Hippocampal inputs engage CCK+ interneurons to mediate endocannabinoid-modulated feed-forward inhibition in the prefrontal cortex
Figures
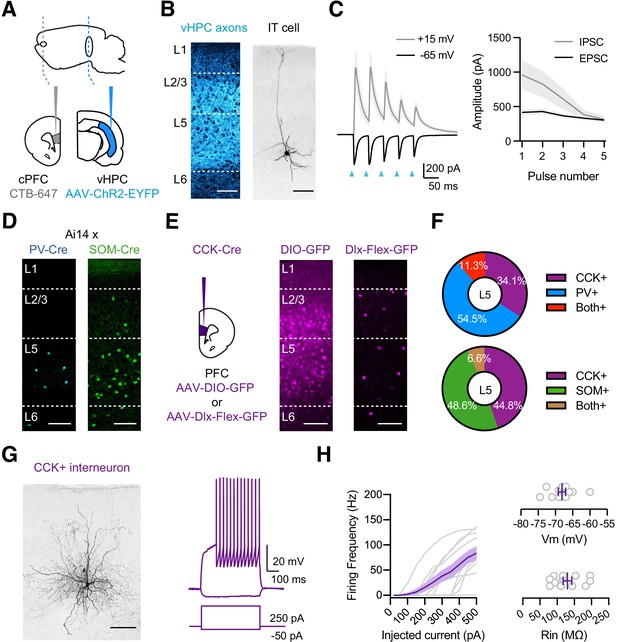
vHPC-evoked feed-forward inhibition and CCK+ interneurons.
(A) Schematic for injections of AAV-ChR2-EYFP into vHPC and CTB-647 into cPFC. (B) Left, Confocal image of vHPC axons (blue) in IL PFC. Scale bar = 100 µm. Right, Confocal image of biocytin-filled L5 IT cell in IL PFC. Scale bar = 100 µm. (C) Left, Average vHPC-evoked EPSCs at −65 mV (black) and IPSCs at +15 mV (gray). Blue arrows = 5 pulses at 20 Hz. Right, Average response amplitudes as a function of pulse number (n = 7 cells, 3 animals). (D) Td-tomato labeling of PV+ (blue) and SOM+ (green) interneurons in PV-Cre × Ai14 and SOM-Cre × Ai14 animals, respectively. Scale bar = 100 µm. (E) Left, Schematic for injections of viruses into PFC of CCK-Cre mice. Middle, Injection of AAV-DIO-GFP labels CCK+ interneurons and pyramidal cells (n = 3 animals). Right, Injection of AAV-Dlx-Flex-GFP labels CCK+ interneurons (n = 3 animals). Scale bars = 100 µm. (F) Quantification of co-labeling of CCK+ interneurons with PV (top) and SOM (bottom) (PV staining, n = 308 cells, 17 slices, 6 animals; SOM staining, n = 105 cells, 8 slices, 3 animals). (G) Left, Confocal image of a biocytin-filled CCK+ interneuron in L5 of IL PFC. Scale bar = 100 µm. Right, Response to positive and negative current injections. (H) Left, Firing frequency (F) versus current (I) curve for CCK+ cells. Right, Summary of membrane resting potential (Vrest) and input resistance (Rin) of CCK+ interneurons (n = 12 cells, 4 animals).
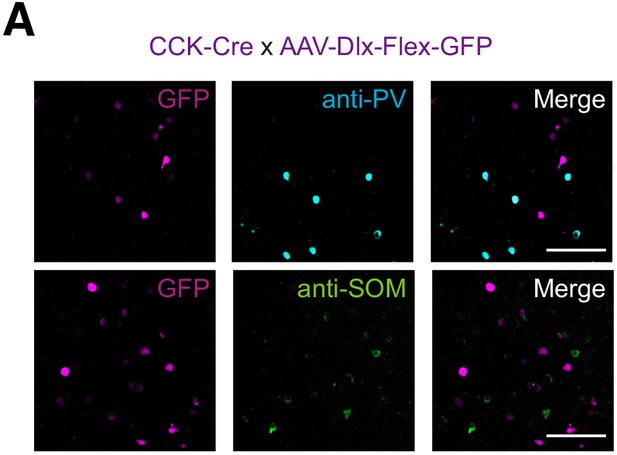
Anatomy of CCK+ and other interneurons in L5 IL PFC.
(A) Co-labeling of GFP-expressing CCK+ interneurons (purple) with PV (blue) and SOM (green). Immunohistochemistry of GFP and either PV (top) or SOM (bottom) in PFC slices from CCK-Cre animals injected with AAV-Dlx-Flex-GFP. From left to right: GFP; PV or SOM; merged. Scale bar = 100 µm.
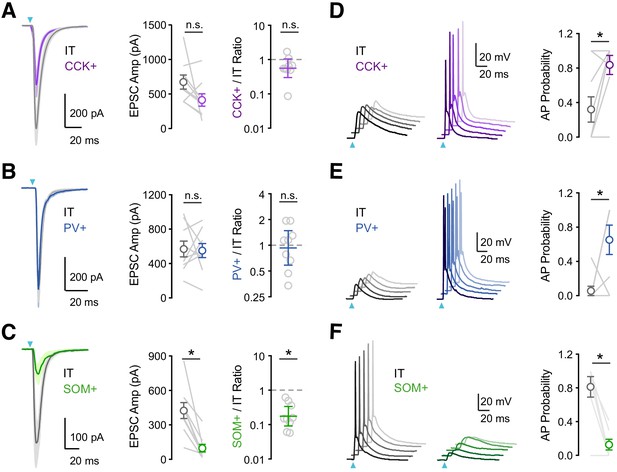
vHPC inputs differentially engage PV+, SOM+, and CCK+ interneurons.
(A) Left, Average vHPC-evoked EPSCs at pairs of L5 IT (gray) and CCK+ (purple) cells in IL PFC. Blue arrow = light pulse. Middle, Summary of EPSC amplitudes. Right, Summary of CCK+/IT EPSC amplitude ratios (n = 9 pairs, 4 animals). (B – C) Similar to (A) for pairs of IT and PV+ (blue) cells (n = 9 pairs, 4 animals) or pairs of IT and SOM+ (green) cells (n = 9 pairs, 4 animals). (D) Left, vHPC-evoked EPSPs and APs recorded in current-clamp from resting membrane potential at pairs of L5 IT (gray) and CCK+ (purple) cells in IL PFC, with 5 traces offset for each cell. Right, Summary of AP probability at pairs of IT and CCK+ cells. Blue arrow = 3.5 mW light pulse (n = 9 pairs, 4 animals). (E – F) Similar to (D) for pairs of IT and PV+ cells (3.5 mW light pulses, n = 8 pairs, 4 animals) or pairs of IT and SOM+ cells (4.8 mW light pulses, n = 7 pairs, 3 animals). *p<0.05.
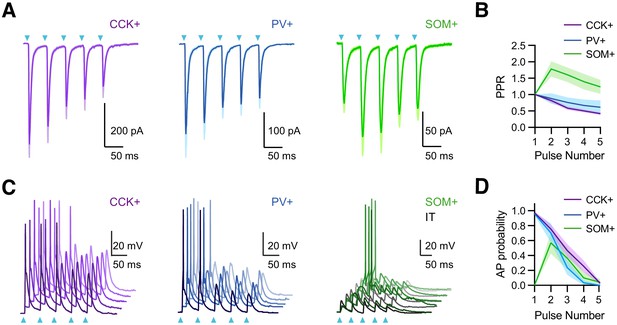
vHPC inputs drive interneurons with distinct temporal dynamics.
(A) Left, Average vHPC-evoked EPSCs at CCK+ interneurons, recorded in voltage-clamp at −65 mV (5 pulses at 20 Hz). Blue arrows = light pulses (n = 6 cells, 3 animals). Middle, For PV+ interneurons (n = 7 cells, 3 animals). Right, For SOM+ interneurons (n = 8 cells, 3 animals). (B) Average paired-pulse ratio (PPR) of vHPC-evoked EPSCs at CCK+, PV+, and SOM+ interneurons. (C) Left, vHPC-evoked EPSPs and APs, recorded in current-clamp from resting membrane potential at an example CCK+ interneuron in L5 of IL PFC, with 5 traces offset for the cell. Blue arrows = light pulses (n = 12 cells, 5 animals). Middle, For PV+ interneurons (n = 6 cells, 3 animals). Right, For SOM+ interneurons. Note that each SOM+ cell was studied with a nearby IT cell under the same recording and stimulation conditions, where the absence of AP firing at IT cells indicated subthreshold of network recurrent activation (n = 6 cells, 3 animals). (D) Summary of average AP probability as a function of pulse number for CCK+, PV+, and SOM+ interneurons.
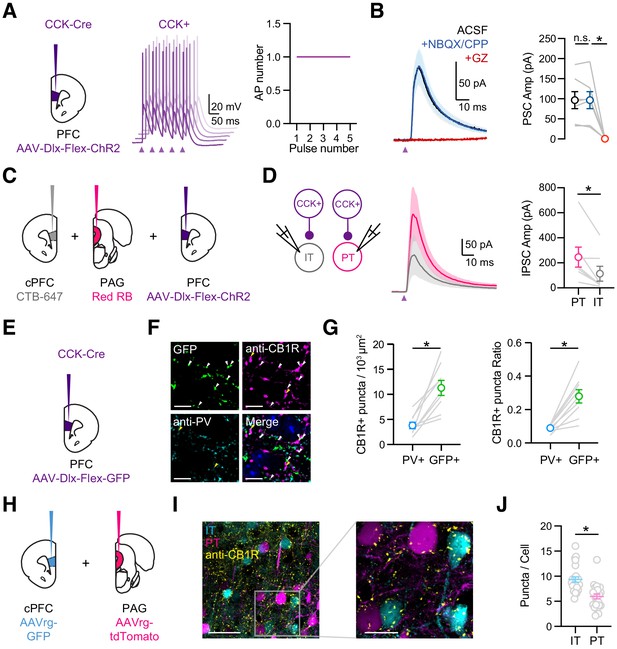
CCK+ interneurons make connections onto pyramidal cells.
(A) Left, Injections of AAV-Dlx-Flex-ChR2 into the PFC of CCK-Cre animals. Middle, Example AP traces from a ChR2-expressing CCK+ interneuron, stimulated with 2 ms light pulses at 20 Hz, showing 5 offset trials. Purple arrow = light pulse. Right, Average AP numbers across stimulation pulses (n = 6 cells, 3 animals). (B) Left, Average CCK+-evoked IPSCs at L5 pyramidal cells in IL PFC. When recording at −50 mV with a low Cl- internal solution, only outward IPSCs were observed (black). IPSCs were unchanged after wash-in of NBQX + CPP (blue) but abolished by wash-in of gabazine (red). Right, Summary of IPSC amplitudes in the different conditions. Purple arrow = light pulse. (C) Schematic of triple injections, with CTB-647 into cPFC, red retrobeads into PAG, and AAV-Dlx-Flex-ChR2 into PFC. (D) Left, Recording schematic of CCK+ inputs onto IT and PT cells. Middle, CCK+-evoked IPSCs at PT and IT cells. Right, Summary of IPSC amplitudes at PT and IT cells (7 cells, 4 animals). (E) Injection schematic. (F) Confocal images of GFP (green), anti-CB1R staining (purple), anti-PV staining (cyan), and merge. Blue labeling in merged image = DAPI. Arrow heads: white = GFP+CB1R+ co-labeling, yellow = PV+CB1R+ co-labeling. (G) Left, Quantification of PV+CB1R+ and GFP+CB1R+ quanta per 103 µm2. Right, Quantification of the ratios of CB1R+ puncta among PV+ and GFP+ puncta. Each line represents counts from one slice (n = 9 slices, 3 animals). (H) Injection schematic of AAVrg-GFP into PFC and AAVrg-tdTomato into PAG. (I) Left, Confocal image of IT cells (cyan), PT cells (magenta), and CB1 receptors (yellow). Scale bar = 50 µm. Right, Magnification of region on left. Scale bar = 20 µm. (J) Quantification of CB1R puncta in IT and PT cells, each dot represents the average puncta number per cell in a slice (n = 247 IT cells, 207 PT cells, 4 animals). *p<0.05.
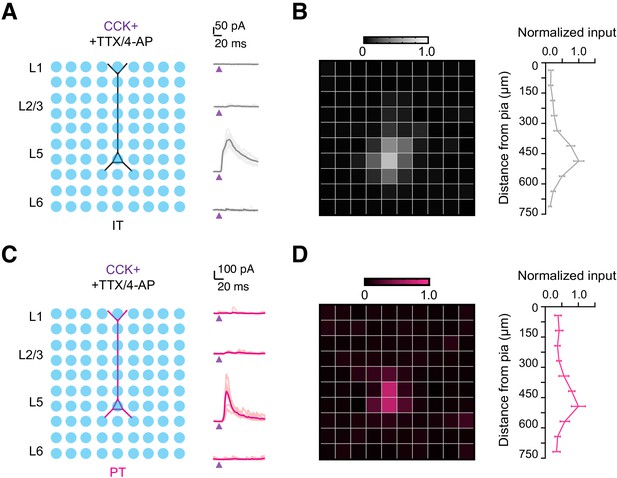
Subcellular targeting of CCK+ interneurons to IT and PT cells in L5 IL PFC.
(A) Left, Schematic for examining the subcellular targeting of CCK+ interneurons to IL L5 IT cells. 75 µm light pixels were delivered at 1 Hz using a pseudo-random 10 × 10 gridded pattern, yielding an effective mapping area of 750 µm × 750 µm, covering the whole dendritic field of the L5 cell. Right, IPSCs recorded at +15 mV at the soma when light spots at L1, L2/3, L5, and L6 were illuminated. Dark traces: average responses; Light traces: responses from individual cells. Purple arrow = 2 ms LED stimulation. (B) Left, Heatmap of normalized average light-evoked IPSC amplitudes across the whole grid. Right, Normalized input amplitudes across the cortical depth (n = 8 cells, 5 animals). (C – D) Similar to (A – B) but for PT cells (n = 7 cells, 5 animals).
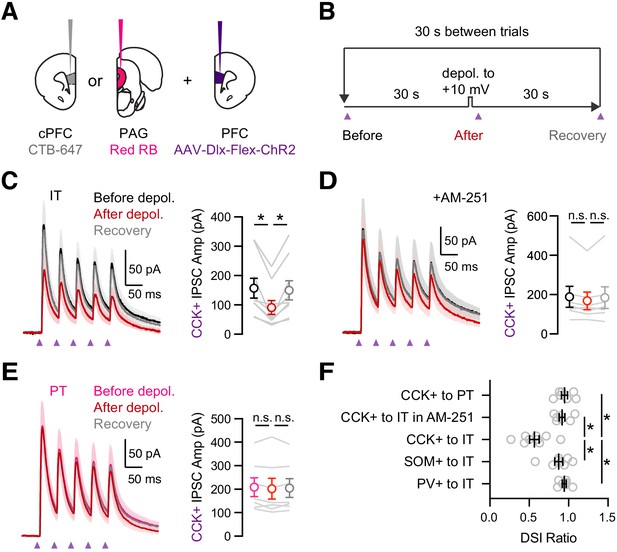
Prominent DSI of CCK+ inputs onto IT cells.
(A) Injection schematic of CTB-647 into cPFC and red retrobeads (RB) into PAG, along with AAV-Dlx-Flex-ChR2 into PFC of CCK-Cre mice. (B) Experimental procedure for depolarization-induced suppression of inhibition (DSI), with 30 s baseline, followed by 5 s depolarization to +10 mV, and 30 s recovery, repeated every 30 s. (C) Left, Average CCK+-evoked IPSCs at IT cells before (black), after (red), and recovery (gray) from the brief depolarization. Purple arrows = 2 ms pulses at 20 Hz. Right, Summary of amplitudes of the first IPSC, showing robust DSI (n = 9 cells, 5 animals). (D) Similar to (C) in the presence of 10 µM AM-251, which abolished DSI (n = 7 cells, 4 animals). (E) Similar to (C) for CCK+-evoked IPSCs at PT cells, showing no DSI (n = 7 cells, 3 animals). (F) Summary of DSI ratios (IPSC after depolarization/IPSC before depolarization) across experiments in (C – E). *p<0.05.
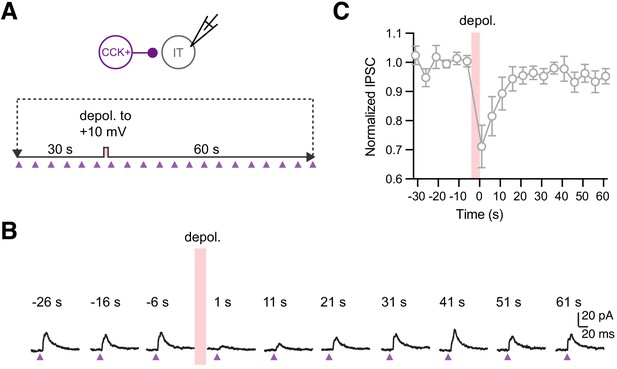
Time course of CCK inputs to IT cells during DSI protocol.
(A) Schematic for examining the time course of DSI. An IT cell was held at −50 mV and 2 ms lights stimulations were delivered at 5 s intervals to establish the baseline input strengths. A brief 5 s depolarization to +10 mV was delivered to trigger DSI. After 1 s, continuous stimuli were applied for 60 s at 5 s intervals to determine the recovery rate. (B) Average traces of an example cell at selected time points. The strengths of IPSCs recovered slowly after the depolarization. Red bar = depolarization period; purple arrow = 2 ms LED stimulation. (C) Normalized IPSC amplitudes showing the average responses across the DSI protocol. Red bar = depolarization period (n = 10 cells, 4 animals).
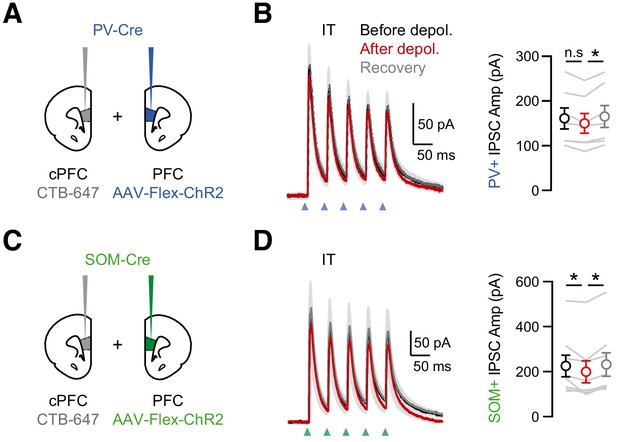
Minimal DSI at PV+ or SOM+ interneuron connections onto IT cells.
(A) Injection schematics of CTB-647 into cPFC and AAV-Flex-ChR2 in PFC of PV-Cre mice. (B) Left, Average PV+ inputs onto IT cells, evoked by 5 pulses at 20 Hz, before (black), immediately after the brief depolarization (red) and after recovery (gray). Blue arrow = light stimulation. Right, Amplitudes of the first IPSCs (n = 7 cells, 3 animals). (C – D) Similar to (A – B) for SOM+ inputs onto IT cells (n = 8 cells, 3 animals).
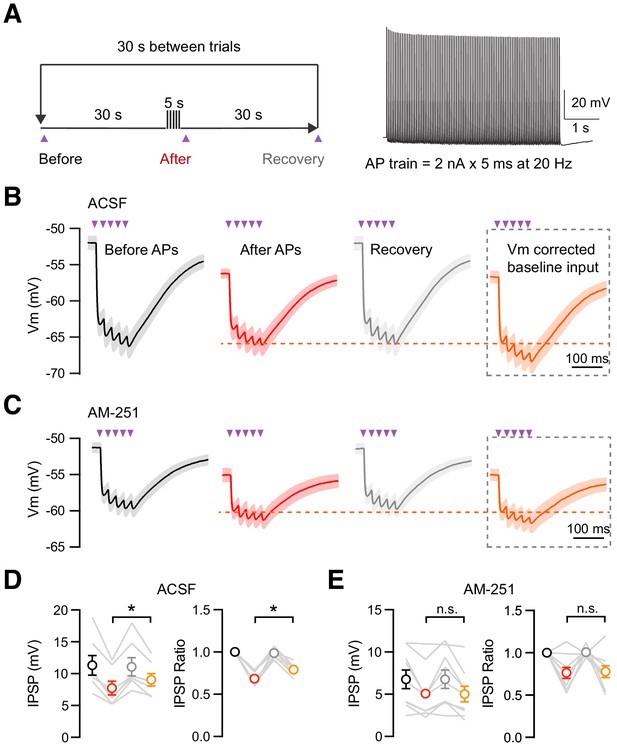
Suppression of inhibition induced by IT cell firing.
(A) Experimental procedure for suppression of inhibition induced by IT cell firing. The procedure was similar to the voltage-clamp DSI protocol shown in Figure 5. IT cells were injected with holding currents to maintain a −50 mV resting potential. During the 5 s firing period, IT cells were injected with 5 ms 2 nA current steps at 20 Hz to maintain a prolonged train of APs. (B) IPSC responses to CCK+ inputs before APs (black), after APs (red), and after recovery (gray) in ACSF. Membrane potentials (Vm) are indicated by the axis on the left. Because cells inevitably showed more hyperpolarized membrane potentials after the prolonged period of spiking, each cell was then held at the corresponding hyperpolarized membrane potential to determine the Vm corrected baseline IPSP strengths (orange). Purple arrow = 2 ms LED stimulation (n = 7 cells, 4 animals). (C) Similar to (B), but in 10 µM AM-251 (n = 9 cells, 4 animals). (D – E) Summary of IPSP amplitudes and ratios in ACSF (D) and AM-251 (E) before APs (black), after APs (red), recovery (gray), and the baseline input when Vm was corrected (orange). *p<0.05.
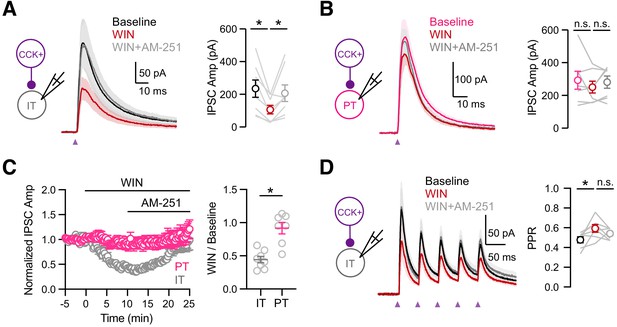
Cell-type specific modulation by CB1 receptors.
(A) Left, Schematic of recordings from IT cells in L5 of IL PFC. Middle, Average CCK+-evoked IPSCs at IT cells at baseline (black), 10 min after wash-in of 1 µM WIN 55,212–2 (red), and 15 min after additional wash-in of 10 µM AM-251 (gray). Purple arrow = light stimulation. Right, Summary of IPSC amplitudes (n = 8 cells, 5 animals). (B) Similar to (A) for CCK+-evoked IPSCs at PT cells, showing lack of modulation by CB1R (n = 7 cells, 4 animals). (C) Left, Summary of time course of modulation at IT and PT cells, with IPSC amplitudes normalized to the average response during the first 5 min. Right, Summary of normalized IPSC amplitudes after WIN wash-in. (D) Similar to (C) for trains of CCK+ inputs onto IT cells (5 pulses at 20 Hz), showing small increase in PPR after wash-in of 1 µM WIN 55,212–2 (n = 7 cells, 4 animals). *p<0.05.
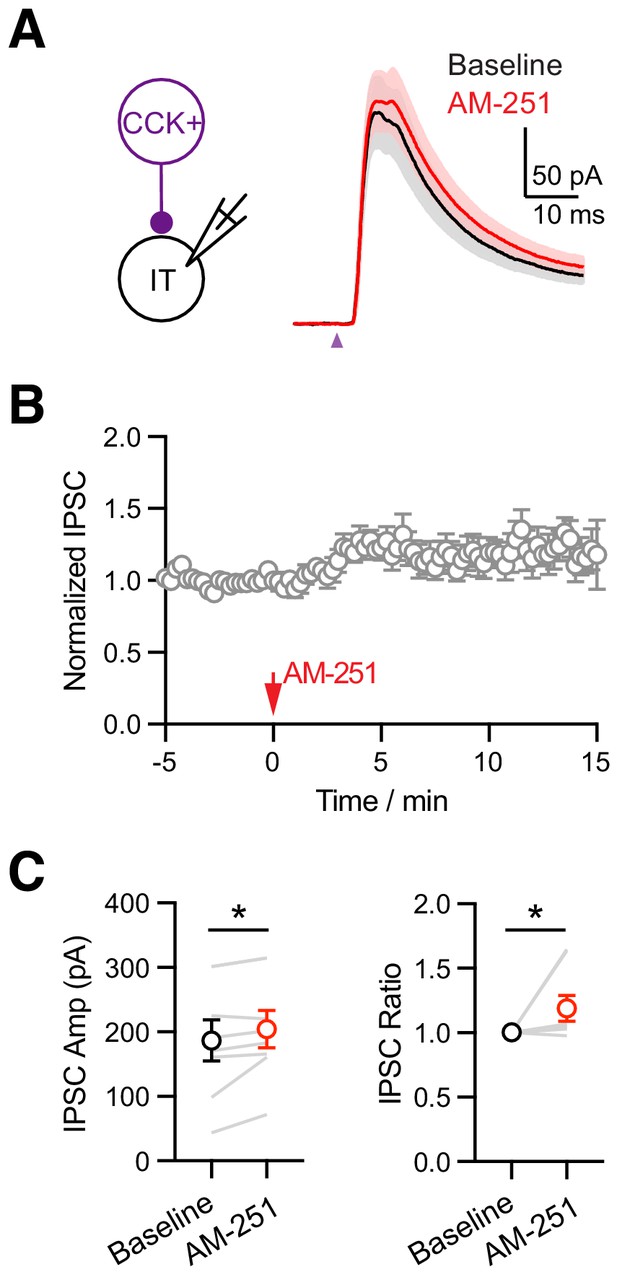
The effect of AM-251 on CCK+ inputs to IL L5 IT cells.
(A) Left, Recording schematic. Right, Average CCK+-evoked IPSCs at IT cells at baseline (black) and 10 min after wash-in of 10 µM AM-251 (red). Purple arrow = 2 ms LED stimulation. (B) Summary of the time course of IPSCs at IT cells in response to AM-251, with IPSC amplitudes normalized to the average baseline responses during the first 5 min. (C) Summary of IPSC amplitudes and ratio comparisons between baseline and 10 min after AM-251 wash-in (n = 8 cells, 5 animals). *p<0.05.
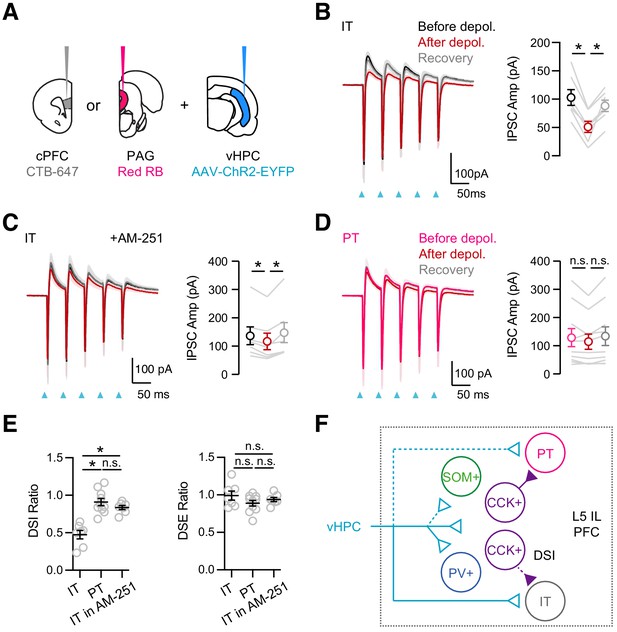
vHPC-evoked feed-forward inhibition at IT cells undergoes DSI.
(A) Injection schematic, showing CTB-647 in cPFC, red retrobeads (RB) in PAG, along with AAV-ChR2-EYFP in vHPC. (B) Left, Average vHPC-evoked EPSCs and IPSCs at IT cells in L5 IL PFC, before (black), immediately after depolarization (red), and after recovery (gray) (same paradigm as Figure 5). Blue arrows = light stimulation. Right, Summary of amplitudes of first vHPC-evoked IPSCs (n = 7 cells, 4 animals). (C) Similar to (B) in the presence of 10 µM AM-251, which reduces DSI (n = 7 cells, 3 animals). (D) Similar to (B) for PT cells, showing no DSI (n = 10 cells, 4 animals). (E) Summary of DSI and DSE ratios (amplitude ratios of IPSCs or EPSCs after/before the depolarizations) across the different experiments. (F) Summary schematic for vHPC-evoked feed-forward inhibition in IL PFC. vHPC inputs directly contact IT over PT cells to evoke EPSCs. vHPC inputs also engage multiple interneurons to evoke local inhibition. Inhibition mediated by CCK+ interneurons displays robust endocannabinoid-mediated DSI, but only at IT cells, and not neighboring PT cells. *p<0.05.
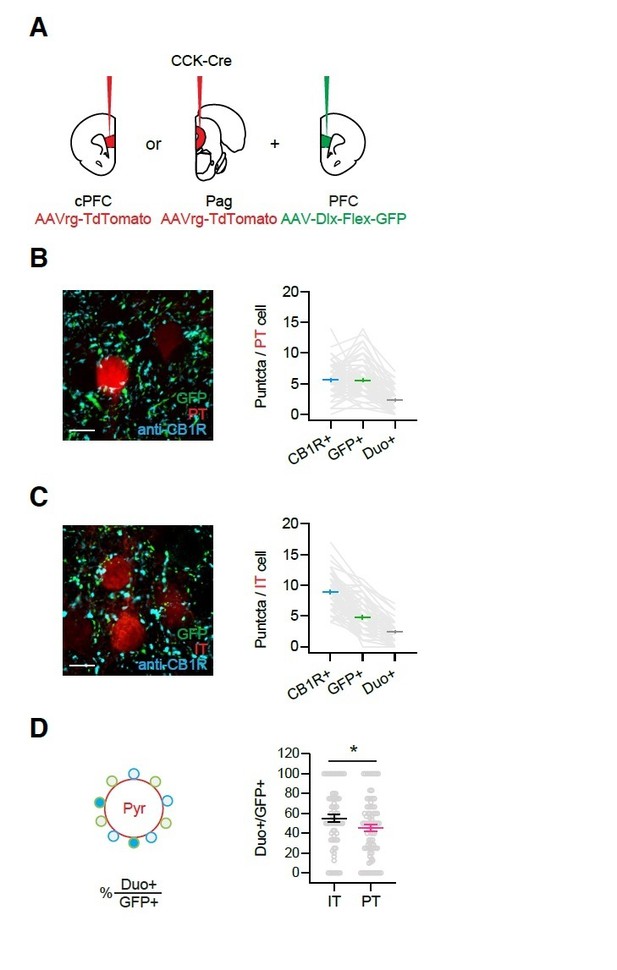
Quantification of CB1R+ and CCK+ puncta around IT and PT soma.
A) Injection schematic. AAVrg-TdTomato was injected to either the cPFC or the Pag along with AAV-Dlx-Flex-GFP to the PFC in CCK-Cre animals, to label IL IT or PT cells with Td-Tomato and CCK+ axons with GFP. Slices were subsequently stained with anti-CB1R antibody. B) Left, Images of GFP and CB1R staining around PT cells. Scale bar = 10 µm. Right, Quantification of average CB1R+, GFP+ and co-labeled puncta (duo+) numbers around IT cells. Each gray line links the counts from one cell. (n = 82 cells, 3 animals) C) Similar to (B), for IT cells. (n = 71 cells, 3 animals) D) Left, Quantification schematic. Right, The percentages of co-labeled puncta (duo+) among GFP+ puncta for IT and PT cells.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (M. musculus, both sexes) | C57BL/6J wild type | Jackson Labs | Stock #: 000664 RRID:IMSR_JAX:000664 | Both sexes |
| Strain, strain background (M. musculus, male) | Pvalbtm1(cre)Arbr (PV-Cre) | Jackson Labs | Stock #: 008069 RRID:IMSR_JAX:008069 | Homozygote male breeder |
| Strain, strain background (M. musculus, male) | Ssttm2.1(cre)Zjh (SOM-Cre) | Jackson Labs | Stock #: 013044 RRID:IMSR_JAX:013044 | Homozygote male breeder |
| Strain, strain background (M. musculus, male) | Ccktm1.1(cre)Zjh (CCK-Cre) | Jackson Labs | Stock #: 012706 RRID:IMSR_JAX:012706 | Homozygote male breeder |
| Strain, strain background (M. musculus, female) | Gt(ROSA)26Sortm14(CAG-tdTomato)Hze (Ai14) | Jackson Labs | Stock #: 007914 RRID:IMSR_JAX:007914 | Homozygote female breeder |
| Other | AAV1-DIO-ChR2-eYFP | UPenn | Cat #: AV-1–20298P | AAV virus expressing Cre-dependent ChR2 |
| Other | AAV1-ChR2-eYFP | UPenn | Cat #: AV-26973P | AAV virus expressing ChR2 |
| Other | AAV1-DIO-eYFP | UPenn | Cat #: AV-1–27056 | AAV virus expressing Cre-dependent eYFP |
| Other | AAVrg-GFP | Addgene | Cat #: 37825-AAVrg RRID:Addgene_37825 | Retrograde virus expressing GFP |
| Other | AAVrg-TdTomato | Addgene | Cat #: 59462-AAVrg RRID:Addgene_59462 | Retrograde virus expressing TdTomato |
| Other | AAV-Dlx-Flex-GFP | Addgene | Cat #: 83900 RRID:Addgene_83900 | AAV virus expressing Cre-dependent GFP in interneurons |
| Other | AAV-Dlx-Flex-ChR2-mCherry | This paper | AAV virus expressing Cre-dependent ChR2 in interneurons | |
| Antibody | Anti-PV (mouse, monoclonal) | Millipore | Cat#: MAB1572 RRID:AB_2174013 | (1:2000) |
| Antibody | Anti-CB1R (guinea pig, polyclonal) | Frontier Institute | Cat#: Af530 RRID:AB_2314113 | (1:500) |
| Antibody | Anti-SOM (rat, monoclonal) | Millipore | Cat#: MAB354 RRID:AB_2255365 | (1:200) |
| Chemical compound, drug | WIN 55,212–2 | Tocris | Cat#: 1038 | 1 µM |
| Chemical compound, drug | AM-251 | Tocris | Cat#: 1117 | 10 µM |