Controlling motor neurons of every muscle for fly proboscis reaching
Figures
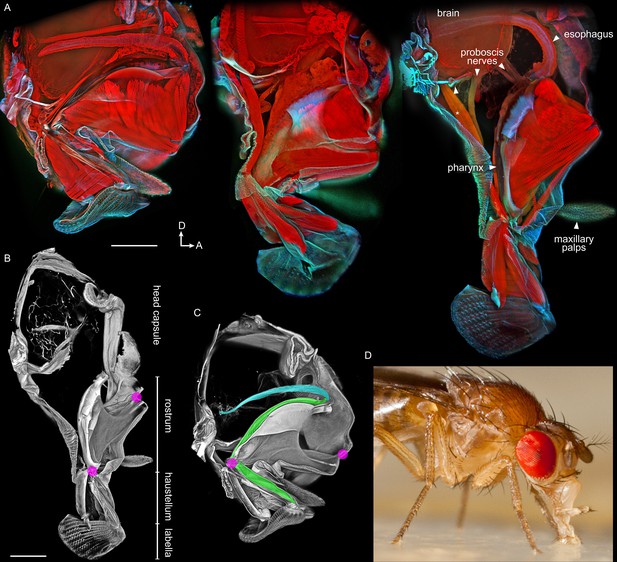
Anatomy of the proboscis.
(A–C) Thick sagittal sections of fly heads with proboscis in various states of extension. Scale bars: 100 µm. Arrows, D: dorsal, A: anterior. (A) Muscles and other internal tissues stained with phalloidin (red). Cuticle and sclerites stained with calcofluor white (cyan). (B–C) Soft tissues digested away to reveal rigid external and internal cuticle. Magenta: locations of proboscis joints. Green: pharynx. Cyan: esophagus. (D) Lateral view of a feeding fly, showing proboscis touching surface near forelegs.
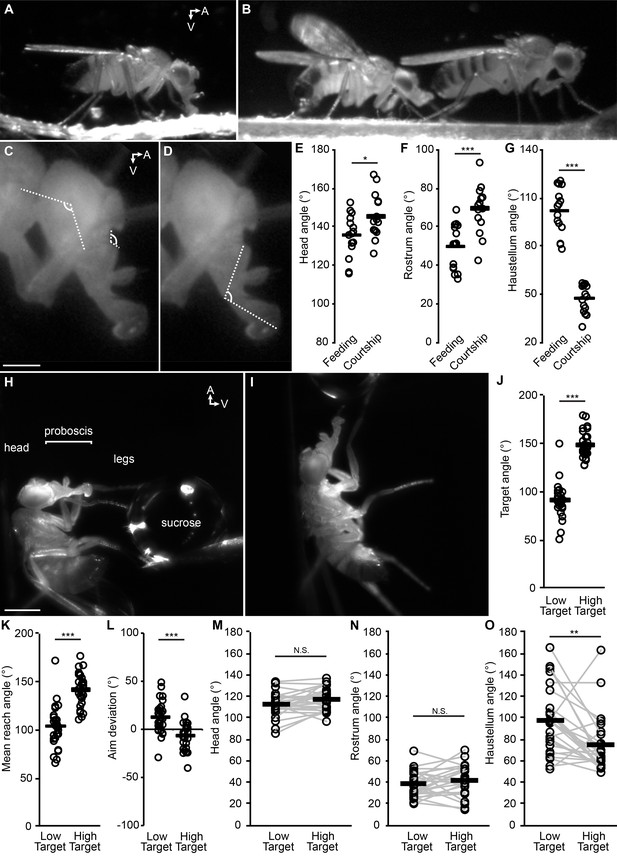
The fly proboscis as a model system for directed reaching.
(A) A fly extending the proboscis towards food on the surface of an experimental chamber (sagittal view). (B) A male fly (left) courting a female (right), extending his proboscis towards the female’s posterior. (C) Points used for measurement of angles of the head (connected by the longer lines) and rostrum (shorter lines). Scale bar: 200 µm. (D) Measurement of haustellum angle. (E–G) Angle of head (E), rostrum (F) and haustellum (G) in males feeding or courting on a flat surface. n = 15 flies per condition. T-test (unpaired) *p<0.05, **p<0.01, ***p<0.001, N.S.: not significant. (H–I) Proboscis extension in response to a low (H) or high (I) sucrose droplet presented to the legs of a tethered fly. Scale bar: 500 µm. (J–L) n = 28 males each presented with sucrose once in low position, once in high. T-test (paired). (J) Angle of target from the fly at frame of first leg contact, when target placed in low or high positions, where 90° would be directly ventral to the eye. (K) Mean reach angle: angle from the posterior-anterior axis of the fly to the proboscis tip, averaged over proboscis extension bout. (L) Aim deviation: reach angle minus target angle. (M–O) Joint angles scored 200 ms after beginning of PE: head (M), rostrum (N), haustellum (O).
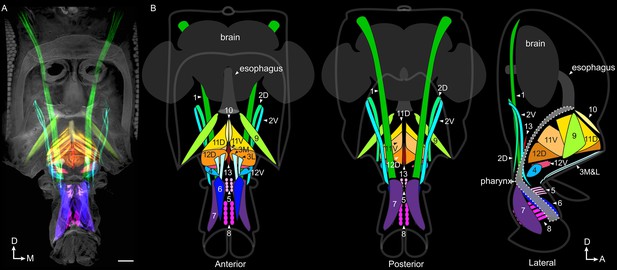
Proboscis muscles.
(A) Frontal view of head with traced proboscis muscles, from clearing technique and segmentation software. Eyes removed at sides, and antennae removed, leaving two holes seen in upper center. Scale bar: 50 µm. (B) Schematics from different views as noted, showing proboscis muscles, brain, esophagus and pharynx. Pharynx superimposed for visibility (approximate outline: dotted line). D: dorsal, M: medial, A: anterior.
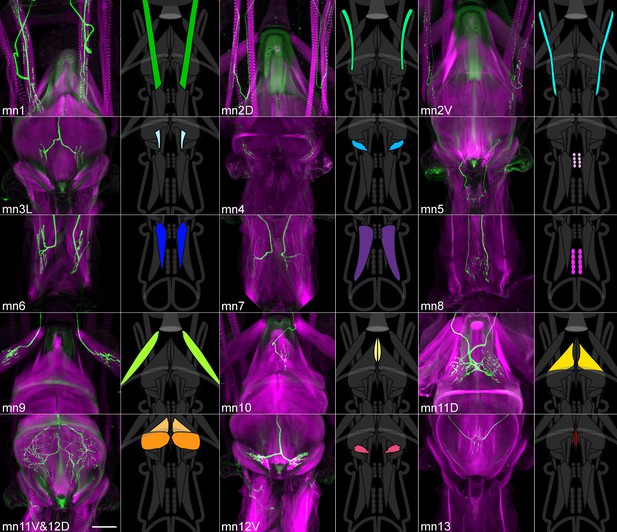
A collection of fly strains to genetically control every proboscis muscle.
Confocal stacks of split GAL4 lines showing the proboscis muscles (magenta) targeted by the motor neurons of the collection (left images; green). (Note: cuticular structures can also autofluoresce green). Scale bar: 50 µm. Gain and contrast adjusted. Right images: location of those muscles in the head schematic from Figure 3, at a reduced scale.
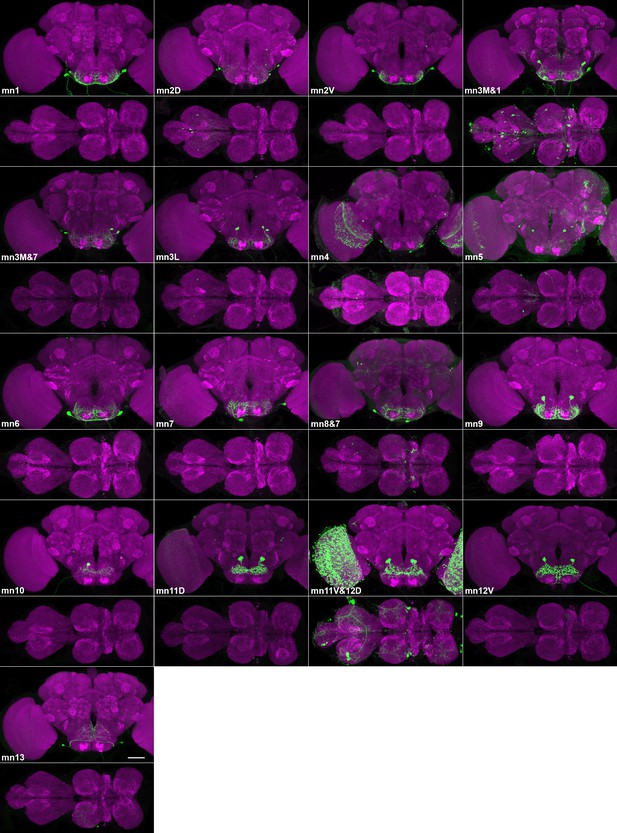
Sparse lines providing genetic access to specific proboscis motor neurons.
Confocal maximum projections of brains (above) and VNCs (below) of split GAL4 lines targeting proboscis motor neurons. Targeted neurons: green (GFP). Counterstain: magenta (nc82). Most lines contain a single motor neuron type in SEZ; a few contain more than one (numbers in Table 1, along with split GAL4s used). Gain and contrast adjusted. Scale bar: 50 µm.
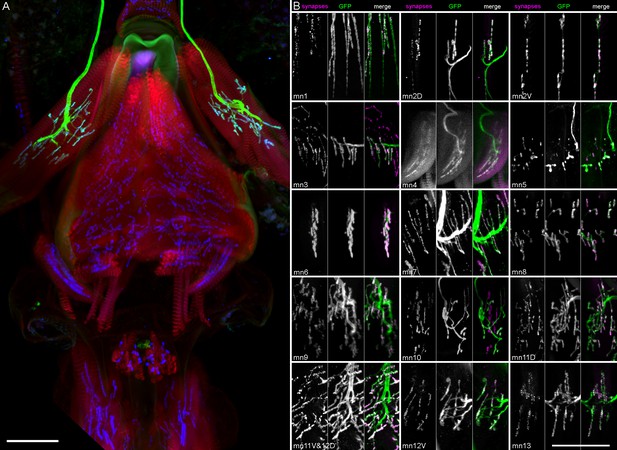
Completeness of motor neuron coverage in split GAL4 collection.
(A) Example proboscis stained for muscles (phalloidin, red), genetically targeted motor neuron (mn9, GFP, green), and neuromuscular junctions (nc82, blue), to determine whether all synaptic sites on the appropriate muscle are occupied by the incoming green axon(s) in that line. (More examples of this staining are shown in Video 9 and in B). Frontal view, dorsal at top. (B) Partial stack projections cropped in the region of each muscle showing neuromuscular junction synapses (nc82, left panels within each group, magenta in overlay) and motor neuron terminals (GFP, middle panels, green in overlay). Gain and contrast adjusted. Scale bars: 50 µm.
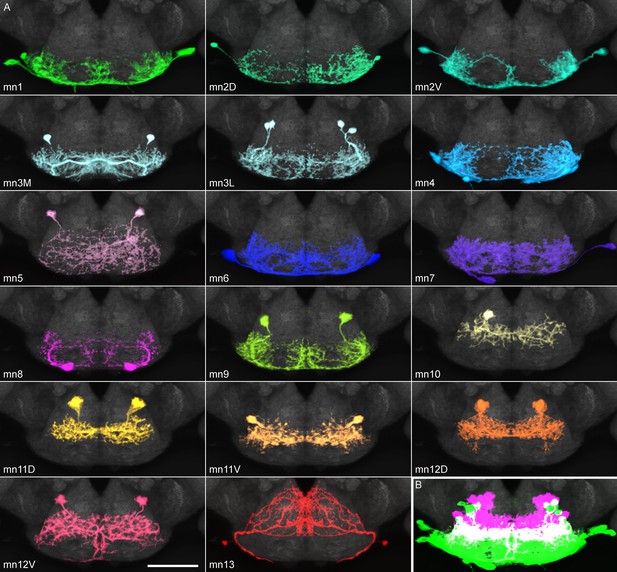
Proboscis motor neuron collection: arbors in brain (subesophageal zone).
(A) Motor neurons from the split GAL4 lines in Table 1, segmented to show arbors in isolation. Colors match muscles in previous figures. Most motor neurons are segmented from split GAL4 combinations in which their arbors are clearly distinguishable, with the exception that mn3M, 8, 11V, 12D and 13 are segmented from stochastic staining in order to separate them from nearby cells. Single neurons from stochastic staining are superimposed upon their mirror images to show bilateral arbors, for comparison with the neurons segmented bilaterally. mn10 is shown unilaterally, since it was never found bilaterally in any split GAL4 combination. Scale bar: 50 µm. (B) Motor neurons colored according to whether their dendrites are primarily dorsal (magenta) or ventral (green). Magenta: 10, 11D, 11V, 12D, 12V (13: not shown). Rest: ventral.
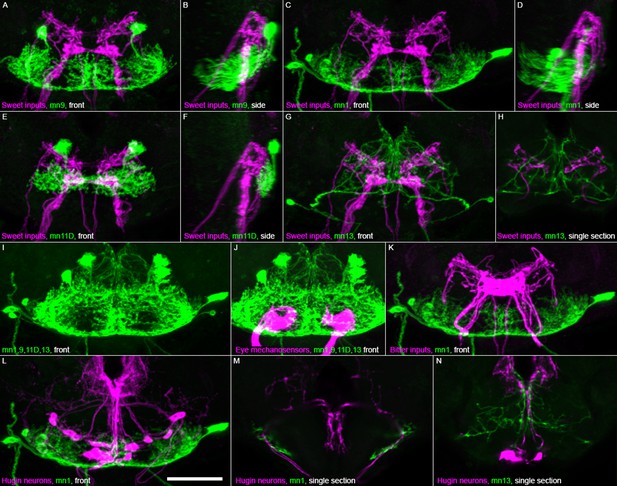
Location of motor neuron dendrites relative to other cell types in brain.
Motor neuron confocal images (green) from brains computationally aligned with brains stained for other cell types in magenta: (A–H) sweet taste inputs (Gr64f-GAL4), (J) eye mechanosensory inputs (VT017251-LexA), (K) bitter taste inputs (Gr66a-GAL4), and (L–N) Hugin neurons (HUGS3-GAL4). Frontal view (‘front’) or sagittal view (‘side’) of confocal maximum projections, except single sections where noted. Each cell type stained individually for GFP then aligned and overlaid. Gain and contrast adjusted. Scale bar: 50 µm.
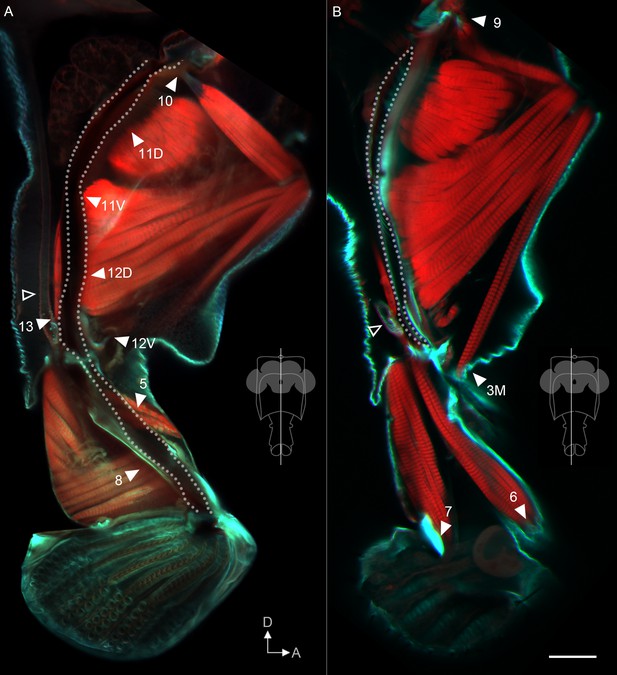
Muscle insertion sites predict function.
Sagittal view of proboscis, showing phalloidin-stained muscles (red) and calcofluor white-stained cuticle (cyan). Single optical slices from the planes shown in insets, from 300 µm vibratome sections. Dotted line: path of pharynx. Arrowheads: insertion sites of muscles that do contact the pharynx (A), and that do not (B), predicted to be involved in pumping vs. proboscis positioning, respectively. The salivary muscle 13 can be seen inserting at the junction of the salivary duct (hollow tube – open arrowheads) with the pharynx. 12V is out of the plane of view in (A) but its tendon inserts on the pharynx. Rest of muscle insertions shown in Figure 6—figure supplement 1. Scale bar: 50 µm.
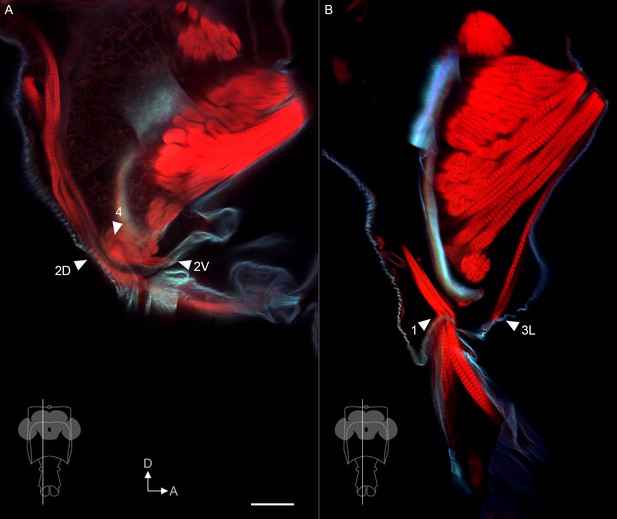
Additional muscle insertion sites.
Sagittal view of proboscis, showing phalloidin-stained muscles (red) and calcofluor white-stained cuticle (cyan). (A,B) Single optical slices from the planes shown in insets, from 300 µm vibratome sections. Arrowheads: insertion sites of additional muscles that do not contact the pharynx, predicted to be involved in proboscis positioning. Scale bar: 50 µm.
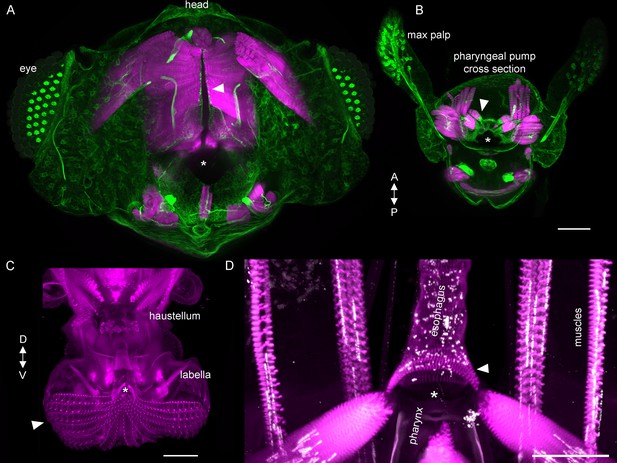
Features of the alimentary canal.
(A,B) Vibratome horizontal sections of the head and proboscis cut at proximal (A) or distal (B) levels of the rostrum, stained for muscles (phalloidin, magenta) and connective tissue at muscle attachments (acetylated tubulin, green; also labels neurons). Some pump muscles (A, arrowhead) insert directly on the wall of the pharynx (dark vertical opening and tube at center, asterisk), and others use tendons (B, arrowhead) to insert onto the pharynx (B, asterisk). Note that the dark cavity of the pharynx is not simply a cylindrical tube but widens into complex cavities within the rostrum. Maximum projections of approximately horizontal ~200 µm slices in 7% agarose. (A) Is close to the head and shows parts of the compound eyes at the sides (grids of bright dots), and (B) Is close to the distal end of the rostrum, where the maxillary palps can be seen as two structures protruding from the anterior surface of the proboscis, filled with sensory neurons. (C) Frontal view of distal proboscis from whole cleared head prep, with labella in open position, showing how pseudotrachea (furrows; arrowhead) lead food to the opening of the pharynx (asterisk). Phalloidin imaged at higher gain to show muscles as well as cuticle. (D) Frontal view of the opening of the esophagus (asterisk) at the base of the rostrum inside the head. Muscles (phalloidin, magenta) and neuromuscular junctions (nc82, white). Arrowhead: circular muscles surround the esophagus, enabling peristalsis. Scale bars: 50 µm. Gain and contrast adjusted.
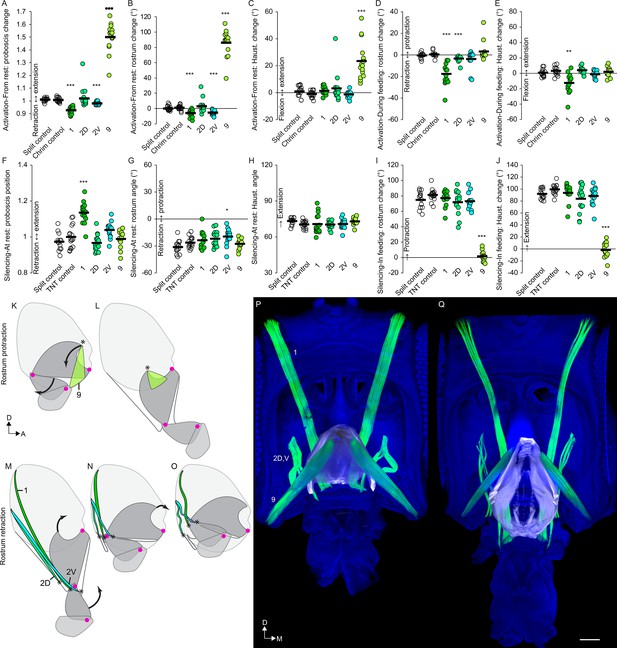
Motor control of the rostrum.
(A–E) CsChrimson activation of split GAL4s for rostrum motor neurons, compared to controls (unfilled), quantifying change in proboscis position from rest (total extension of proboscis at maximum movement divided by at rest; therefore no movement = 1) (A), change in rostrum angle from rest (B), change in haustellum angle from rest (C), change in rostrum angle from a protracted position during feeding (D), and change in haustellum angle from an extended position during feeding (E). (F–J) TNT silencing of split GAL4s for rostrum motor neurons, compared to controls, quantifying proboscis position at rest (F), rostrum angle at rest (G), haustellum angle at rest (H), change in rostrum angle from rest to feeding position (I), and change in haustellum angle from rest to feeding position (J). Bar: mean. Biological replicates, n = 14–16 flies/genotype. Asterisks: unpaired t-tests, experimental (colored) vs. each control (showing least significant), with multiple testing correction. *p<0.05, **p<0.01, ***p<0.001, no asterisk, not signficant. See Methods for further explanations of metrics shown. (K–L) Sagittal schematics of proboscis movements controlled by muscle 9 (green): protraction of rostrum and extension of haustellum (arrows). Muscle 9 origin: ventral wall of head. Muscle 9 insertion: internal part of rostrum cuticle (asterisk). Proboscis segments (dark gray) pivot around joints (magenta dots). (M–O) Proboscis movements controlled by muscles 1, 2D and 2V (colored): retraction of rostrum and haustellum (arrows). Muscle origins: posterior wall of head. (P–Q) Frontal view of whole-mount heads (blue) with segmented muscles (green, numbered) and the apodeme within the rostrum (white) that swings outward during rostrum extension, shown with rostrum retracted (P) or extended (Q). Scale bar: 50 µm.
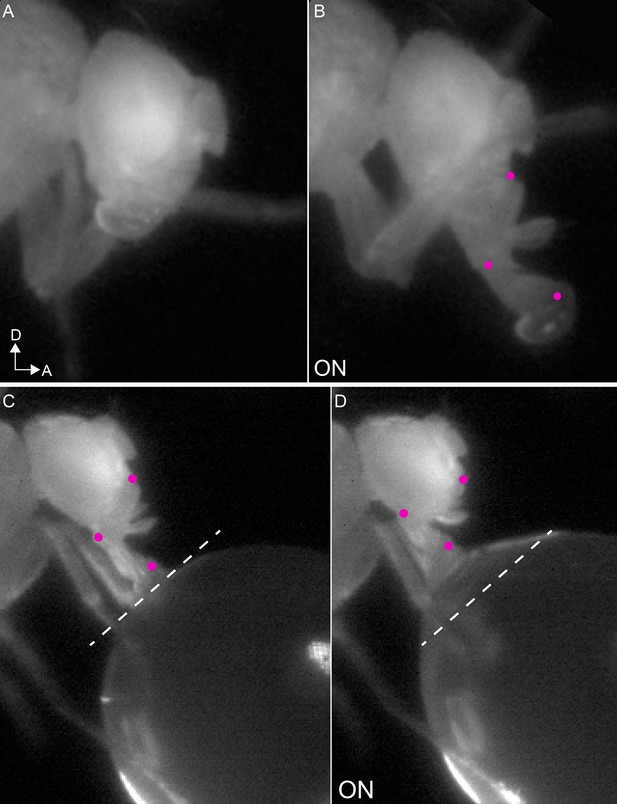
Examples of rostrum muscle actions.
(A–B) Example mn9 phenotype: extension of both the rostrum and haustellum in response to CsChrimson activation (B), compared to resting proboscis before the stimulus (A). (C–D) Example retractor phenotype: retraction of the rostrum in response to CsChrimson activation of mn1 (D), compared to feeding position before the stimulus (dotted line) (C).
Raw joint angles, not normalized, for all motor neurons from Figures 7–9.
(A–B) CsChrimson activation of split GAL4s for the motor neurons shown, quantifying raw rostrum angle in a protracted position during feeding (A) and raw haustellum angle in an extended position during feeding (B). (C–D) TNT silencing of the same split GAL4s, quantifying maximum raw rostrum angle during feeding (C) and maximum raw haustellum angle during feeding (D). Bar: mean. Biological replicates, n = 14–16 flies/genotype. Asterisks: unpaired t-tests, experimental (colored) vs. each control (unfilled), showing least significant, with multiple testing correction. *p<0.05, **p<0.01, ***p<0.001, no asterisk, not significant.
Motor control of the haustellum.
(A–E) CsChrimson activation of haustellum split GAL4s, compared to controls (unfilled), quantifying change in proboscis position from rest (A), change in rostrum angle from rest (B), change in haustellum angle from rest (C), change in rostrum angle from a protracted position during feeding (D), and change in haustellum angle from an extended position during feeding (E). (F–J) TNT silencing of haustellum split GAL4, compared to controls, quantifying proboscis position at rest (F), rostrum angle at rest (G), haustellum angle at rest (H), change in rostrum angle from rest to feeding position (I), and change in haustellum angle from rest to feeding position (J). Bar: mean. Biological replicates, n = 14–16 flies/genotype. Asterisks: unpaired t-tests, experimental (colored) vs. each control (showing least significant), with multiple testing correction. *p<0.05, **p<0.01, ***p<0.001, no asterisk, not signficant. (K–L) Sagittal schematics of proboscis movement controlled by muscle 4 (blue): extension of haustellum (arrow). Muscle 4 location: near haustellum joint. Proboscis segments (dark gray) pivot around joints (magenta dots). (M–N) Proboscis movement controlled by muscle 3 (light blue): flexion of haustellum (arrow). Muscle 3 origin: anterior rostrum. Insertion: asterisk. (O–P) Haustellum mechanism. Lateral view of proboscis (blue) with segmented muscles (green, numbered) and a Y-shaped apodeme (red in composite, white below), from thick sections with haustellum partly flexed (O) or partly extended (P). Muscle 4 inserts on the free dorsal arm of the apodeme (asterisk). Muscle 2V inserts on the anterior apodeme arm (plus sign). Muscle 3 inserts in the haustellum (via tendons not stained here). Arrows: rotation of apodeme, controlling extension of haustellum. Scale bar: 50 µm.
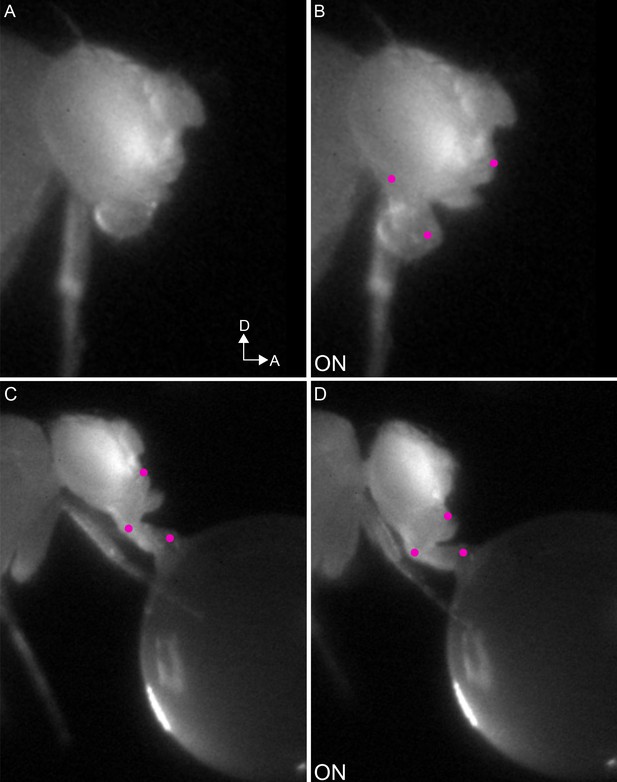
Examples of haustellum muscle actions.
(A–B) Example mn4 phenotype: extension of the haustellum in response to CsChrimson activation (B), compared to resting proboscis before the stimulus (A). (C–D) Example mn3 phenotype: flexion of the haustellum in response to CsChrimson activation of mn3L (D), compared to proboscis in feeding position before the stimulus (C).
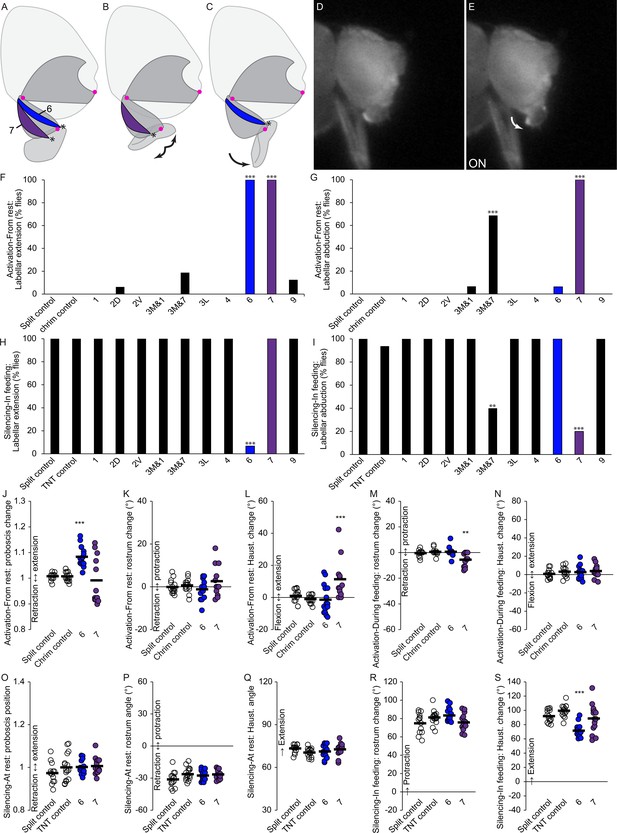
Motor control of the labella.
(A–C) Sagittal schematics of labellar movements controlled by muscles 7 and 6 (colored): abduction (B) and extension (C) of labella (arrows). Muscle origins: dorsal haustellum. Insertions: at labella (asterisks). Proboscis segments (dark gray) pivot around joints (magenta dots). (D–E) Example phenotype: extension of labella in response to CsChrimson activation of mn6 (E), compared to resting proboscis before the stimulus (D). (F–I) % flies showing labellar extension (F,H) or abduction (G,I) in response to CsChrimson activation (F,G) or TNT silencing (H,I) of motor neurons listed, compared to controls. Fisher’s exact test. Biological replicates, n = 16 flies/genotype. (J–N) CsChrimson activation of split GAL4s listed compared to controls (unfilled), quantifying change in proboscis position from rest (J), change in rostrum angle from rest (K), change in haustellum angle from rest (L), change in rostrum angle from a protracted position during feeding (M), and change in haustellum angle from an extended position during feeding (N). (O–S) TNT silencing of split GAL4s listed compared to controls, quantifying proboscis position at rest (O), rostrum angle at rest (P), haustellum angle at rest (Q), change in rostrum angle from rest to feeding position (R), and change in haustellum angle from rest to feeding position (S). Bar: mean. Biological replicates, n = 14–16 flies/genotype. Asterisks: unpaired t-tests, experimental (colored) vs. each control (showing least significant), with multiple testing correction. *p<0.05, **p<0.01, ***p<0.001, no asterisk, not signficant.
Videos
Synchrotron x-ray video of proboscis extension.
Looping video of proboscis extension in a fly tethered with head glued, in a synchrotron x-ray beam, sagittal view, 1/100 speed. This example primarily shows movement of the rostrum (labeled magenta in the later portion of the video), pivoting around the joint marked by crosshairs, with little extension of the haustellum and labella (green).
Proboscis joint movements in response to sucrose.
Sagittal view of a tethered fly (anterior: up) reaching the proboscis towards a droplet of sucrose presented to the legs. 1/30 speed. Magenta dots: approximate locations of two proboscis joints.
Rostrum joint movement.
Proboscis movement restricted to rostrum joint by gluing haustellum (in or out) and head. Sagittal view of tethered fly, anterior: up, 1/3 speed. Magenta dot: approximate location of rostrum joint.
Haustellum joint movement.
Proboscis movement restricted mainly to haustellum joint by gluing rostrum (in or out) and head. (Rostrum glued along one surface, not completely immobilized). Sagittal view of tethered fly, anterior: up, 1/3 speed. Magenta dot: approximate location of haustellum joint.
Proboscis extension during feeding.
A wildtype male feeding from sucrose painted onto wall of chamber (bright region) extends the proboscis ventrally towards the food. 1/1 speed.
Proboscis extension during courtship.
A male (left) courting a female (right) on a flat surface extends the proboscis anteriorly towards the female. 1/10 speed, wildtype flies.
Proboscis reaching to targets in different locations.
Proboscis and head movements reaching towards a sucrose target in high or low (anterior or ventral) locations. Sagittal view of tethered fly, anterior: up, 1/1 speed.
Head movement.
Head movement towards sucrose when both rostrum and haustellum joints are glued (not labella). Sagittal view of tethered fly, anterior: up, 1/3 speed.
Confocal stack showing an example of raw data from the head clearing technique, first in composite then each channel individually.
Red: muscles stained with phalloidin. Green: GFP stain of several motor neuron types genetically targeted in this example, projecting to the rostrum. Blue: synapses stained with nc82.
A fly head imaged by cuticle autofluorescence (gray) with proboscis pointing downwards and eyes cropped out of image at sides.
All muscles were stained with phalloidin (stain not shown), then traced in segmentation software to display every proboscis muscle in a different color, first overlaid, then individually. Last: approximate path of pharynx. (Pharynx is a more complex shape than shown here but is only visible as negative space with this staining, difficult to trace). The brain can be seen in dark gray within the head, with hook-shaped sclerites underneath it. Fat body and air sacs not shown.
Each proboscis motor neuron type, isolated using segmentation software.
Cropped to show only SEZ at the bottom of brain. Hole at top: esophageal foramen.
Translation through computational alignment of feeding command-like neuron ‘Fdg’ (magenta) with motor neurons 1, 9 and 11D (green) shown sequentially.
Motor neurons: confocal stacks. Fdg: manually segmented from a line with a broader expression pattern, NP883-GAL4. Central brain, with SEZ at bottom. Gain and contrast adjusted.
Predicted mechanism of pumping food (blue) through the pharynx (white) by sequential activation of the seven muscles that insert on the pharynx wall (colored as in Figures 3 and 4).
Sagittal view of head.
Behavioral phenotypes of rostrum protractor.
First part: activation of rostrum protractor (mn9) with CsChrimson, in frames noted, compared to CsChrimson control, beginning with the proboscis in the resting (retracted) position. Second part: silencing of mn9 with TNT, compared to TNT control, in a feeding assay where normal flies fully extend the proboscis towards a droplet of sucrose. Tethered males, sagittal view (dorsal up), 1/30 speed.
Behavioral roles of rostrum retractor.
First part: activation of the main rostrum retractor (mn1) with CsChrimson, in frames noted, compared to CsChrimson control, beginning with the proboscis in the extended position during feeding. Second part: silencing of mn1 with TNT, compared to TNT control, in the resting proboscis position to demonstrate that mn1 silencing results in incomplete proboscis retraction. Tethered males, sagittal view (dorsal up), 1/30 speed.
Mechanism of rostrum movement.
Head (blue), muscles involved in rostrum movement (green), and apodeme within rostrum (white). First part: schematic of rostrum movement (pivot point: red crosshairs). Muscles: 1 (long, at left), 2V and 2D (short, at left), and 9 (right). Dorsal up, anterior at right. Second part: same structures segmented from confocal images (sagittal view, maximum projection) with rostrum more retracted (left) or extended (right), showing direction of muscle action (arrows). Third part: same segmented structures in rotating views with rostrum more retracted followed by more extended.
Behavioral roles of haustellum extensor.
First part: activation of haustellum extensor (mn4) with CsChrimson, in frames noted, compared to CsChrimson control, beginning with the proboscis in the resting (retracted) position. Second part: silencing of mn4 with TNT, compared to TNT control, in a feeding assay where normal flies fully extend the proboscis towards a droplet of sucrose. Tethered males, sagittal view (dorsal up), 1/30 speed.
Behavioral roles of haustellum flexor.
First part: activation of one of the two m3 haustellum flexors (using line mn3M&7) with CsChrimson, in frames noted, compared to CsChrimson control, beginning with the proboscis in the extended position during feeding. Second part: silencing of mn3M&7 with TNT, compared to TNT control, in the resting proboscis position to demonstrate that mn3M&7 silencing results in incomplete proboscis retraction. Tethered males, sagittal view (dorsal up), 1/30 speed.
Behavioral roles of labellar extensor.
First part: activation of labellar extensor (mn6) with CsChrimson, in frames noted, compared to CsChrimson control, beginning with the proboscis in the resting (retracted) position. Second part: silencing of mn6 with TNT, compared to TNT control, in a feeding assay. Labella marked in blue in certain frames to show difference in labellar angle. Tethered males, sagittal view (dorsal: up), 1/30 speed.
Behavioral roles of labellar abductor.
First part: activation of labellar abductor (mn7) with CsChrimson, in frames noted, compared to CsChrimson control, beginning from the resting position (proboscis retracted, labella closed). Labella marked in blue in certain frames. Second part: silencing of mn7 with TNT, compared to TNT control, in a feeding assay where normal flies open the labella towards a droplet of sucrose. Tethered males, anterior view (dorsal up), 1/30 speed.
Tables
Muscle names and the best split GAL4 lines that target their motor neurons.
Muscle names in different papers, plus our split GAL4s targeting each muscle, motor neurons present in those lines, and location of motor neuron dendrites, soma and which proboscis nerve the axon uses. In split GAL4 names, D = dorsal, V = ventral, M = medial, L = lateral. In almost every case, when the motor neurons were present, they occupied 100% of the NMJs on the relevant muscle, with the exceptions of 3 and 10, described in the text. Former 12–1 is more similar to 11 than to 12–2; renamed as 11V. 12D and 12V are named for proximity, not implying related function. Yellow: positioning muscles that do not insert on the pharynx. Green: pharyngeal muscles.
| Miller, 1950 | Rajashekhar and Singh, 1994 | Flood et al., 2013 | Schwarz et al., 2017 | Muscle location | Split GAL4 | AD split half | DBD split half | MNs in # proboscis sides | Dend- rites | Soma | Nerve |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1,lateral labial adductor m. | retractor of rostrum | 1 | rostrum | mn1 | VT043075 | VT019731 | mn1 in 18/18 | vent | vent | vent | |
| 2,maxillary retractor m. | not investigated | 2–1 | rostrum | mn2D | GMR11F07 | VT064563 | mn2D in 16/16 | vent | vent | vent | |
| not known | not known | 2–2 | rostrum | mn2V | VT064563 | GMR13E04 | mn2V in 12/12 | vent | vent | vent | |
| 3 | flexor of labrum | 3 | rostrum | mn3M &1 | VT025784 | VT063630 | mn3M in 4/4 mn1 in 4/4 | vent | dors | ND | |
| 3 | flexor of labrum | 3 | rostrum | mn3M&7 | VT063630 | GMR75F02 | mn3M in 12/12 mn7 in 6/12 | vent | dors | ND | |
| not known | flexor of labrum | not known | rostrum | mn3L | VT031145 | GMR89F06 | mn3L in 10/10 | vent | dors | dors | |
| 4,maxillary m. | not investigated | 4 | rostrum | mn4 | GMR48H12 | GMR45G01 | mn4 in 10/10 | vent | vent | vent | |
| 5,labral compressor m. | not investigated | 5 | haustellum | mn5 | VT033616 | VT043145 | mn5 in 10/10 | vent | dors | dors | |
| 6,palpal m. | retractor of paraphysis | 6 | haustellum | mn6 | GMR18B07 | GMR81B12 | mn6 in 10/10 | vent | vent | vent | |
| 7,palpal m. | retractor of furca | 7 | haustellum | mn7 | VT014959 | VT001484 | mn7 in 9/10 | vent | vent | vent | |
| 8,transverse labial m. | transverse m. of haust. | 8 | haustellum | mn8 and 7 | VT027168 | VT015822 | mn8 in 15/16 mn7 in 7/16 | vent | vent | vent | |
| 9,lateral pharyngeal m. | protractor of fulcrum | 9 | rostrum | mn9 | VT061715 | VT005008 | mn9 in 12/12 | vent | dors | dors | |
| 10,median pharyngeal m. | median pharyngeal m. | 10 | rostrum | mn10 | GMR14H09 | VT020713 | mn10 in 6/12 | dors | dors | dors | |
| 11,pharyngeal m. | dorsal pharyng. dilator | m.11 | 11–1,11-2 | rostrum | mn11D | VT020737 | GMR10B11 | mn11D in 10/10 | dors | dors | dors |
| 12,cibarial m. | ventral pharyng. dilator | m.12–1 | 12–1 | rostrum | mn11V and 12D | VT050240 | GMR10E04 | mn11V in 21/22 mn12D in 21/22 | dors | dors | dors |
| 12,cibarial m. | not known | m.12–2 | 11–3 | rostrum | mn11V and 12D | VT050240 | GMR10E04 | mn11V in 21/22 mn12D in 21/22 | dors | dors | dors |
| not known | not known | 12–2 | rostrum | mn12V | GMR80D06 | GMR75F02 | mn12V in 14/14 | dors | dors | dors | |
| 13,dorsal salivary m. | not investigated | 13 | rostrum | mn13 | VT043700 | VT034258 | mn13 in 12/12 mn4 in 4/12 | dors | vent | vent |
Head stain results from 100 GAL4 lines.
Lines from Rubin (GMR) and Dickson (VT) collections, in attP2 landing site, showing motor neurons found in proboscis. Note: this clearing technique showed more motor neuron types than were previously known in two lines, GMR18B07 and GMR81B12 (Schwarz et al., 2017).
| GAL4 line | Motor neurons in proboscis | GAL4 line | Motor neurons in proboscis | GAL4 line | Motor neurons in proboscis |
|---|---|---|---|---|---|
| GMR10B11 | 11 or 12D,12V | GMR32D10 | none | VT037554 | none |
| GMR10E04 | 2V,7,8,11,12 | GMR41G04 | 1 or 2?,6,7, 8, 9, 11, 12? | VT037583 | 1,6 |
| GMR10E06 | none | GMR70E08 | 9 | VT037859 | 1,4,7? |
| GMR11B04 | 1,2V,4,6,7,10,11,12,13 | GMR72D06 | 2V | VT038335 | none |
| GMR11C05 | 9,11D | GMR75F02 | 3M,7,12V | VT039475 | none |
| GMR11D03 | 11 or 12? (stochastic) | GMR78E09 | 5,9,3? | VT041887 | none |
| GMR11D04 | 2(D?),7,8 | GMR81B12 | 6,10,1 stochastic | VT042739 | none |
| GMR11D09 | none | GMR89F06 | 3,5 | VT043075 | 1 |
| GMR11F07 | 1,2D,4,9,11,12V | VT000831 | 6,7,8 | VT043088 | 1,7 |
| GMR11F12 | 6 | VT001484 | 3,7 | VT043145 | 5,9,12V |
| GMR11G01 | 4,8 | VT003229 | 1,4,11 | VT043147 | 2V |
| GMR12E03 | 2V | VT005008 | 9 | VT043700 | 2D,6,13 |
| GMR13B02 | 3L,4 | VT011965 | 1,6,7 | VT049356 | none |
| GMR13E04 | 2V, 8? | VT013506 | 11 | VT049481 | 1,4,8,10 |
| GMR13F07 | none | VT013605 | 1,2V,6,7,9,10, (12 or 3),13 | VT049727 | none |
| GMR14H09 | 10 | VT014959 | 6,7,8 | VT050217 | 11 |
| GMR15E02 | 1,2D,2V,3L,4,5,6,7,12V | VT015822 | 1,7,8 | VT050240 | 3,11,12 |
| GMR15E10 | none | VT017933 | 8 | VT050663 | 4,6,7 |
| GMR17D03 | 9,10 | VT019731 | 1,7 | VT055404 | none |
| GMR18A08 | none | VT020713 | 10 | VT056658 | none |
| GMR18B07 | 7(stochastic),8(stoch.),9,10(dim),11,12,12V | VT020737 | 11 | VT057137 | 2D,2V |
| GMR18D09 | none (in brains, one stochastic MN) | VT022244 | none | VT057237 | 6,7,8 |
| GMR18G02 | 1,2D,2V,4,5,6,7,8 | VT023789 | 1,7,8,9 | VT057379 | 6 |
| GMR19A06 | 11 | VT025784 | 1,3 | VT058488 | 1,4,7,13 |
| GMR19G04 | 1,2D,2V,3L,4,5,7,10,11,12V | VT026026 | 8 | VT059784 | 10,11,12 |
| GMR20E07 | 6 | VT027168 | 7,8 | VT061715 | 9 |
| GMR20E09 | 2D,2V | VT031145 | 2V,3 | VT062553 | 1,6,7,13 |
| GMR20G03 | none | VT031157 | 11,12V | VT063219 | 1,6,13 |
| GMR21A10 | none | VT031562 | 4,12V | VT063302 | none |
| GMR23C08 | 4 | VT032912 | 2D,2V,4,8 | VT063630 | 1,2V(stochastic),3,4,7,8 |
| GMR24A06 | none | VT033616 | 5 | VT063635 | 2V |
| GMR27G06 | 9,10,11 | VT034258 | 4,13 | VT064563 | 2D,2V |
| GMR29F03 | none | VT037492 | 7,8 | VT065306 | none |
| GMR32A11 | 1,3,4,5,6,7,8,9,10,11,12,13,12V |
Summary of motor neuron phenotypes from activation and silencing experiments (also informed by insertion site information). Asterisks: findings that were previously undescribed, or that differ from a previous study (Schwarz et al., 2017).
| mn | Activation phenotypes | Silencing phenotypes |
|---|---|---|
| 1 | proboscis retraction | *impaired proboscis retraction |
| 2D | *rostrum retraction from extended | no phenotype (expected redundancy) |
| 2V | *rostrum retraction from rest | *impaired rostrum retraction |
| 3 | *haustellum flexion | *impaired proboscis retraction |
| 4 | *haustellum extension | *impaired haustellum extension |
| 6 | labellar extension | impaired labellar extension |
| 7 | *labellar abduction | *impaired labellar abduction |
| 9 | rostrum protract. and *haust. exten. | impaired rostrum protract. and *haust. exten. |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information | |
|---|---|---|---|---|---|
| Antibody | mouse mAb anti-bruchpilot (nc82) | Developmental Studies Hybridoma Bank | Cat#nc82; RRID:AB_2314865 | (1:50) | |
| Antibody | rabbit anti-GFP | Thermo Fisher Scientific | Cat#A11122; RRID:AB_221569 | (1:500) | |
| Antibody | rabbit anti-GFP | Thermo Fisher Scientific | Cat#A10262; RRID:AB_2534023 | (1:1000) | |
| Antibody | rat mAb anti-FLAG | Novus Biologicals | Cat# NBP1-06712; RRID:AB_1625981 | (1:200) | |
| Antibody | rabbit anti-HA | Cell Signaling Technology | Cat# 3724S; RRID:AB_1549585 | (1:300) | |
| Antibody | mouse anti-V5 | AbD Serotec | Cat# MCA1360; RRID:AB_322378 | (1:300) | |
| Antibody | goat anti-rabbit AlexaFluor-488 | Thermo Fisher Scientific | Cat#A11034; RRID:AB_2576217 | (1:500) | |
| Antibody | goat anti-chicken AlexaFluor-488 | Thermo Fisher Scientific | Cat#A11039; RRID:AB_142924 | (1:500) | |
| Antibody | goat anti-mouse AlexaFluor-488 | Thermo Fisher Scientific | Cat #A11001; RRID:AB_2534069 | (1:500) | |
| Antibody | goat anti-mouse AlexaFluor-568 | Thermo Fisher Scientific | Cat#A11031; RRID:AB_144696 | (1:500) | |
| Antibody | goat anti-rabbit Alexa Fluor-488 | Thermo Fisher Scientific | Cat#A32731; RRID:AB_2633280 | (1:1000) | |
| Antibody | goat anti-mouse Cy3 | Jackson Immunoresearch | Cat#115-166-003; RRID:AB_2338699 | (1:250) | |
| Antibody | goat anti-rat AlexaFluor-568 | Thermo Fisher Scientific | Cat #A11077; RRID:AB_2534121 | (1:500) | |
| Antibody | goat anti-rat AlexaFluor-633 | Thermo Fisher Scientific | Cat#A21094; RRID:AB_141553 | (1:500) | |
| Chemical compound, drug | Texas Red-X Phalloidin | Life Technologies | Cat#T7471 | ||
| Chemical compound, drug | Calcofluor White | Sigma-Aldrich | Cat#F3543 | ||
| Chemical compound, drug | Congo Red | Sigma-Aldrich | Cat#C676 | ||
| Chemical compound, drug | Alexa Fluor 633 Phalloidin | Life Technologies | Cat#A22284 | ||
| Chemical compound, drug | All-trans retinal | Toronto Research Chemical | Cat# R240000 | ||
| Genetic reagent Drosophila melanogaster | Canton S | Bloomington Stock Center | RRID:BDSC_64349 | ||
| Genetic reagent (D. melanogaster) | Rubin and VT GAL4 lines listed in Table 2 | Jenett et al., 2012; Pfeiffer et al., 2010; Tirian and Dickson, 2017 | N/A | ||
| Genetic reagent (D. melanogaster) | Split GAL4 lines targeting proboscis muscles, listed in Table 1 | This paper | N/A | Split GAL halves from G. Rubin and B. Dickson (Jenett et al., 2012; Pfeiffer et al., 2010; Tirian and Dickson, 2017) | |
| Genetic reagent (D. melanogaster) | 10XUAS-IVS-mCD8::GFP in su(Hw)attP5 (pJFRC2) | Gerald Rubin (Pfeiffer et al., 2010) | N/A | ||
| Genetic reagent (D. melanogaster) | 20XUAS-CsChrimson-mCherry in su(Hw)attP5 | Insertion from Vivek Jayaraman, construct from Klapoetke et al., 2014 | N/A | ||
| Genetic reagent (D. melanogaster) | UAS-TeTxLC.TNT | Sweeney et al., 1995 | N/A | ||
| Genetic reagent (D. melanogaster) | pBPhsFlp2::PEST in attP3;; pJFRC201-10XUAS-FRT > STOP > FRT-myr::smGFP-HA in VK00005 | Nern et al., 2015 | N/A | ||
| Genetic reagent (D. melanogaster) | pJFRC240-10XUAS-FRT > STOP > FRT-myr::smGFP-V5-THS-10XUAS-FRT > STOP > FRT-myr::smGFP-FLAG in su(Hw)attP1 | Nern et al., 2015 | N/A | ||
| Genetic reagent ' (D. melanogaster) | VT017251-LexA | Hampel et al., 2017 | N/A | ||
| Genetic reagent (D. melanogaster) | HUGS3-GAL4 | Melcher and Pankratz, 2005 | N/A | ||
| Genetic reagent (D. melanogaster) | Gr64f-GAL4 [737-5];Gr64f-GAL4 [737-1] | Dahanukar et al., 2007 | N/A | ||
| Genetic reagent (D. melanogaster) | Gr66a-GAL4 (II) | Dunipace et al., 2001 | N/A | ||
| Genetic reagent (D. melanogaster) | NP883-GAL4 | Flood et al., 2013 | N/A | ||
| Genetic reagent (D. melanogaster) | NP5137-GAL4 | Flood et al., 2013 | N/A | ||
| Software, algorithm | Adobe Photoshop | Adobe Systems (www.adobe.com/products/photoshop.html) | RRID:SCR_014199 | ||
| Software, algorithm | Adobe Illustrator | Adobe Systems (https://www.adobe.com/products/illustrator.html) | RRID:SCR_010279 | ||
| Software, algorithm | Computational Morphometry Toolkit | Jefferis et al., 2007 | RRID:SCR_002234 | ||
| Software, algorithm | LabView | National Instruments (www.labview.com) | RRID:SCR_014325 | ||
| Software, algorithm | ImageJ | https://imagej.nih.gov/ij/ | RRID:SCR_003070 | ||
| Software, algorithm | Icy | http://icy.bioimageanalysis.org/ | RRID:SCR_010587 | ||
| Software, algorithm | gVision | Gus Lott (http://gvision-hhmi.sourceforge.net/) | N/A | ||
| Software, algorithm | G*Power | http://www.gpower.hhu.de/ | RRID:SCR_013726 | ||
| Software, algorithm | VVDViewer | https://github.com/takashi310/VVD_Viewer | N/A | ||





