The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling
Figures
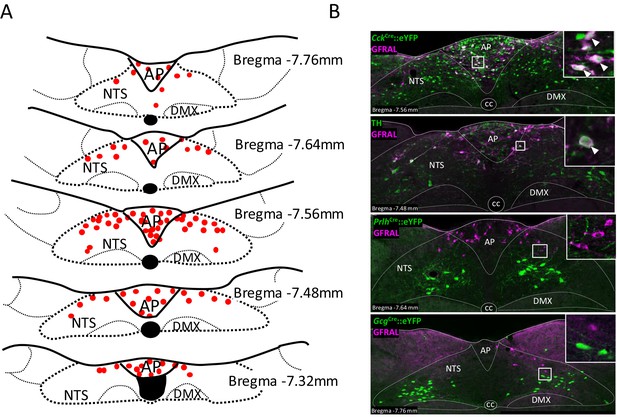
GFRAL-positive neurons in the AP and NTS co-localise with CCK.
(A) Schematic describing the distribution of GFRAL-immunoreactive cell bodies in the AP and dorsal NTS at different rostrocaudal levels relative to bregma. (B) Dual-fluorescence labelling for GFRAL (magenta) with TH or eYFP (staining using antibody raised against green fluorescent protein) in three reporter mice, CckCre::eYFP, PrlhCre::eYFP or GcgCre::eYFP. GFRAL co-localised with CCK and TH, but not PrRP or PPG (the latter being located more caudal to the majority of GFRAL neurons). White arrows in higher magnification inset indicate co-labelled cells. AP (area postrema), cc (central canal), DMX (dorsal motor nucleus of the tenth cranial nerve, vagus), NTS (nucleus of the tractus solitarius).
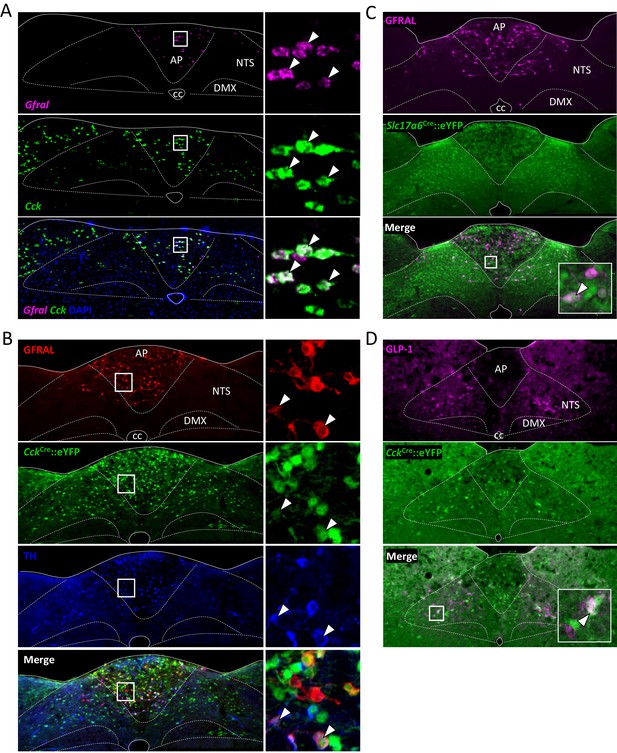
Further histological analysis of GFRAL/CCK neurons.
(A) Dual-label fluorescence in situ hybridisation showing co-localisation of GFRAL (magenta) with CCK (green CckCre::eYFP). Nuclear staining (DAPI, blue) used to identify brain regions. High-magnification photomicrograph of AP neurons showing overlap of cellular markers. White arrows indicate double-labelled cells. (B) Triple-label fluorescence immunohistochemistry showing some co-localisation of GFRAL (red), CCK (green CckCre::eYFP) and TH (blue). Inset, high-magnification photomicrograph of AP showing overlap of cellular markers. White arrows indicate triple-labelled cells. (C) Dual-label fluorescence immunohistochemistry showing co-localisation of GFRAL (magenta) with VGlut2 (green Slc17a6Cre::eYFP). Inset, high-magnification photomicrograph showing overlap of cellular markers. White arrow indicates a double-labelled cell. (D) Using an antibody raised against GLP-1 (magenta), it was seen that some CCK neurons must also contain PPG (white arrow). AP (area postrema), cc (central canal), DMX (dorsal motor nucleus of the tenth cranial nerve, vagus), NTS (nucleus of the tractus solitarius).
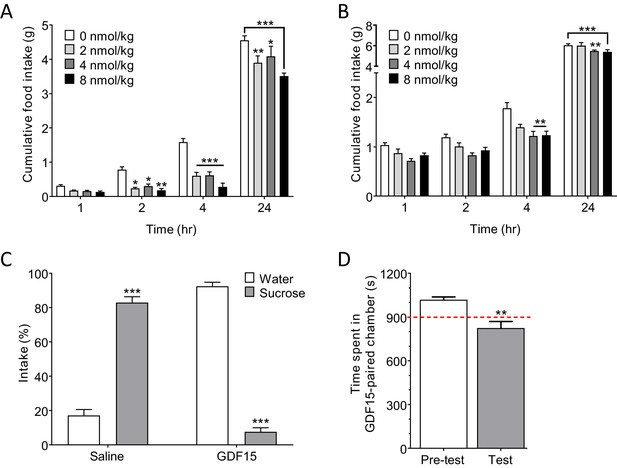
GDF15 produces anorexia and a negative affective valence.
(A) Subcutaneous administration of GDF15, just before ‘lights out,’ decreased normal, night-time feeding (n = 6 per group; *p<0.05, **p<0.01, ***p<0.001, compared with 0 nmol/kg group; two-way ANOVA followed by Tukey’s multiple comparison test). (B) GDF15 also decreased fast-induced, day-time re-feeding (n = 5–6 per group; **p<0.01, ***p<0.001, compared with 0 nmol/kg group). (C) GDF15 supported a conditioned taste aversion in mice when paired with sucrose as the conditioned stimulus. Data show two-bottle fluid intake 24 hr following a single conditioning to GDF15 (n = 6 per group; ***p<0.001, water versus sucrose intake for saline- and GDF15-treated groups; two-way ANOVA followed by Sidak’s multiple comparison test). (D) GDF15 supported a conditioned place aversion in mice. Mice showed a preference for one side of the arena measured as time spent (seconds) in preferred side. During conditioning, mice received an injection of GDF15 on their preferred (dark) side and saline on their non-preferred side. On the test day, the mice displayed a decreased preference for the side on which they received GDF15 (n = 12; **p<0.01, time spent in preferred side; paired t-test).
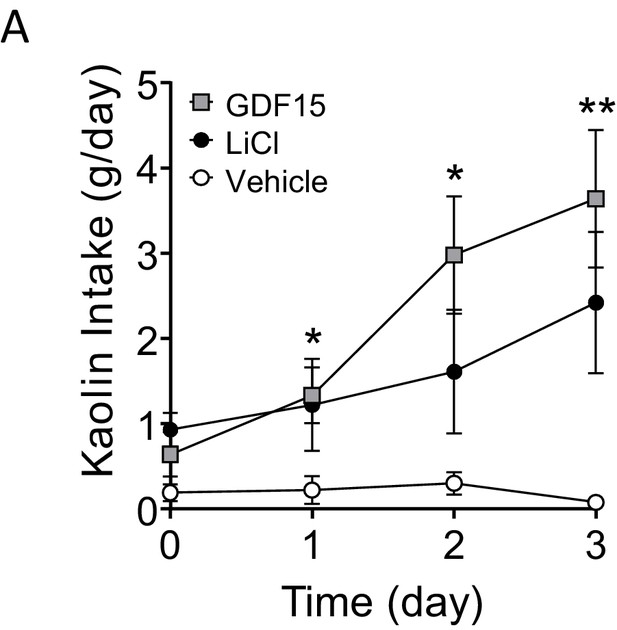
GDF15 supports sickness behaviour in rats.
(A) Daily kaolin intake following injections of vehicle, LiCl or GDF15 on three consecutive days (n = 9–10 per group; *p<0.05, **p<0.01, GDF15 compared with vehicle; two-way ANOVA, followed by a post hoc Tukey test).
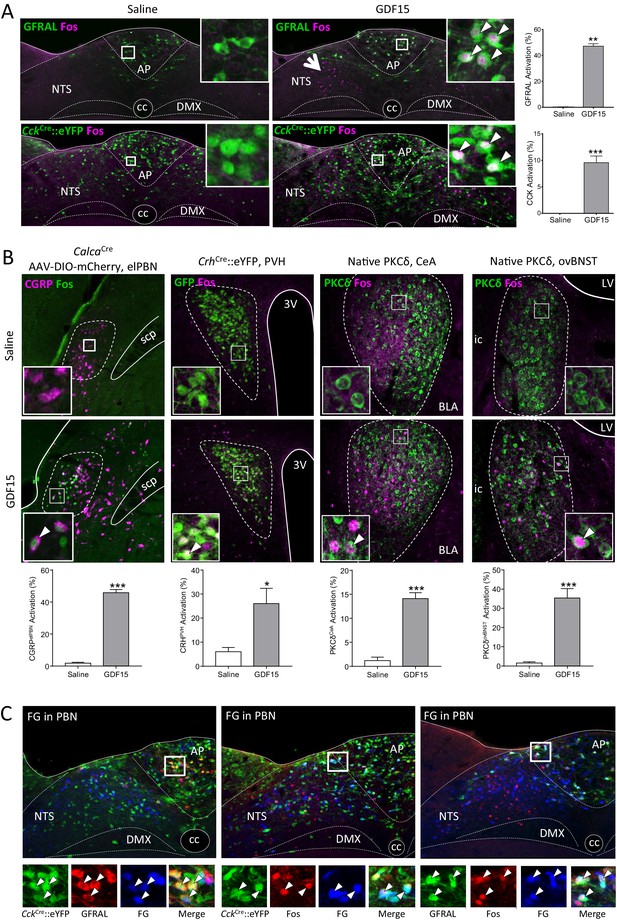
GDF15 activates GFRAL+ve/CCK neurons in the AP/NTS.
(A) Fluorescence photomicrographs showing Fos expression (magenta) in GFRAL and CCK-positive neurons (green) in the AP/NTS following a minimal effective dose of GDF15. For triple labelling, see Figure 3—figure supplement 1A. The percentage of activated GFRAL-immunopositive or CckCre::eYFP neurons is presented on the right (n = 6–7 per group). White arrows in higher magnification insets indicate co-labelled cells. Note that GDF15 administration activated a group of cells in the medial NTS which are GFRAL-ve and CCK-ve (large arrow head). (B) Dual-label immunofluorescence for Fos and downstream neuronal targets. CGRP neurons were visualised by injecting CalcaCre mice with AAV-DIO-mCherry (Fos green; CGRP magenta). In the other pictures, PKC-δ+ or CrhCre::eYFP cells are coloured green. Quantification is provided below the relevant photomicrographs (CalcaCren = 4 per group; CrhCren = 6–7 per group; PKC-δ+n = 6–7 per group). (C) GFRAL neurons, which were activated by GDF15, project directly to the parabrachial nucleus, as demonstrated using Fluoro-Gold retrotracing. White arrows in higher magnification insets indicate triple-labelled cells. aca (anterior part of the anterior commissure), AP (area postrema), BLA (basolateral amygdala), ovBNST (bed nucleus of the stria terminalis, oval sub-nucleus), cc (central canal), CeA (central nucleus of the amygdala), DMX (dorsal motor nucleus of the tenth cranial nerve, vagus), ic (internal capsule), LV (lateral ventricle), NTS (nucleus of the tractus solitarius), PBN (parabrachial nucleus), PVH (paraventricular nucleus of the hypothalamus), scp (superior cerebellar peduncle), 3V (third ventricle). *p<0.05, **p<0.01, ***p<0.001; unpaired t-test.
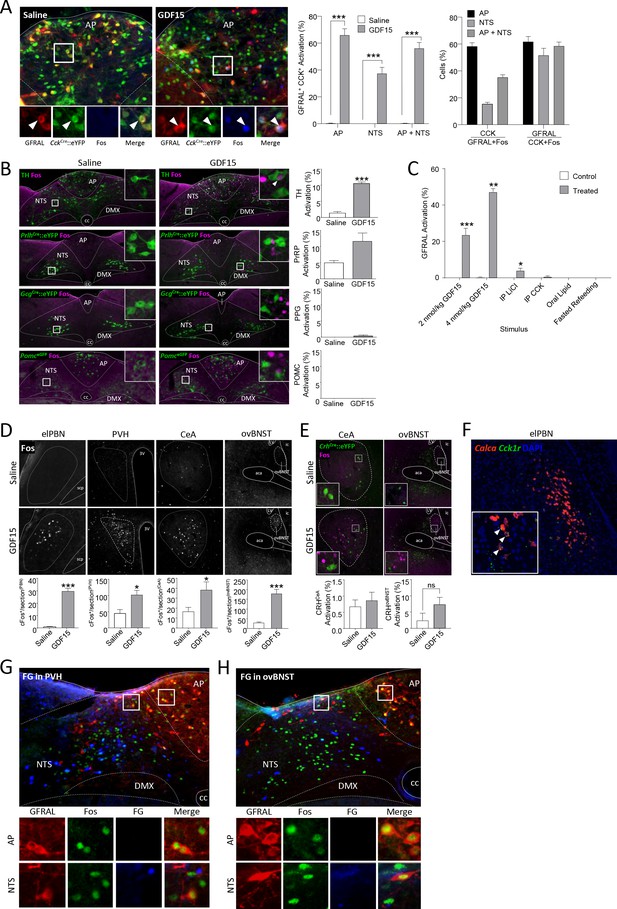
Neuronal activation by GDF15.
(A) Triple-label fluorescence immunohistochemistry showing co-localisation of Fos (blue), GFRAL (red) and CCK (green CckCre::eYFP), with high-magnification photomicrograph of AP showing overlap of cellular markers, and with quantification to the right (n = 4 per group; ***p<0.001, unpaired t-test). (B) GDF15 did not activate either GcgCre::eYFP (n = 2/3 per group), PrlhCre::eYFP (n = 4/5 per group) or PomceGFP (n = 4 per group) cells. There was a statistically significant activation of TH-positive cells (n = 6–7 per group). (C) GDF15 caused a dose-dependent increase in Fos staining in GFRAL neurons, whereas neither LiCl, systemic CCK, oral lipid or fast re-feeding induced Fos in GFRAL neurons. In each case, the control is administration of saline, except for when fasted and re-fed mice are compared (n = 4–7 per group). (D) Single-label immunofluorescence for Fos (pictures as in Figure 3B with counterstaining removed), showing the activation of non-GFRAL neurons in other regions of the brain. Quantification is provided below the relevant photomicrographs (PBN: n = 4 per group; PVH, CeA, ovBNST: n = 6–7 per group). (E) CRH neurons in the ovBNST and CeA are not activated by GDF15 (n = 6/7 per group). (F) Dual-label in situ hybridisation histology showed co-localisation of the CCK1 receptor (green) on Calca (CGRP) neurons (red) in the PBN. Nuclear staining (DAPI, blue) included to identify brain regions. Inset, high-magnification photomicrograph of PBN neurons showing overlap of cellular markers. White arrows indicate double-labelled cells. (G and H) Triple labelling demonstrating that the PVH and ovBNST are not direct targets for GFRAL+ve cells that are activated by GDF15. aca (anterior part of the anterior commissure), AP (area postrema), ovBNST (bed nuceus of the stria terminalis, oval sub-nucleus), cc (central canal), CeA (central nucleus of the amygdala), DMX (dorsal motor nucleus of the tenth cranial nerve, vagus), ic (internal capsule), le (lateral, external region of the PBN), LV (lateral ventricle), NTS (nucleus of the tractus solitarius), PBN (parabrachial nucleus), PVH (paraventricular nucleus of the hypothalamus), 3V (third ventricle). *p<0.05, **p<0.01, ***p<0.001; unpaired t-test.
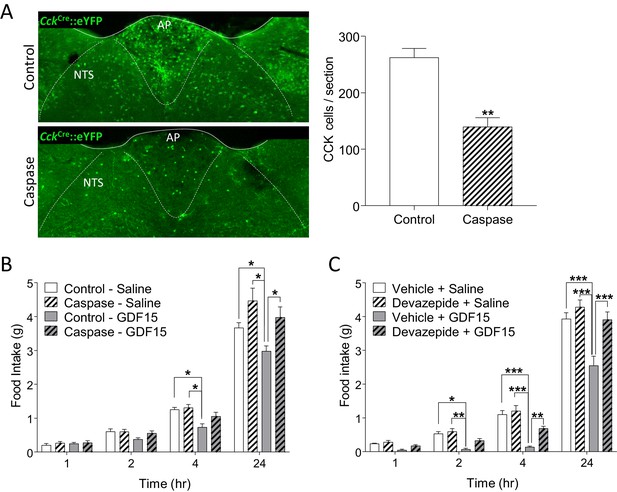
GDF15-induced anorexia is dependent on CCK signalling.
(A) Injection of AAV-caspase into the AP and dorsal NTS of CckCre::eYFP mice caused a reduction in the number of eYFP cells as assessed by immunohistochemistry (n = 7 per group; **p<0.01, unpaired t-test). (B) CckCre::eYFP mice transduced with control AAV displayed a significant decrease in food intake following GDF15 administration, while those transduced with AAV-caspase showed reduced anorexia (n = 7 per group; *p<0.05; two-way ANOVA, followed by a post hoc Tukey test). (C) Pre-administration of the CCK receptor antagonist, devazepide, attenuated the anorectic response to GDF15 (n = 6 per group; *p<0.05, **p<0.01, ***p<0.001; two-way ANOVA, followed by a post hoc Tukey test).
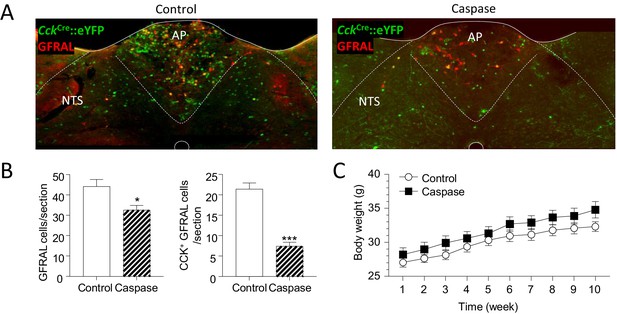
Treatment with caspase leads to a significant loss of GFRAL neurons.
(A) Dual immunostaining showing CCK- and GFRAL-positive neurons following treatment with control AAV or AAV-caspase. (B) Number of GFRAL cells and CCK-positive GFRAL cells per section (n = 7 per group; *p<0.05, ***p<0.001, unpaired t-test). (C) There is no difference in body weight of CckCre::eYFP mice over 10 weeks following injection of either control or caspase virus into the AP/NTS (n = 7 per group; repeated measures two-way ANOVA with a post hoc Sidak test).
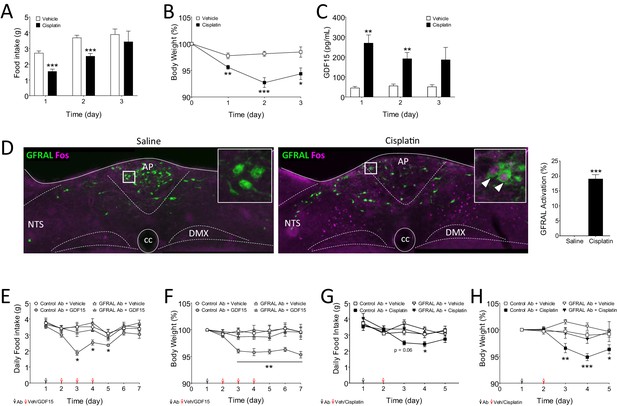
The anorectic action of the cancer therapeutic drug, cisplatin, is blocked by inhibition of signalling through GFRAL.
(A) A single dose of cisplatin reduced food intake and (B) body weight over the following 3 days (n = 6 per time point; *p<0.05, **p<0.01, ***p<0.001; two-way ANOVA, followed by a post hoc Tukey test). (C) This corresponded with an increase in circulating GDF15 (n = 6 per time point, ***p<0.001, unpaired t-test) and (D) induction of Fos (magenta) in immunopositive GFRAL neurons (green) at 24 hr after administration (n = 5–6 per group; ***p<0.001, unpaired t-test). (E) Three injections of GDF15, on days 2–4, led to a decrease in cumulative food intake and (F) body weight (n = 6 per group; *p<0.05, **p<0.01; repeated measures ANOVA, followed by a post hoc Tukey test, control Ab + GDF15 versus all other groups). The actions of GDF15 were blocked completely by pre-administration of a monoclonal antibody against GFRAL (10 mg/kg) on day 1. The GFRAL mAb had no effect on food intake or body weight by itself. For full data set, using different concentrations of GFRAL mAb, see Figure 5—figure supplement 1B and C. (G) Pre-administration of 10 mg/kg GFRAL mAb the day before, completely blocked the reduction of food intake and (H) body weight caused by cisplatin (n = 5–6 per group; *p<0.05, **p<0.01, ***p<0.001; repeated measures ANOVA, followed by a post hoc Tukey test).
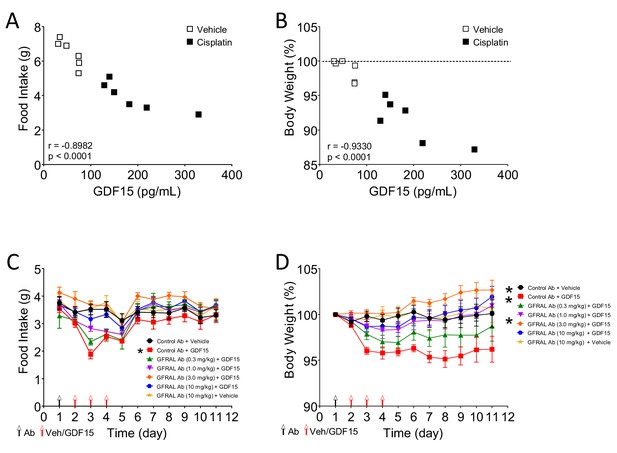
The anorectic action of the cancer therapeutic drug, cisplatin, is blocked by inhibition of signalling through GFRAL.
(A) 48 hr after saline (open squares) or cisplatin (closed squares), there was an inverse correlation between circulating GDF15 and both cumulative food intake and (B) body weight. (n = 12; Pearson’s r) (C) Three injections of GDF15, on days 2–4, led to a decrease in cumulative food intake and (D) body weight (red squares). The actions of GDF15 were abrogated by pre-administration (day 1) of increasing doses of the monoclonal antibody raised against GFRAL. The GFRAL mAb had no effect on food intake or body weight by itself (yellow triangles) (n = 6 per group *p<0.05, two-way ANOVA).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus) | C57Bl/6J (Mouse, male) | Envigo | Stock #057 | MGI:2164189 |
| Genetic reagent (Mus musculus) | C57Bl/6NHsd (Mouse, male) | Envigo | Stock #044 | MGI:2161078 |
| Genetic reagent (M. musculus) | C57Bl/6J (Mouse, male) | Charles River | Stock #632 | MGI:3028467 |
| Genetic reagent (M. musculus) | C57Bl/6J (Mouse, male) | Janvier labs | N/A | MGI:2670020 |
| Genetic reagent (M. musculus) | PomceGFP (Mouse, male) | Jackson Laboratories | Stock #: 009593 | MGI:3851684 |
| Genetic reagent (M. musculus) | Cckires-Cre (Mouse, male) | Jackson Laboratories | Stock #: 012706 | MGI:5014249 |
| Genetic reagent (M. musculus) | Crhires-Cre (Mouse, male) | Jackson Laboratories | Stock #: 012704 | MGI:4452101 |
| Genetic reagent (M. musculus) | Slc17a6ires-Cre (Mouse, male) | Jackson Laboratories | Stock #: 016963 | MGI:5300532 |
| Genetic reagent (M. musculus) | Rosa26-loxSTOPlox-eYFP (Mouse, male) | Jackson Laboratories | Stock #: 006148 | MGI:3621481 |
| Genetic reagent (M. musculus) | GcgiCre (Mouse, male) | Parker et al., 2012 PMID:22638549 | N/A | MGI:5432481 |
| Genetic reagent (M. musculus) | Prlhires-Cre (Mouse, male) | Dodd et al., 2014 PMID:25176149 | N/A | MGI:5634277 |
| Genetic reagent (M. musculus) | CalcaCre (Mouse, male) | Carter et al., 2013 PMID:24121436 | N/A | MGI:5559692 |
| Genetic reagent (Rattus norvegicus) | Sprague Dawley (Rat, male) | Envigo | Stock #: SD-002 | N/A |
| Antibody | anti-cFos (Rabbit polyclonal) | Santa Cruz | Cat.# SC52 RRID:AB_2106783 | Primary antibody (1:500) IHC |
| Antibody | anti-DS Red (Goat polyclonal) | Santa Cruz | Cat.# 33353 RRID:AB_639924 | Primary antibody (1:500) IHC |
| Antibody | anti-GFP (Chicken polyclonal) | Abcam | Cat.# 13970 RRID:AB_300798 | Primary antibody (1:2000) IHC |
| Antibody | anti-GFRAL (Sheep polyclonal) | Thermofisher | Cat.# PA5-47769 RRID:AB_2607220 | Primary antibody (1:200) IHC |
| Antibody | anti-GLP1 (Rabbit polyclonal) | PenLabs | Cat.#. T-4363 RRID:AB_518978 | Primary antibody (1:2000) IHC |
| Antibody | anti-PKCδ (Mouse monoclonal) | BD Biosciences | Cat.#. 610398 RRID:AB_397781 | Primary antibody (1:500) IHC |
| Antibody | anti-TH (Rabbit polyclonal) | AbCam | Cat.# AB112 RRID:AB_297840 | Primary antibody (1:2000) IHC |
| Antibody | anti-TH (Sheep polyclonal) | Millipore | Cat.# AB1542 RRID:AB_90755 | Primary antibody (1:1000) IHC |
| Antibody | anti-chicken, Alexa Fluor 488 (Donkey polyclonal) | Jackson ImmunoResearch | Cat.# 703-545-155 RRID:AB_2340375 | Secondary antibody (1:1000) IHC |
| Antibody | anti-mouse, Alexa Fluor 594 (Donkey polyclonal) | Jackson ImmunoResearch | Cat.# 715-585-150 RRID:AB_2340854 | Secondary antibody (1:1000) IHC |
| Antibody | anti-rabbit, Alexa Fluor 350 (Donkey polyclonal) | Molecular Probes | Cat.# A10039 RRID:AB_2534015 | Secondary antibody (1:1000) IHC |
| Antibody | anti-Sheep, Alexa Fluor 350 (Donkey polyclonal) | Molecular Probes | Cat.# A21097 RRID:AB_10376162 | Secondary antibody (1:1000) IHC |
| Antibody | anti-sheep, Alexa Fluor 594 (Donkey polyclonal) | Molecular Probes | Cat.# A11016 RRID:AB_10562537 | Secondary antibody (1:1000) IHC |
| Antibody | Anti-GFRAL (Mouse monoclonal) | Emmerson et al., 2017 PMID:28846098 | mIgG1 GFRAL 8A2 | Subcutaneous injection (0–10 mg/kg) |
| Recombinant DNA reagent | AAV8-hSyn-DIO-mCherry | Dr Bryan Roth Addgene | Cat.# 50459-AAV8 | N/A |
| Recombinant DNA reagent | AAV5-flex-taCasp3-TEVp | Dr Nirao Shah University of North Carolina Vector Core | N/A | PMID:23663785 |
| Sequence-based reagent | Gfral | Advanced Cell Diagnostics | Cat.# 417021-C3 | RNAScope mRNA probe |
| Sequence-based reagent | Cck | Advanced Cell Diagnostics | Cat.# 402271-C1 | RNAScope mRNA probe |
| Sequence-based reagent | Cckr1 | Advanced Cell Diagnostics | Cat.# 313751-C1 | RNAScope mRNA probe |
| Sequence-based reagent | Calca | Advanced Cell Diagnostics | Cat.# 420361-C2 | RNAScope mRNA probe |
| Peptide, recombinant protein | GDF15 | R and D Systems | Cat.# 9279-GD | (4 nmol/kg) |
| Peptide, recombinant protein | Streptavadin 488 | Jackson ImmunoResearch | Cat.# 016-540-084 RRID:AB_2337249 | (1:1000) IHC |
| Peptide, recombinant protein | Streptavadin 594 | Jackson ImmunoResearch | Cat.# 016-580-084 RRID:AB_2337250 | (1:1000) IHC |
| Commercial assay or kit | Mouse/rat GDF15 ELISA | R and D Systems | Cat.# MGD-150 | N/A |
| Commercial assay or kit | RNAscope Multiplex Fluorescent Assay | Advanced Cell Diagnostics | Cat # 323100 | |
| Chemical compound, drug | Devazepide | Tocris Bioscience | Cat.# 2304 | (1 mg/kg) |
| Chemical compound, drug | Lithium chloride (LiCl) | Sigma | Cat # L9650 (mouse) Cat.# 73036 (rat) | Mouse (128 mg/kg) Rat (128 mg/kg) |
| Chemical compound, drug | Hydroxystibamidine (Fluoro-Gold) | Invitrogen, Thermofisher | Cat.# H22845 | 4% in H2O |
| Chemical compound, drug | Cisplatin | Sigma Aldrich | Cat.# PHR1624 | (4 mg/kg) |
| Software, algorithm | Prism | GraphPad | RRID:SCR_002798 | Version 7 |
| Software, algorithm | Fiji | ImageJ | RRID:SCR_002285 | Version 2.0.0-rc-69/1.52 p |
| Software, algorithm | Micromanager | ImageJ | RRID:SCR_016865 | Version 1.4.23 |
| Software, algorithm | Smart | Panlab, Harvard Biosciences/Biochrom Ltd | RRID:SCR_002852 | Version 3.0 |
Additional files
-
Supplementary file 1
A summary table of the quantification of cell counts demonstrating neuropeptide co-expression in the medulla oblongata.
The number of cells per section single-, double- or triple-labelled on sections through the AP and NTS. Values are stated as are mean ± SEM (n = number of animals). Percentage co-expression is written in the text. Methods involved either immunohistochemistry (top) or in situ hybridisation histology (bottom).
- https://cdn.elifesciences.org/articles/55164/elife-55164-supp1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/55164/elife-55164-transrepform-v2.docx





