Cooperative enzymatic control of N-acyl amino acids by PM20D1 and FAAH
Figures
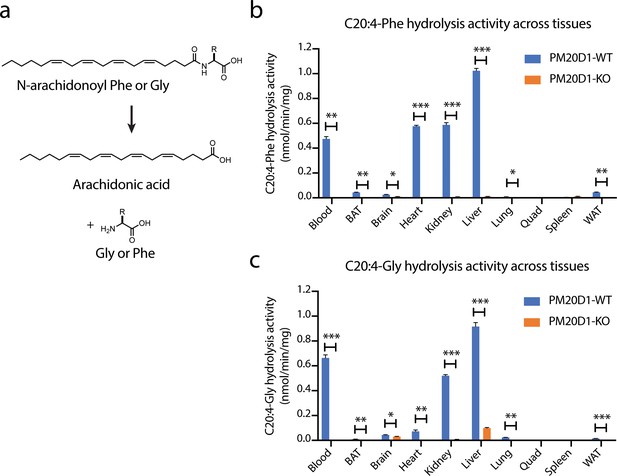
Detection of a residual N-acyl amino acid hydrolase activity in PM20D1-KO tissues.
(a) Schematic of the enzymatic assay that monitors conversion of C20:4-Phe or C20:4-Gly into arachidonic acid. (b, c) C20:4-Phe (b) and C20:4-Gly (c) hydrolysis activities across the indicated wild-type (blue) or PM20D1-KO (orange) tissues. For (b) and (c), activity assays were conducted with 100 µM substrates and 100 µg tissue lysate in phosphate-buffered saline (PBS) for 1 hr at 37°C. Data are shown as means ± SEM, N = 3/group. All experiments were performed once, with N corresponding to biological replicates. *, p<0.05; **, p<0.01, ***, p<0.001 for the indicated comparison.
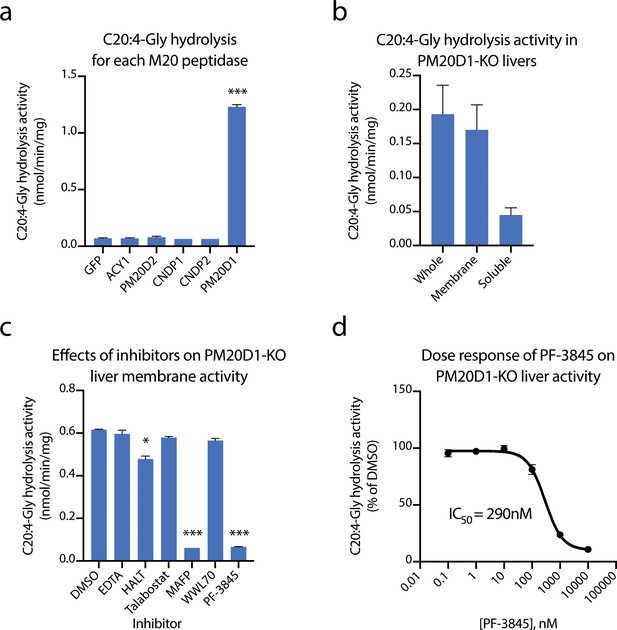
Identification of fatty acid amide hydrolase (FAAH) as the enzyme responsible for the PM20D1-independent N-acyl amino acid hydrolase activity.
(a, b) C20:4-Gly hydrolysis activity of cell lysates transfected with the indicated mammalian M20 peptidase (a) or of the indicated liver homogenate fraction from PM20D1-KO animals (b). (c, d) Effect on the C20:4-Gly hydrolysis activity from PM20D1-KO liver membranes of the indicated inhibitors. Activity assays were conducted with 100 µM substrates and 100 µg tissue lysate in PBS for 1 hr at 37°C. For panel (b), membrane and soluble fractions of liver lysate were separated by centrifugation at 100,000 x g for 1 hr. For panel (c), inhibitors were pre-incubated at 1 mM for EDTA and 10 µM for all other compounds for 10 min before the start of the assay. Data are shown as means ± SEM, N = 3/group. All experiments were performed once, with N biological replicates. *, p<0.05; ***, p<0.001 for the comparison versus DMSO or GFP control.
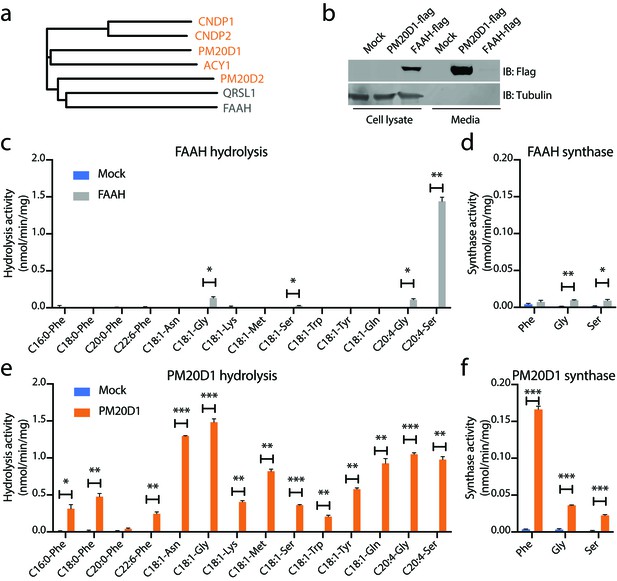
N-acyl amino acid hydrolase and synthase substrate scope in vitro for FAAH and PM20D1.
(a) Phylogenetic alignment of the five murine M20 peptidases with mouse FAAH and a FAAH-related enzyme, QRSL1. Orange, M20 peptidases; gray, FAAH-related sequences. (b) Anti-flag western blot for cell lysates (left) and conditioned media (right) transfected with the indicated plasmids. (c–f) N-acyl amino acid hydrolysis and synthase activities of FAAH- and mock-transfected cell lysates (b, c) or PM20D1-transfected and mock-transfected conditioned media (d, e). Activity assays were conducted with 100 µM substrates and 100 µg protein in PBS for 1 hr at 37°C. Data are shown as means ± SEM, N = 3/group. All experiments were performed once, with N biological replicates. *, p<0.05; **, p<0.01; ***, p<0.001 for the indicated comparison.
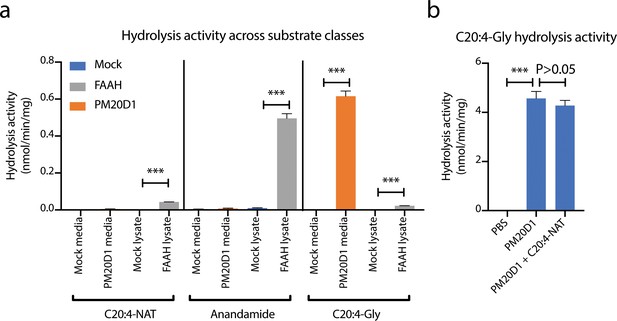
Additional characterization of FAAH and PM20D1 enzyme activities in vitro.
(a) Hydrolysis activities using the indicated substrate from transfected cell lysates (FAAH) or transfected conditioned media (PM20D1). (b) C20:4-Gly hydrolysis activity with purified PM20D1 in the presence or absence of C20:4-NAT competitor (100 µM). Activity assays were conducted with 100 µM substrates and 100 µg protein in PBS for 1 hr at 37°C. Data are shown as means ± SEM, N = 3/group. All experiments were performed once, with N biological replicates. ***, p<0.001 for the indicated comparison.
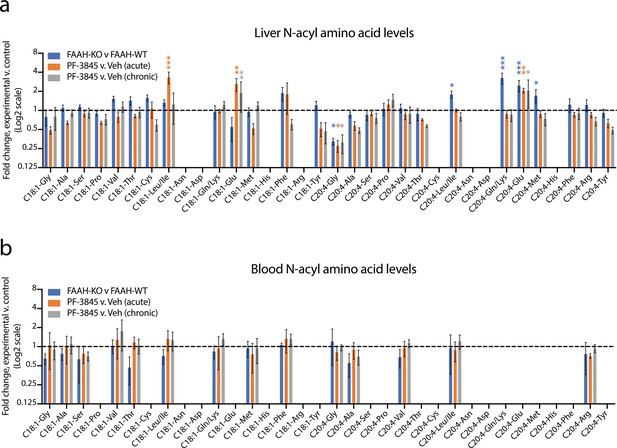
Changes in N-acyl amino acids upon selective blockade of FAAH in vivo.
(a, b) Fold change of the indicated N-acyl amino acids compared to the control for each of the indicated comparisons from liver (a) or blood (b). For drug treatment, PF-3845 was administered intraperitoneally at 10 mg/kg once (acute) or for three consecutive days (chronic). Tissues were harvested 3 hr after the final dose. No bars are shown for N-acyl amino acids that were below the limit of detection. Data are shown as means ± SEM, N = 4–5 mice/group for each of the indicated comparisons. All experiments were performed once, with N biological replicates. *, p<0.05; **, p<0.01; ***, p<0.001 by ANOVA with Dunnett’s multiple comparisons test versus control animals.
-
Figure 4—source data 1
Absolute quantitation of N-acyl amino acids in liver and plasma following FAAH blockade.
- https://cdn.elifesciences.org/articles/55211/elife-55211-fig4-data1-v1.xlsx
-
Figure 4—source data 2
Absolute quantitation of N-acyl ethanolamines and N-acyl taurines in wild-type mouse liver.
- https://cdn.elifesciences.org/articles/55211/elife-55211-fig4-data2-v1.xlsx
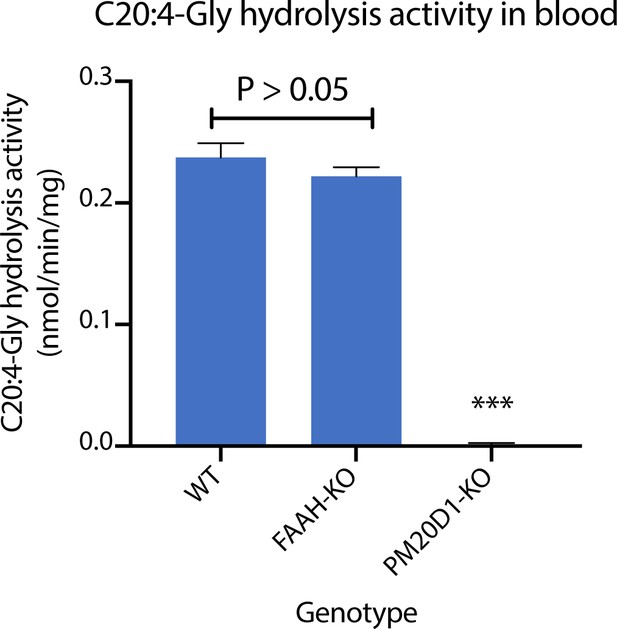
PM20D1 activity in FAAH-KO plasma.
C20:4-Gly hydrolysis activities in from WT, FAAH-KO, or PM20D1-KO plasma. Activity assays were conducted with 100 µM substrates and 100 µg plasma in PBS for 1 hr at 37°C. Data are shown as means ± SEM, N = 3/group. All experiments were performed once, with N biological replicates. ***, p<0.001 versus WT.
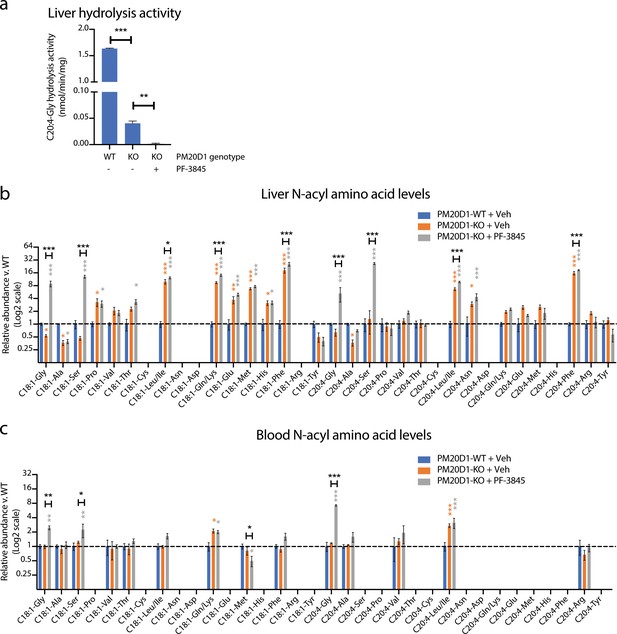
Cooperative interactions between PM20D1 and FAAH regulate endogenous N-acyl amino acid levels.
(a) C20:4-Gly hydrolysis activity in livers from PM20D1-WT, PM20D1-KO, or PM20D1-KO treated with PF-3845. (b, c) Relative fold change of the indicated N-acyl amino acids in PM20D1-KO mice or in PM20D1-KO mice treated with PF-3845 versus wild-type mice in liver (b) or blood (c). For drug treatment, PF-3845 was administered intraperitoneally at 10 mg/kg for three consecutive days and tissues were harvested 3 hr after the final dose. No bars are shown for N-acyl amino acids that were below the limit of detection. Data are shown as means ± SEM, N = 4–5 mice/group for each of the indicated comparisons. All experiments were performed once, with N biological replicates. *, p<0.05; **, p<0.01; ***, p<0.001 in color are versus PM20D1-WT levels, whereas those in black are for the indicated comparison by ANOVA with Tukey’s multiple comparison test.
-
Figure 5—source data 1
Absolute quantitation of N-acyl amino acids in liver and plasma following PM20D1 or dual PM20D1/FAAH blockade.
- https://cdn.elifesciences.org/articles/55211/elife-55211-fig5-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Mouse line (Mus musculus) | PM20D1-KO | Long et al., 2018 (PMID:29967167) | ||
| Mouse line (M. musculus) | C57BL/6J | Jackson Labs | 000664 | |
| Transfected construct (M. musculus) | PM20D1-flag | Addgene | 84566 | |
| Transfected construct (M. musculus) | FAAH-flag | Origene | MR209084 | |
| Transfected construct (M. musculus) | ACY1-flag | Origene | MR206415 | |
| Transfected construct (M. musculus) | CNDP1-flag | Origene | MR219018 | |
| Transfected construct (M. musculus) | CNDP2-flag | Origene | MR207616 | |
| Transfected construct (M. musculus) | PM20D2-flag | Origene | MR222068 | |
| Cell line (Homo sapiens) | HEK293T | ATCC | CRL-3216 | |
| Antibody | Anti-flag M2, mouse monoclonal | Sigma | F1804 | (1:1000) |
| Antibody | Anti-tubulin, rabbit polyclonal | Abcam | Ab6046 | (1:1000) |
| Chemical compound | PF-3845 | Selleckchem | S2666 | |
| Chemical compound | C20:4-Gly | Cayman | 90051 | |
| Chemical compound | C20:4-Ser | Cayman | 10005455 | |
| Chemical compound | C20:4-Phe | Abcam | Ab141612 | |
| Chemical compound | Arachidonic acid | Sigma-Aldrich | 10931 | |
| Chemical compound | WWL70 | Sigma-Aldrich | SML1641 | |
| Chemical compound | Talabostat | R and D | 3719 | |
| Chemical compound | MAFP | Fisher Scientific | 14-21-5 | |
| Chemical compound | C20:4-NAT | Cayman | 10005537 | |
| Chemical compound | Anandamide | Sigma-Aldrich | A0580 | |
| Chemical compound | C16:0-Phe | Lin et al., 2018 (PMID:29533650) | ||
| Chemical compound | C18:0-Phe | Lin et al., 2018 (PMID:29533650) | ||
| Chemical compound | C20:0-Phe | Lin et al., 2018 (PMID:29533650) | ||
| Chemical compound | C22:6-Phe | Lin et al., 2018 (PMID:29533650) | ||
| Chemical compound | C18:1-Asn | Lin et al., 2018 (PMID:29533650) | ||
| Chemical compound | C18:1-Gly | Cayman | 90269 | |
| Chemical compound | C18:1-Lys | Lin et al., 2018 (PMID:29533650) | ||
| Chemical compound | C18:1-Met | Lin et al., 2018 (PMID:29533650) | ||
| Chemical compound | C18:1-Ser | Cayman | 13058 | |
| Chemical compound | C18:1-Trp | Lin et al., 2018 (PMID:29533650) | ||
| Chemical compound | C18:1-Tyr | Lin et al., 2018 (PMID:29533650) | ||
| Chemical compound | C18:1-Gln | Lin et al., 2018 (PMID:29533650) |





