Host sirtuin 2 as an immunotherapeutic target against tuberculosis
Figures
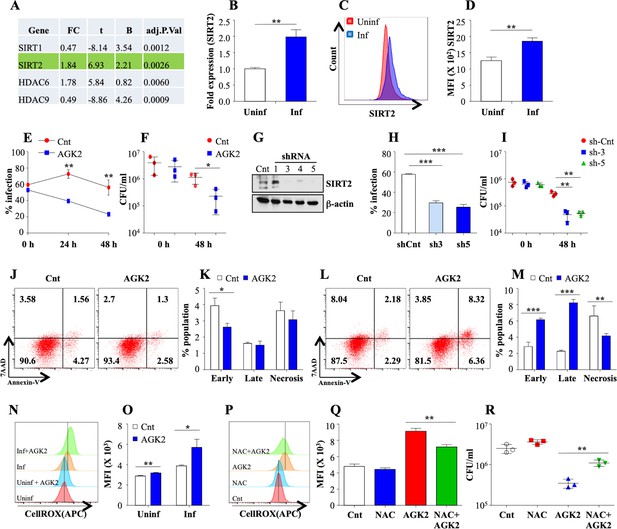
SIRT2 aids in mycobacterial survival ex vivo.
(A) Analysis of microarray data from Mehta et al., 2016 reveals differential expression of SIRT1, SIRT2, HDAC6 and HDAC9 in THP1 cells 24 hr pi. FC: Fold Change in the gene expression in infected THP1 cells as compared to uninfected control, t: moderated t-statistics, B: B-statistics or log-odds that the gene is differentially expressed, adj.p.val: p-value after adjustment for multiple testing. (B) Mouse peritoneal macrophages were infected with Mtb laboratory strain H37Rv for 24 hr and SIRT2 expression was assessed through RT-PCR. (C–D) Mouse peritoneal macrophages infected with Mtb for 24 hr were stained with anti SIRT2 antibody followed by FACS analysis. (E and F) Mouse peritoneal macrophages, pre-treated with AGK2 for 2 hr, were infected with GFP expressing H37Rv and maintained in 10 µM of AGK2 for 48 hr. (E) Percentage of infected cells as analyzed by flow cytometry. (F) In a parallel experiment, cell lysates were plated for bacterial CFU at 0 hr and 48 hr pi. (G–I) RAW 264.7 macrophages were transfected with shRNAs specific for SIRT2. (G) 48 hr post transfection, SIRT2 protein levels were assessed by western blot. Transfected cells were infected with GFP expressing H37Rv for 48 hr. (H) Percentage of infected cells as analyzed by flow cytometry at 48 hr pi. (I) In a parallel experiment, cell lysate was plated for enumeration of CFU. (J–M) Representative dot plots and bar graphs to show the percentage of necrotic and apoptotic cell populations in the uninfected (J and K) and Mtb infected (L and M) peritoneal macrophages with or without AGK2 treatment as analyzed by FACS analysis. (N and O) Representative overlay plots and quantification of cellular ROS measured by staining cells with CellROX followed by flow cytometry. Mouse peritoneal macrophages treated with AGK2 (10 µM), NAC (10 mM) or both were infected H37Rv for 48 hr. (P and Q) Representative overlay plots and bar graph depicting cellular ROS in these cells 48 hr pi. (R) In a parallel experiment, cell lysates were plated for CFU enumeration. Data shown is representative of at least two independent experiments performed in triplicates. The data values represent mean ± SD (n = 3). *p<0.05, **p<0.005, ***p<0.0005.
-
Figure 1—source data 1
SIRT2 inhibition enhances anti-mycobacterial potential of host macrophages.
Source data for Figure 1B, D–F, H, I, K, M, O, Q and R.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig1-data1-v2.xlsx
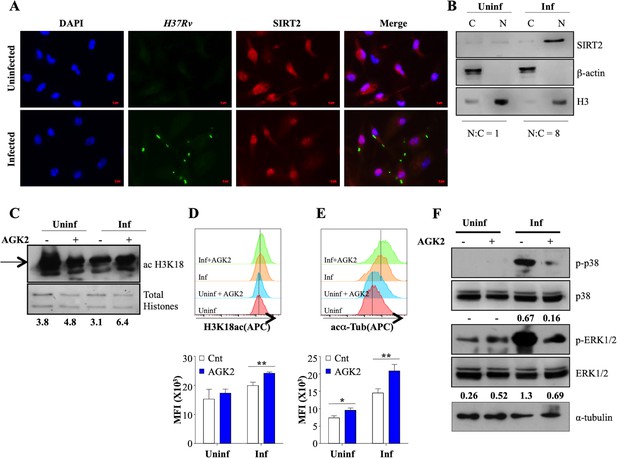
SIRT2 translocate to the nucleus and modulates histone deacetylations and cellular signaling during Mtb infection.
(A) Endogenous SIRT2 was detected by immunofluorescence in mouse peritoneal macrophages uninfected or infected with H37Rv for 4 hr. (B) Uninfected macrophages or macrophages infected with H37Rv for 24 hr were fractionated for cytosol and nucleus followed by immunoblotting for the indicated proteins. Mouse peritoneal macrophages, pre-treated with AGK2 for 2 hr were infected H37Rv followed by AGK2 treatment for 24 hr. Acetylation levels of H3K18 were checked by (C) immunoblotting and (D) intracellular staining followed by flow cytometry. (E) Acetylated α-tubulin levels in uninfected and Mtb-infected cells with or without AGK2 treatment at 24 hr pi. (F) Mouse peritoneal macrophages, pre-treated with AGK2 for 2 hr were infected with H37Rv for 2 hr. Phosphorylation status of the indicated proteins was checked by immunoblotting. Data shown is representative of at least two independent experiments performed in triplicates. Each bar represents mean ± SD (n = 3). *p<0.05, **p<0.005.
-
Figure 2—source data 1
SIRT2 migrates to the nucleus upon Mtb infection.
Source data for Figure 2B and D.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig2-data1-v2.xlsx
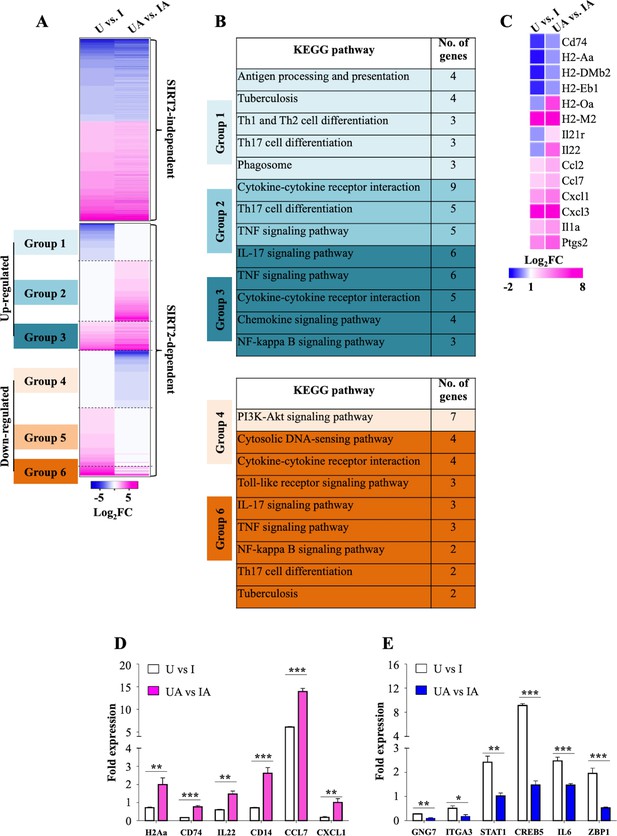
SIRT2 modulates host gene transcription during Mtb infection.
(A) Heatmap interpretation of gene expression changes (Log2 fold) as determined by RNAseq analysis of peritoneal macrophages infected with Mtb for 24 hr. Pink depicts upregulation while blue represents repression. (B) KEGG analysis of molecular signaling pathways affected by AGK2 treatment. (C) Heatmap representing the expression profile of the genes involved in T cell activation. qRT-PCR of candidate genes which are either (D) up- or (E) downregulated in SIRT2 dependent manner. . U: Uninfected. UA: Uninfected treated with AGK2. I: Infected. IA: Infected with AGK2 treatment. RNAseq was performed in three biological replicates. Each bar in (D) and (E) represents mean ± SD (n = 3). *p<0.05, **p<0.005, ***p<0.0005.
-
Figure 3—source data 1
SIRT2 modulates transcriptional landscape of Mtb-infected macrophages.
Source data for Figure 3A and B–E.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig3-data1-v2.xlsx
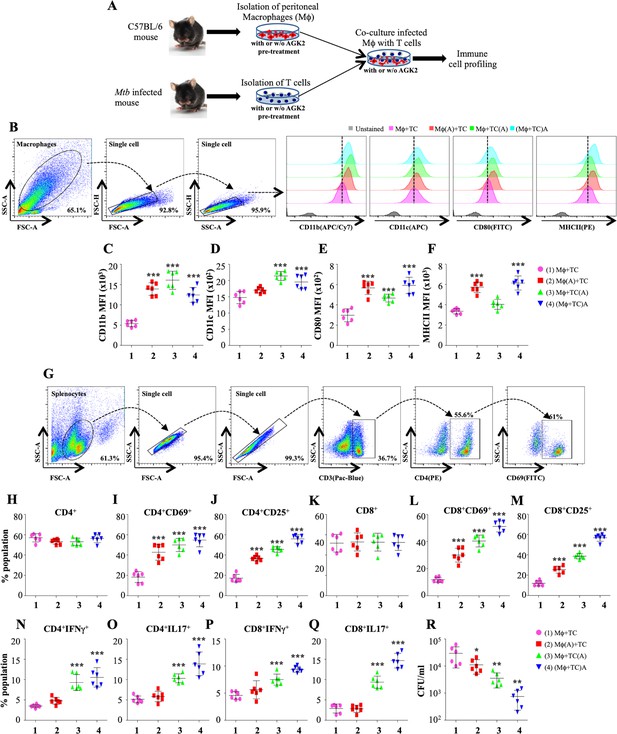
SIRT2 inhibition activates macrophages and T cells to induce pro-inflammatory response.
(A) Schematic representation of the experimental plan (for details see Materials and methods). 48 hr post co-culture, macrophages were stained with CD11b (APC/Cy7), CD11c (APC), CD80 (FITC) and MHC-II (PE) followed by flow cytometry analysis. (B) Gating strategy employed and representative overlay plots to depict macrophage activation. Expression of (C) CD11b, (D) CD11c, (E) CD80 and (F) MHCII on the surface of peritoneal macrophages. (G) Gating strategy for T cell activation. Splenocytes were stained for surface markers CD3 (Pacific Blue), CD4 (PE), CD8 (APC/Cy7), CD69 (FITC) and CD25 (APC) followed by flow cytometry. Percentage of (H) CD4+, (I) CD4+CD69+, (J) CD4+CD25+, (K) CD8+, (L) CD8+CD69+ and (M) CD8+CD25+ T cells. For cytokine analysis, splenocytes were stained with CD3 (Pacific Blue), CD4 (PE), CD8 (APC/Cy7), IFNγ (APC) and Il17 (PE/cy7). (N and O) Intracellular cytokines (IFNγ and IL17) expressed by CD4+ T cells. (P and Q) CD8+ T cells expressing IFNγ and IL17. (R) CFU at 48 hr post co-culture. Combined data from two independent experiments performed in triplicates is shown. Each bar represents mean ± SD (n = 6). Statistical analysis performed using one-way ANOVA followed by Tukey post hoc test. *p<0.05, **p<0.005, ***p<0.0005.
-
Figure 4—source data 1
SIRT2 inhibition leads to the activation of macrophages and T cells upon Mtb infection.
Source data for Figure 4C–F and H–R.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig4-data1-v2.xlsx
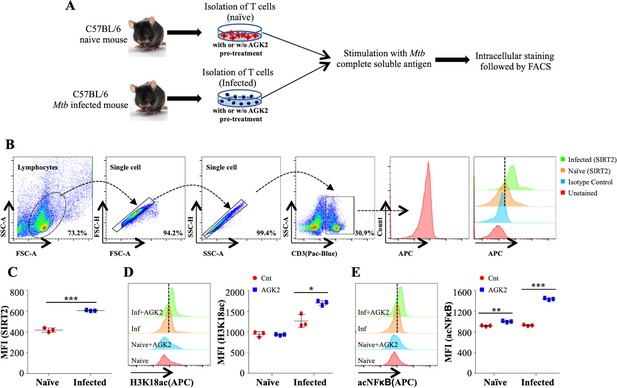
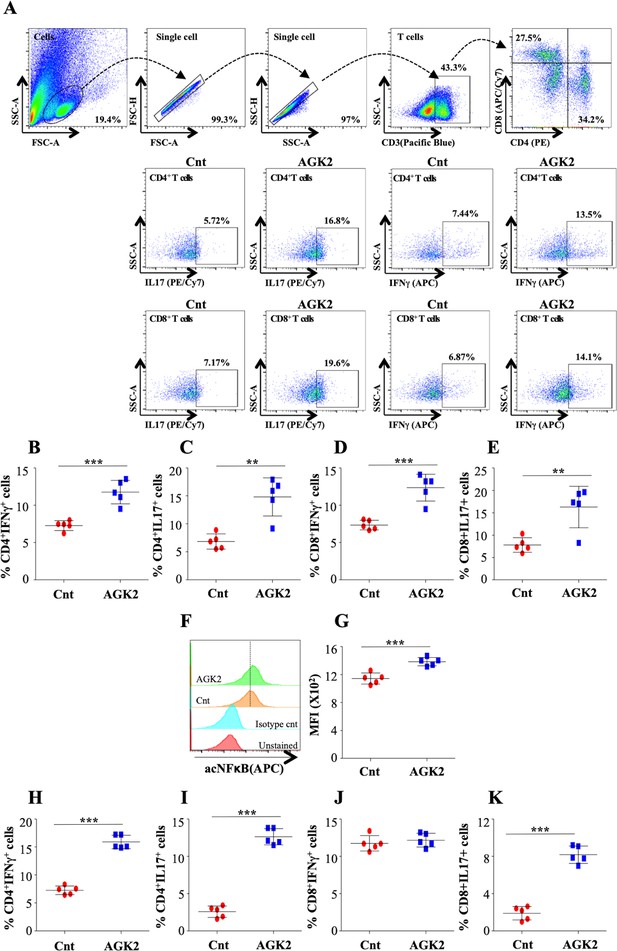
SIRT2 targets histone and non-histone proteins in Mtb-specific T-cells to ameliorate anti-mycobacterial host immune responses.
(A) Schematic representation of the experimental plan. (B) Gating strategy utilized to analyze the intracellular levels of (C) SIRT2, (D) H3K18ac and (E) acetylated NFκB p65. Data shown is representative of at least two independent experiments each performed in triplicates. Data is represented as mean ± SD (n = 3). *p<0.05, **p<0.005, ***p<0.0005.
-
Figure 5—source data 1
SIRT2 deacetylates H3K18 and NFκB-p65 in Mtb-specific T cells.
Source data for Figure 5C, D and E.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig5-data1-v2.xlsx
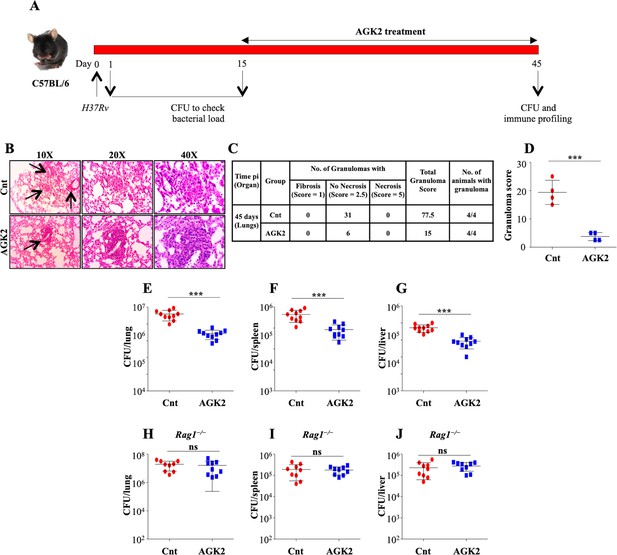
SIRT2 inhibition restricts mycobacterial growth in vivo.
(A) Schematic representation of the murine model of infection. A group of C57BL/6 mice were infected with low dose of H37Rv. After 15 days of disease establishment, mice were either left untreated or were treated with AGK2 (20 mg/kg) for 30 days. (B) Histopathological analysis of infected lungs with arrows indicating the granulomatous lesions. (C and D) Quantification of the number of granulomas (granuloma score) observed in the infected mice. (E–G) Bacterial load in the lungs, spleen and liver of mice at 45 days pi. (H–J) A group of Rag1-/- mice were infected with low dose of H37Rv followed by treatment with AGK2 (20 mg/kg) for 30 days. The graphs represent bacterial burden in the lungs, spleen and liver of Rag1-/- mice. E-J represents combined data from two independent experiments with four to five mice in each group. Granuloma score was obtained from the lungs of four mice per group. Data is represented as mean ± SD. *p<0.05, **p<0.005, ***p<0.0005.
-
Figure 6—source data 1
AGK2 treatment reduces bacterial burden in murine model of TB.
Source data for Figure 6D–J.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig6-data1-v2.xlsx
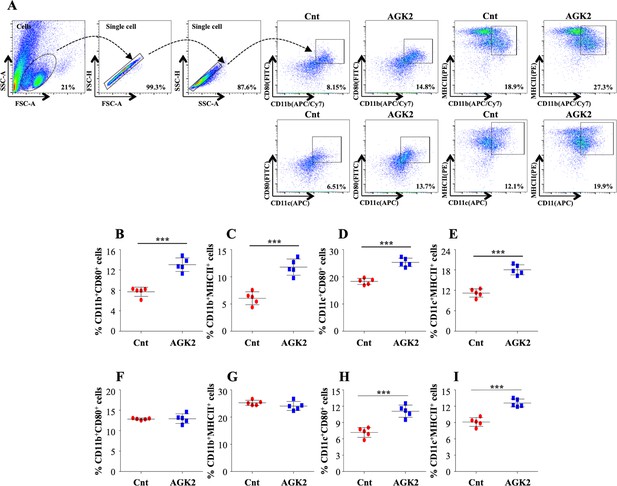
Inhibition of SIRT2 activity enhances macrophage stimulation in the lungs and spleen of infected animals.
(A) After overnight stimulation with CSA, the cells isolated from the lungs of infected C57BL/6 animals were surface stained with CD11b (APC/Cy7), CD11c (APC), CD80 (FITC) and MHC-II (PE) followed by flow cytometry analysis. Gating strategy employed and flow cytometry dot plots of CD11b+CD80+, CD11b+MHCII+, CD11c+CD80+, CD11c+MHCII+ cells in the lungs of infected mice (B–E) Percentage of (B) CD11b+CD80+, (C) CD11b+MHCII+, (D) CD11c+CD80+ and (E) CD11c+MHCII+ cells in the lungs. (F–I) Percentage of CD11b+CD80+, CD11b+MHCII+, CD11c+CD80+, CD11c+MHCII+ cells in the spleen of infected mice. Data is representative of two independent experiments with five mice per group. Each graph represents mean ± SD (n = 5). ***p<0.0005.
-
Figure 7—source data 1
AGK2 treatment enhances macrophage stimulation in the lungs and spleen of infected animals.
Source data for Figure 7B–I.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig7-data1-v2.xlsx
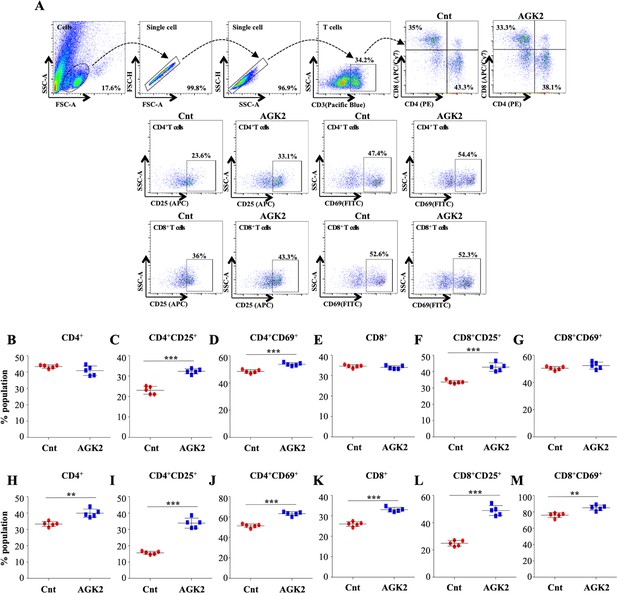
SIRT2 inhibition ameliorates T cell activation in the lungs and spleen of infected mice.
Cells isolated from the lungs of control and AGK2-treated Mtb-infected C57BL/6 animals were subjected to surface staining (CD3-Pacific Blue, CD4-PE, CD8-APC/Cy7, CD69-FITC and CD25-APC) followed by FACS analysis. (A) Gating strategy and representative dot plots. Scatter plots depicting the percentage of CD4+, CD4+CD69+, CD4+CD25+, CD8+, CD8+CD69+ and CD8+CD25+ T cells in the (B–G) lungs and the (H–M) spleen of infected mice. The experiment was performed twice with five mice in each group. Data is represented as mean ± SD (n = 5). **p<0.005, ***p<0.0005.
-
Figure 8—source data 1
SIRT2 inhibition increases T cell activation in vivo.
Source data for Figure 8B–M.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig8-data1-v2.xlsx
AGK2 treatment induces host protective immune responses against TB.
Cells isolated from infected lungs of C57BL/6 mice were stained with CD3 (Pacific Blue), CD4 (PE), CD8 (APC/Cy7), IFNγ (APC) and IL17 (PE/cy7) followed by flow cytometry. (A) Gating strategy and representative flow cytometry dot plots. Percentage of IFNγ and IL17 producing CD4+ (B and C) and CD8+ (D and E) T cells in the lungs of infected mice. (F) Overlay plots and (G) quantification of acetylated NFκB p65 levels in the CD3+ T cells isolated from the lungs of infected mice. (H–K) IFNγ and IL17 expressing CD4+ (H and I) and CD8+ (J and K) T cells in the spleen of infected mice. The experiment was performed twice with five mice in each group. Data is represented as mean ± SD (n = 5). **p<0.005, ***p<0.0005.
-
Figure 9—source data 1
SIRT2 inhibition skews immune response toward Th1/Th17 phenotype Source data for Figure 9B–D and G–K.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig9-data1-v2.xlsx
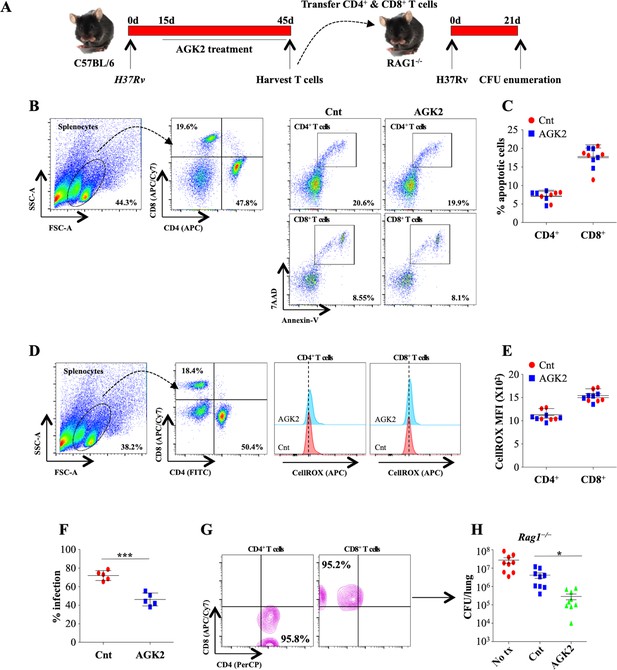
AGK2-treated splenocytes demonstrate increased propensity toward mycobacterial killing and transfer Mtb-specific protective immunity in naive animals.
(A) Schematic representation of the adoptive transfer experiment. Splenocytes isolated from infected and AGK2-treated C57BL/6 mice were analyzed for (B and C) apoptosis by Annexin V staining and (D and E) ROS production via cellROX (see Materials and methods). (F) Splenocytes isolated from infected and AGK2 treated mice were co-cultured with peritoneal macrophages infected with GFP expressing H37Rv. 48 hr pi, percentage of infected cells were analyzed by flow cytometry. CD4+ and CD8+ T cells purified from the spleen of infected and AGK2-treated mice (G) were intravenously transferred into Rag1-/- mice followed by challenge with aerosolized Mtb. (H) CFU enumeration 21 days after adoptive transfer. Data is representative of two independent experiments performed with four to five mice in each group. Data is represented as mean ± SD (n = 5). H shows combined data from two independent experiments (n = 9). *p<0.05, ***p<0.0005.
-
Figure 10—source data 1
AGK2-treated splenocytes transfer Mtb-specific protective immunity in naive animals.
Source data for Figure 10C, E, F and H.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig10-data1-v2.xlsx
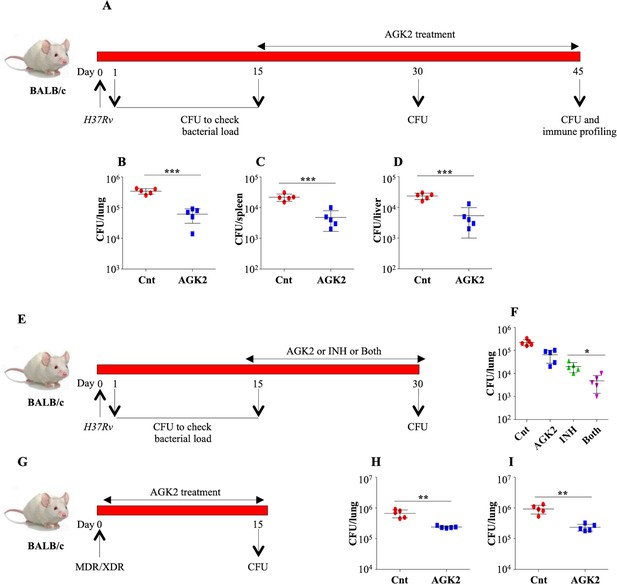
AGK2 treatment increases the efficacy of anti-TB drug INH.
(A) Experimental plan for infection in BALB/c mice. (B–D) CFU enumeration to determine the bacterial burden in the lungs, spleen and liver of infected mice. (E) Schematic representation of the adjunct therapy experiment. A group of mice were infected with low dose of H37Rv. Following a rest of 15 days, mice were either left untreated or were treated with AGK2, INH or both. After 15 days of treatment, mice were euthanized for CFU enumeration in the lungs. (F) CFU from the lung homogenates of respective animals. (G) Schematic representation of the AGK2 treatment infection model. (H and I) CFU obtained from the lung homogenates of mice infected with MDR and XDR strains of Mtb with and without AGK2 treatment. The experiment was performed once with five mice in each group. Data is represented as mean ± SD (n = 5). *p<0.05, **p<0.005, ***p<0.0005.
-
Figure 11—source data 1
Efficacy of AGK2 as an adjunct to current anti-TB drug INH.
Source data for Figure 11B–D, F, H and I.
- https://cdn.elifesciences.org/articles/55415/elife-55415-fig11-data1-v2.xlsx
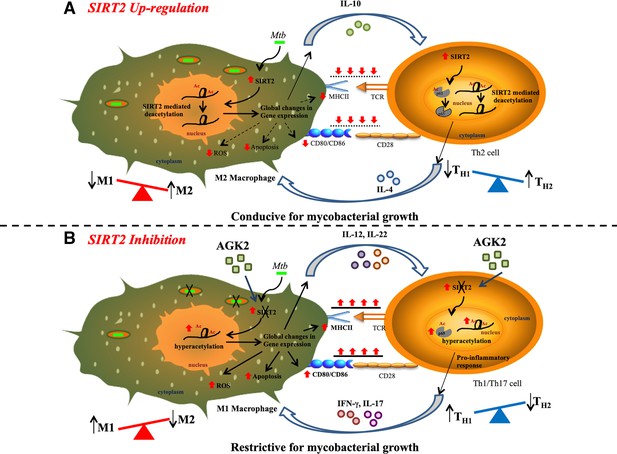
Proposed mechanism of SIRT2 up-regulation and consequence of SIRT2 inhibition on Mtb survival.
(A) Mtb infection induces the expression and translocation of SIRT2 in the nucleus where it deacetylates histone H3K18 to induce consequential changes in host transcriptome leading to decreased activation of macrophages, reduced levels of apoptosis and ROS, and increased expression of anti-inflammatory Th2-polarizing cytokines. Simultaneously, upregulation of SIRT2 in Mtb-specific T-cells specifically deacetylates and deactivates NFκB p65 leading to an anti-inflammatory response. M2 macrophages and Th2 cells preferentially generated provide a more favorable environment for Mtb survival within the host. (B) SIRT2 inhibition in Mtb-infected cells reverses the SIRT2-dependent gene expression changes enhancing the capacity of macrophages to activate T-cells. AGK2 treatment also enhances anti-mycobacterial defense forces in macrophages such as apoptosis, ROS and pro-inflammatory Th1/Th17 polarizing cytokines. Moreover, hyperacetylation of NFκB p65 in Mtb-specific activated T-cells leads to Th1/Th17-mediated pro-inflammatory immune response. Enhanced levels of protective innate and adaptive immunity in AGK2 treated cells restrict mycobacterial survival.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (M. musculus) | B6.129S7-Rag1tm1Mom/J | PMID:1547488 | RRID:IMSR_JAX:002216 | |
| Cell line (M. musculus) | RAW 264.7 | ATCC | RRID:CVCL_0493 | |
| Antibody | Rabbit monoclonal [EP959Y] to Histone H3 (acetyl K18) | Abcam | Cat#ab40888 | 1:10000 (WB) 1:2000 (FC) |
| Antibody | Rabbit polyclonal to NF-kB p65 (acetyl K310) | Abcam | Cat#ab19870 | 1:5000 (WB) 1:1000 (FC) |
| Antibody | Rabbit polyclonal to beta Actin | Abcam | Cat#ab8227 | 1:5000 (WB) |
| Antibody | Mouse monoclonal [GT114] to alpha Tubulin | Abcam | Cat#ab184613 | 1:5000 (WB) |
| Antibody | Rabbit monoclonal [EPR16772] to alpha Tubulin (acetyl K40) | Abcam | Cat#ab179484 | 1:2500 (WB) 1:1000 (FC) |
| Antibody | Rabbit monoclonal [EPR20411] to SIRT2 | Abcam | Cat#ab211033 | 1:2500 (WB) 1:1000 (FC) |
| Antibody | FITC anti-mouse CD4 Antibody | Biolegend | Cat#100510 | 1:250 (FC) |
| Antibody | PerCP anti-mouse CD4 Antibody | Biolegend | Cat#100433 | 1:250 (FC) |
| Antibody | APC anti-mouse CD4 Antibody | Biolegend | Cat#100411 | 1:250 (FC) |
| Antibody | APC/Cyanine7 anti-mouse CD8a Antibody | Biolegend | Cat#100713 | 1:250 (FC) |
| Antibody | FITC anti-mouse CD69 Antibody | Biolegend | Cat#104506 | 1:250 (FC) |
| Antibody | APC anti-mouse CD25 Antibody | Biolegend | Cat#101910 | 1:250 (FC) |
| Antibody | APC/Cy7 anti-mouse/human CD11b Antibody | Biolegend | Cat#101226 | 1:250 (FC) |
| Antibody | APC anti-mouse CD11c Antibody | Biolegend | Cat#117310 | 1:250 (FC) |
| Antibody | APC anti-mouse IFN-γ Antibody | Biolegend | Cat#505810 | 1:250 (FC) |
| Antibody | PE/Cyanine7 anti-mouse IL-17A Antibody | Biolegend | Cat#506922 | 1:250 (FC) |
| Antibody | PE anti-mouse CD4 Antibody | Biolegend | Cat#100512 | 1:250 (FC) |
| Antibody | FITC anti-mouse CD80 Antibody | Biolegend | Cat#104706 | 1:250 (FC) |
| Antibody | PE anti-mouse I-A/I-E Antibody | Biolegend | Cat#107607 | 1:250 (FC) |
| Antibody | Pacific Blue anti-mouse CD3 Antibody | Biolegend | Cat#100214 | 1:250 (FC) |
| Antibody | p44/42 MAPK (Erk1/2) Antibody | Cell Signaling Technology | Cat#9102 | 1:2000 (WB) |
| Antibody | Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) Antibody | Cell Signaling Technology | Cat#9101 | 1:1000 (WB) |
| Antibody | p38 MAPK Antibody | Cell Signaling Technology | Cat#9212 | 1:2000 (WB) |
| Antibody | Phospho-p38 MAPK (Thr180/Tyr182) (D3F9) XP Rabbit mAb | Cell Signaling Technology | Cat#4511 | 1:1000 (WB) |





