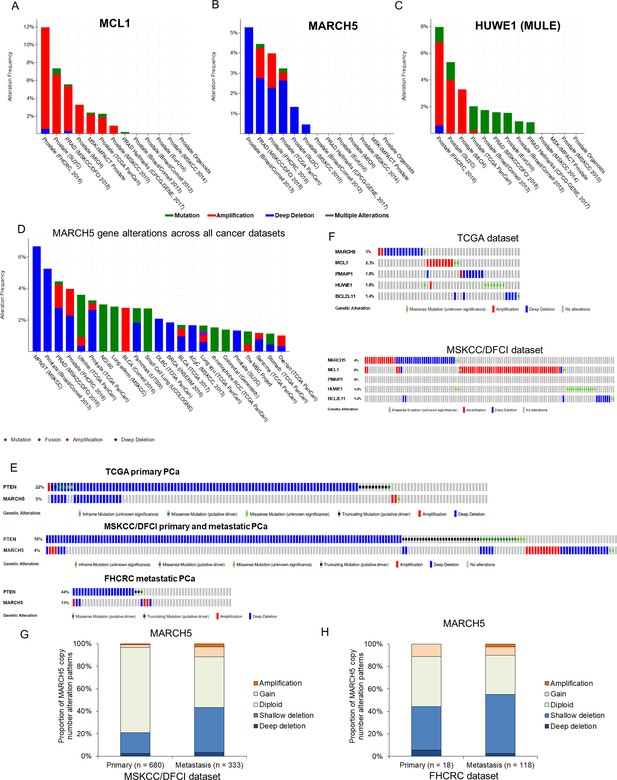
MARCH5 deletion or MCL1 amplification exists in subsets of PCa patients.
(A–C) Molecular profiles (copy number alterations and mutation) of MCL1 (A), MARCH5 (B), and HUWE1 (MULE) (C) among PCa datasets in the cBioPortal for Cancer Genomics (http://cbioportal.org). (D) Frequency and patterns for MARCH5 gene alterations across all cancer datasets, frequency in MPNST (malignant peripheral nerve sheath tumors) reflects only one case. (E) Overlap between genomic alterations in MARCH5 and PTEN. (F) Gene alterations for MARCH5, MCL1, PMAIP (NOXA), HUWE1 (MULE), and BCL2L11 (BIM) in TCGA dataset and MSKCC/DFCI PCa datasets. (G and H) Proportion of copy number alteration patterns for MARCH5 between primary prostate tumor and metastatic prostate tumor samples in MSKCC/DFCI dataset (G) and FHCRC dataset (H).
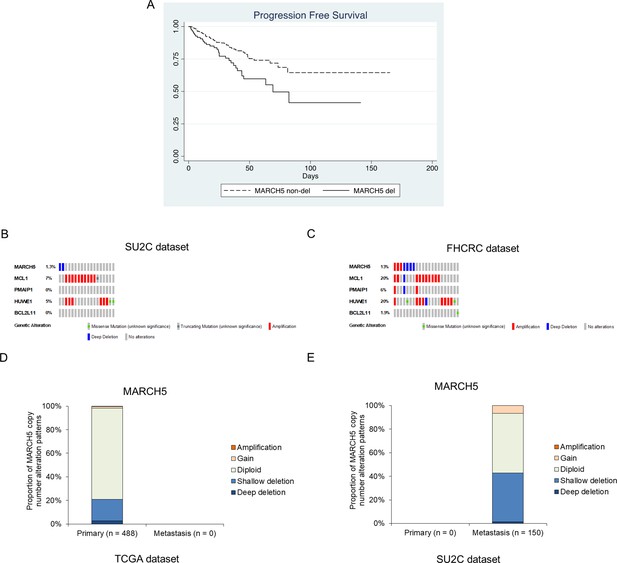
MARCH5 deletion is observed in subsets of PCa patients.
(A) Progression-free survival for tumors with MARCH5 deletion (deep and shallow) in TCGA data set. (B and C) Heatmap of gene alterations for MARCH5, MCL1, NOXA (PMAIP), MULE (HUWE1), and Bim (BCL2L11) in SU2C dataset (B) and FHCRC dataset (C). (D and E) Proportion of copy number alteration patterns for MARCH5 in primary prostate tumor samples in TCGA dataset (D) and in metastatic prostate tumor samples in SU2C dataset (E).
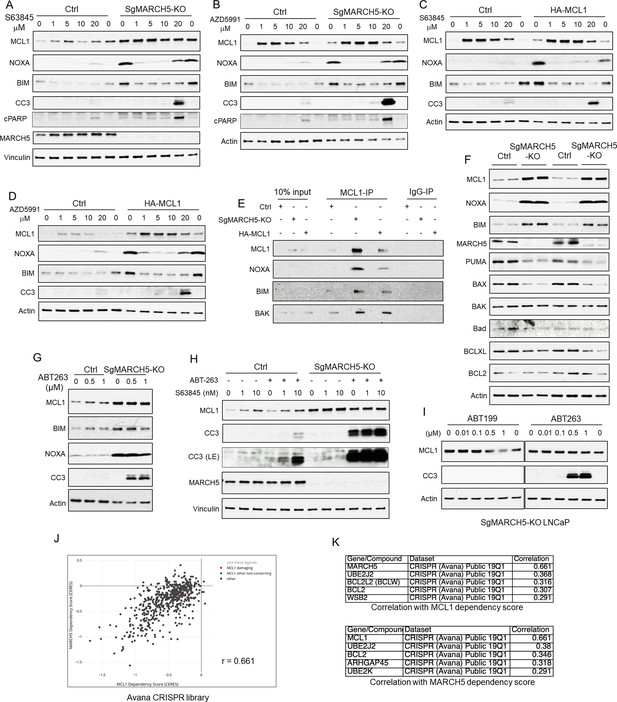
MARCH5 depletion sensitizes BH3 mimetics to drive apoptosis in PCa cells.
(A and B) SgMARCH5-KO or control LNCaP cells were treated with S63845 (0–20 μM) (A) or another MCL1 inhibitor AZD5991 (0–20 μM) (B) for 12 hr. Apoptosis induction was detected with cleaved caspase 3 (CC3) and cleaved PARP (cPARP) signals. (C and D) HA-MCL1 or control LNCaP cells were treated with S63845 (0–20 μM) (C) or AZD5991 (0–20 μM) (D) for 12 hr. (E) Cell lysates of SgMARCH5-KO, HA-MCL1, or control LNCaP with same protein amounts were immunoprecipitated using anti-MCL1 mouse antibody or control mouse IgG with protein G agarose, followed by immunoblotting with rabbit antibodies targeting MCL1, BIM, or BAK, or mouse antibody targeting NOXA. (F) SgMARCH5-KO or control LNCaP cells (biological replicates) were lysed and immunoblotted for indicated proteins. (G) SgMARCH5-KO or control LNCaP cells were treated with BCL2/BCLXL inhibitor ABT-263 (0–1 μM) for 9 hr. (H) sgMARCH5-KO or control LNCaP cells were treated with S63845 (0–10 nM) and ABT-263 (500 nM) or DMSO and for 9 hr. LE, long exposure. (I) SgMARCH5-KO LNCaP cells were treated with BCL2 inhibitor ABT-199 (0–1 μM) or ABT-263 (0–1 μM) for 9 hr. (J) Correlation between MCL1 dependency score and MARCH5 dependency score in AVANA CRISPR screen. (K) Lists of top five genes whose dependency scores are correlated with MCL1 dependency score (upper) or MARCH5 dependency score (lower). Immunoblot in (F) is representative of results obtained in two independent experiments, and the remainder are representative of at least three independent experiments.
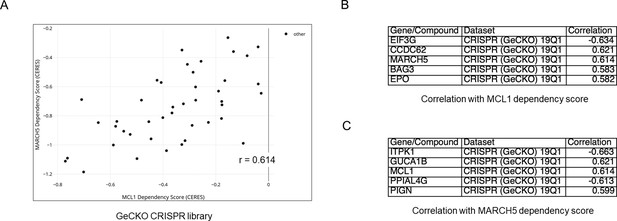
MARCH5 shows codependency with MCL1 in DepMap CRISPR-CAS9 essentiality screens in cancer cells.
(A) Correlation between MCL1 and MARCH5 dependency scores in cancer cells from CRISPR-CAS9 screens using GeCKO libraries. (B and C) Top five genes correlated with MCL1 dependency score (B) or genes correlated with MARCH5 dependency score (C) in cancer cells from CRISPR-CAS9 screens using GeCKO libraries.
Additional files
-
Supplementary file 1
Key Resources Table.
- https://cdn.elifesciences.org/articles/54954/elife-54954-supp1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/54954/elife-54954-transrepform-v2.docx





