Protective role of neuronal and lymphoid cannabinoid CB2 receptors in neuropathic pain
Figures
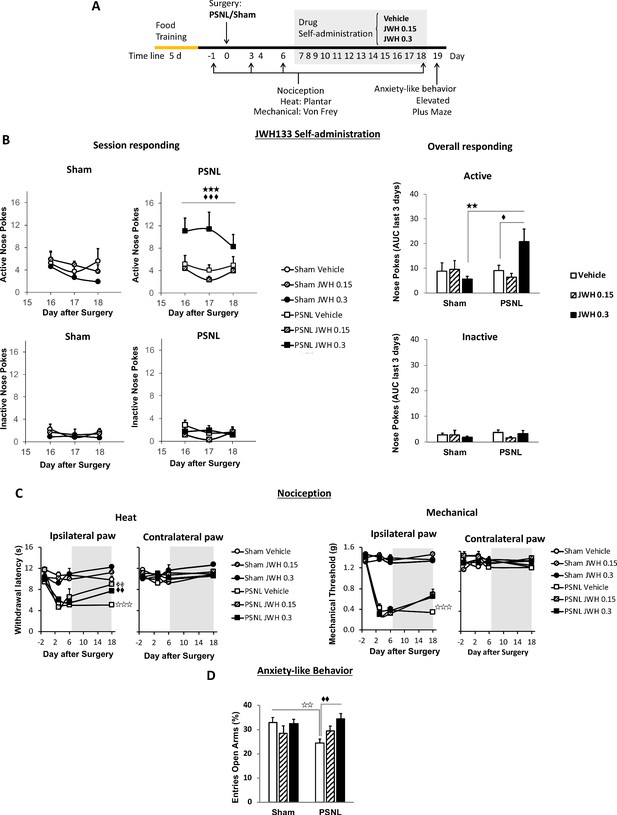
C57BL/6J mice self-administer a CB2 receptor agonist with antinociceptive and anxiolytic-like properties.
(A) Timeline of the drug self-administration paradigm. Mice were trained in Skinner boxes (5 days, 5d) where nose-poking an active sensor elicited delivery of food pellets. Partial sciatic nerve ligation (PSNL) or sham surgery were conducted (day 0) followed by jugular catheterization to allow intravenous (i.v.) drug infusion. From days 7 to 18, mice returned to the operant chambers and food was substituted by i.v. infusions of JWH133 (0.15 or 0.3 mg/kg/inf.). Mechanical and thermal sensitivity were assessed before (−1) and 3, 6 and 18 days after PSNL using Plantar and von Frey tests. Anxiety-like behavior was measured at the end (day 19) with the elevated plus maze. (B) Nerve-injured mice poked the active sensor to consume the high dose of JWH133 (0.3 mg/kg/inf.). (C) PSNL-induced ipsilateral thermal and mechanical sensitization (days 3 and 6). JWH133 inhibited thermal hypersensitivity but the effect on mechanical nociception was not significant (D) Nerve-injured mice receiving vehicle showed decreased percentage of entries to the open arms of the elevated plus maze, whereas PSNL mice receiving JWH133 0.3 mg/kg/inf. did not show this alteration. N = 5–10 mice per group. Shaded areas represent drug self-administration. Mean and error bars representing SEM are shown. Stars represent comparisons vs. sham; diamonds vs. vehicle. *p<0.05; **p<0.01; ***p<0.001.
-
Figure 1—source data 1
JWH133 self-administration, antinociception and anxiolytic-like effects in C57BL6/J mice.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig1-data1-v3.xlsx
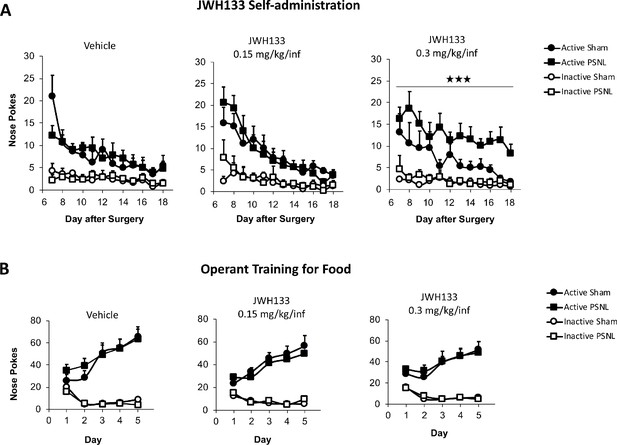
JWH133 self-administration after nerve injury or sham surgery in C57BL6/J mice and food-maintained operant training before the drug self-administration.
(A) The first day of i.v. self-administration, sham-operated mice exposed to the vehicle showed higher active nose pokes than nerve-injured mice exposed to the same treatment. For the rest of the JWH133 self-administration period, mice exposed to the vehicle or to the low dose of JWH133 (0.15 mg/kg/inf) showed similar operant behaviour, regardless of the type of surgery. Nerve-injured mice exposed to the high dose of JWH133 (0.3 mg/kg/inf.) showed higher active nose poking than sham mice exposed to this dose. Inactive responding was similar regardless of type of the surgery and treatment. (B) All groups of mice developed operant behaviour directed to obtain food pellets before the partial sciatic nerve ligation (PSNL) or sham surgery. N = 7–10 mice per group. Mean and error bars represent SEM. Stars represent p<0.001 vs. respective sham group.
-
Figure 1—figure supplement 1—source data 1
Operant training and full JWH133 self-administration in C57BL6/J mice.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig1-figsupp1-data1-v3.xlsx
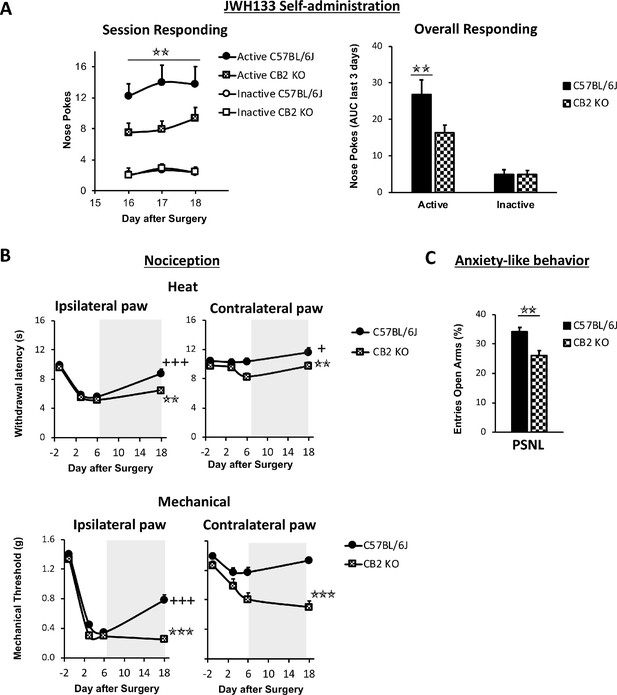
Nerve-injured mice constitutively lacking CB2 receptor show disruption of JWH133 intake and blunted effects of the drug.
CB2 constitutive knockout mice (CB2 KO) and C57BL/6J mice were food-trained in Skinner boxes (Food training, 5 days), subjected to a partial sciatic nerve ligation (PSNL, day 0), catheterized and exposed to high doses of the CB2 agonist JWH133 (0.3 mg/kg/inf., days 7 to 18). Nociceptive sensitivity to heat (Plantar) and mechanical (von Frey) stimulation were measured before and after the nerve injury (−1,3,6,18), and anxiety-like behavior was evaluated at the end (day 19). (A) CB2 KO mice showed decreased active operant responding for the CB2 agonist. (B) The effects of JWH133 on thermal nociception were reduced in constitutive knockout mice. CB2 KO mice showed contralateral mechanical and thermal sensitization and complete abolition of JWH133 effects on mechanical hypersensitivity. (C) Anxiety-like behavior after the treatment worsened in CB2 KO mice. N = 16–19 mice per group. Mean and error bars representing SEM are shown. Shaded areas represent drug self-administration. Stars represent comparisons vs. C57BL/6J mice; crosses represent day effect. *p<0.05; **p<0.01; ***p<0.001.
-
Figure 2—source data 1
JWH133 self-administration, antinociception and anxiolytic-like effects in nerve-injured CB2 constitutive knockout mice.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig2-data1-v3.xlsx
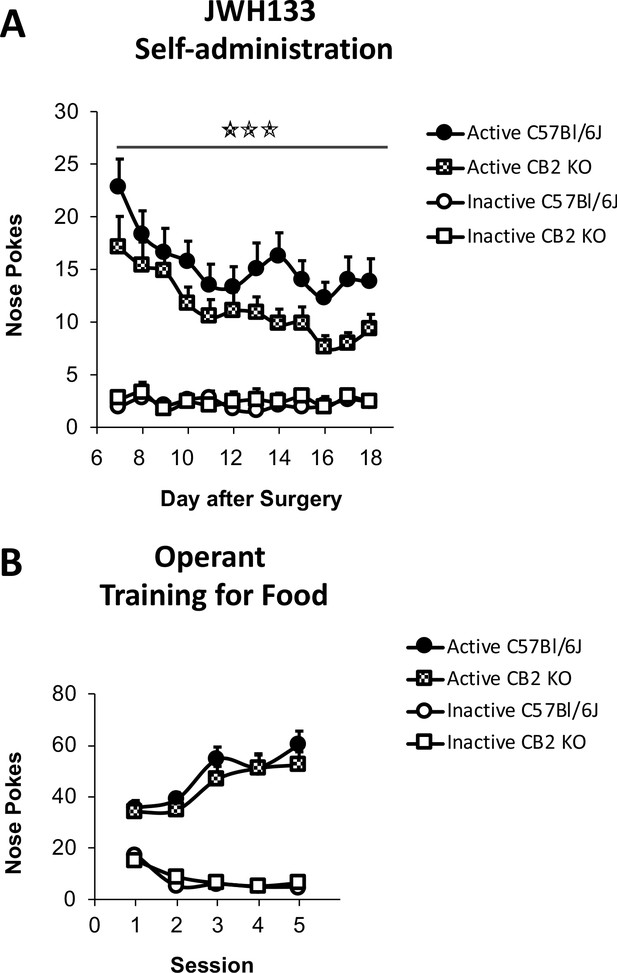
JWH133 self-administration in C57BL6/J and CB2 constitutive knockout (CB2 KO) mice and food-maintained operant training before nerve injury and drug self-administration.
(A) Nerve-injured CB2 KO mice showed a disruption of the active operant behavior directed to obtain high doses of the CB2 agonist JWH133 (0.3 mg/kg/inf.). (B) C57BL6/J and CB2 KO mice developed similar operant behavior for food before the partial sciatic nerve ligation. N = 16–19 mice per group. Stars represent p<0.001 vs. C57Bl6/J.
-
Figure 2—figure supplement 1—source data 1
Operant training and full JWH133 self-administration in CB2 constitutive knockout mice.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig2-figsupp1-data1-v3.xlsx
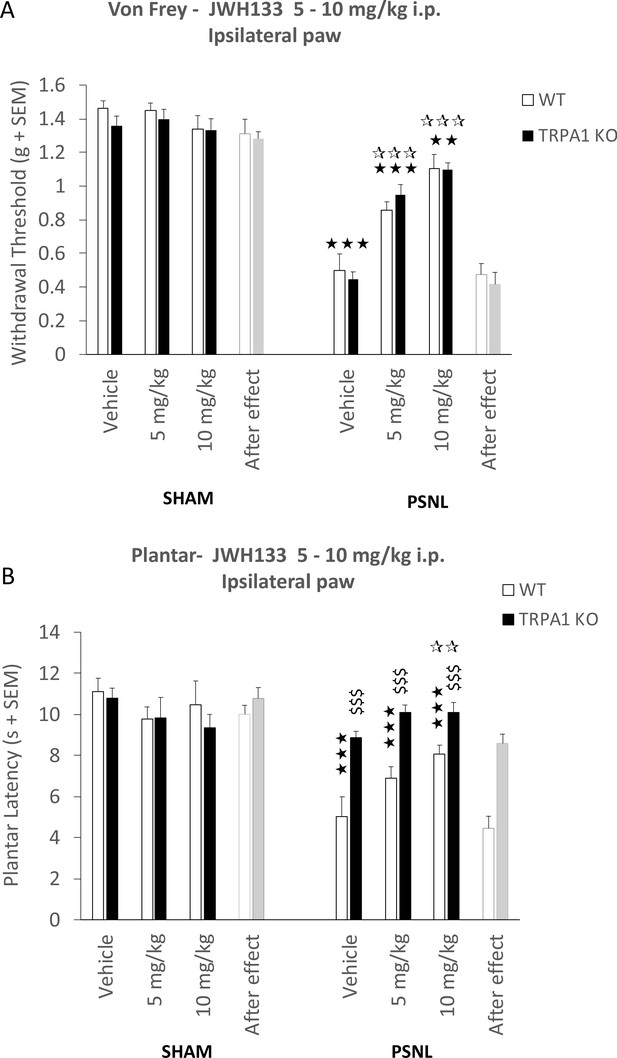
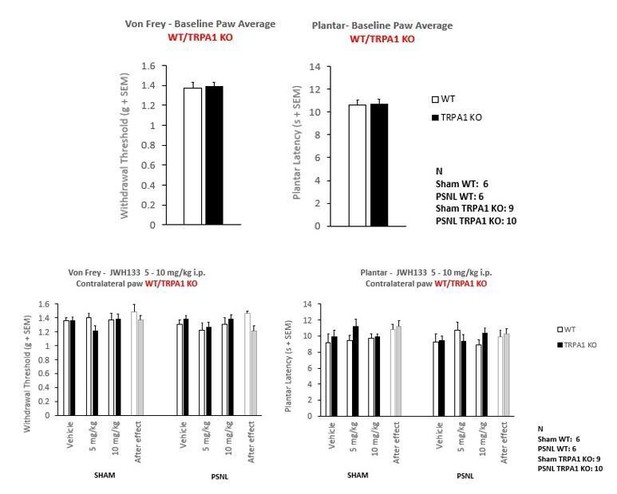
Mice lacking TRPA1 receptor retain JWH133- effects.
(A) Intraperitoneal doses (i.p.) of 5 and 10 mg/kg JWH133 had similar effects on mechanical hypernociception of nerve-injured mice lacking TRPA1 (TRPA1 KO) and nerve-injured wild-type mice (WT). (B) Nerve-injured TRPA1KO mice showed a dramatic reduction of thermal hyperalgesia. A JWH133 effect persisted after the high JWH133 dose (10 mg/kg), regardless of the genotype. White stars are comparisons vs. Vehicle, Black stars vs. Sham, Dollars vs. WT. *p<0.05, **p<0.01,***p<0.001. N = 6–10 mice per group. Means and SEM are shown.
-
Figure 2—figure supplement 2—source data 1
Antinociceptive effect of JWH133 in TRPA1 knockout mice.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig2-figsupp2-data1-v3.xlsx
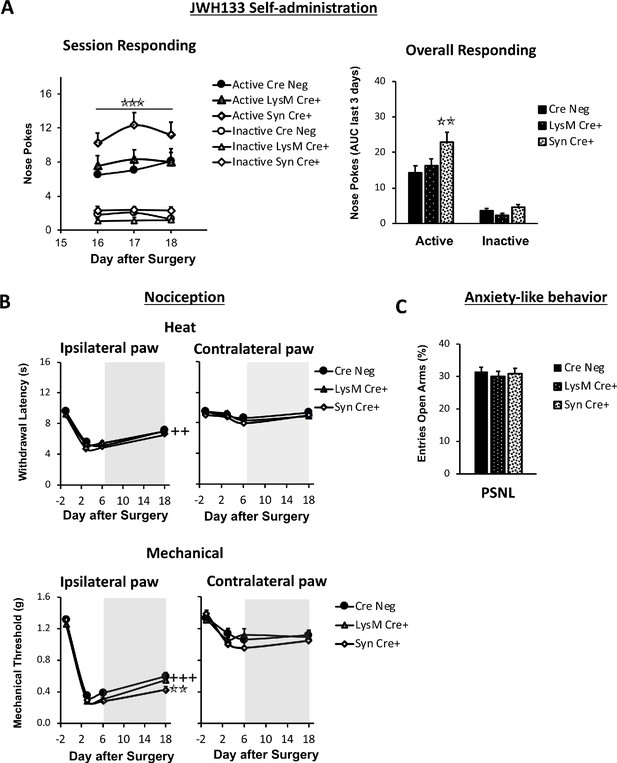
Nerve-injured mice defective in neuronal CB2 receptor show increased self-administration of the CB2 agonist JWH133 and a decrease in the antinociceptive effects of the drug.
Mice lacking CB2 in neurons (Syn-Cre+), in monocytes (LysM-Cre+) or their wild-type littermates (Cre Neg) were food-trained in Skinner boxes (Food training, 5 days), subjected to partial sciatic nerve ligation (PSNL, day 0), catheterized and exposed to JWH133 (0.3 mg/kg/inf., days 7 to 18). Nociceptive sensitivity to heat (Plantar) and mechanical (von Frey) stimulation were measured before and after nerve injury (−1,3,6,18), anxiety-like behavior was evaluated at the end (day 19). (A) Syn-Cre+ mice showed increased active operant responding for JWH133 in the last sessions of the self-administration period (B) All mouse strains showed decreased heat nociception after JWH133 treatment, and Syn-Cre+ mice showed reduced effects of JWH133 on mechanical nociception. (C) Every mouse strain showed similar anxiety-like behavior after JWH133 self-administration. No significant differences were found between LysM-Cre+ and Cre Neg mice. N = 18–36 mice per group. Mean and error bars representing SEM are shown. Shaded areas represent drug self-administration. Stars represent comparisons vs. Cre Neg mice; crosses represent day effect. *p<0.05; **p<0.01; ***p<0.001.
-
Figure 3—source data 1
JWH133 self-administration, antinociception and anxiolytic-like effects in nerve-injured neuronal or microglial CB2 knockout mice.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig3-data1-v3.xlsx
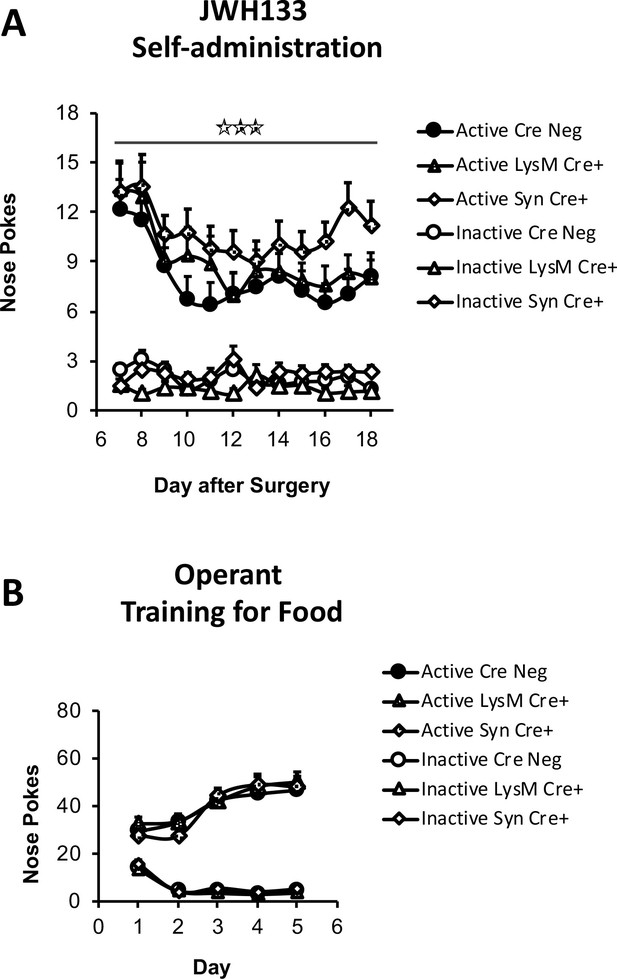
JWH133 self-administration in mice lacking CB2 in neurons or monocytes and their wild-type littermates and food-maintained operant training before nerve injury and drug self-administration.
(A) Mice lacking CB2 in neurons (Syn-Cre+) mice showed increased active operant behavior directed to obtain high doses of the CB2 agonist JWH133 (0.3 mg/kg/inf.). Operant responding for the CB2 agonist was similar between mice lacking CB2 in monocytes (LysM-Cre+) mice and their wild-type littermates (Cre Neg). (B) Syn-Cre+, LysM-Cre+ and Cre Neg mice developed similar operant behavior for food before the partial sciatic nerve ligation. N = 18–36 mice per group. Stars represent p<0.001 vs. Cre Neg.
-
Figure 3—figure supplement 1—source data 1
Operant training and full JWH133 self-administration in neuronal or microglial CB2 knockout mice.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig3-figsupp1-data1-v3.xlsx
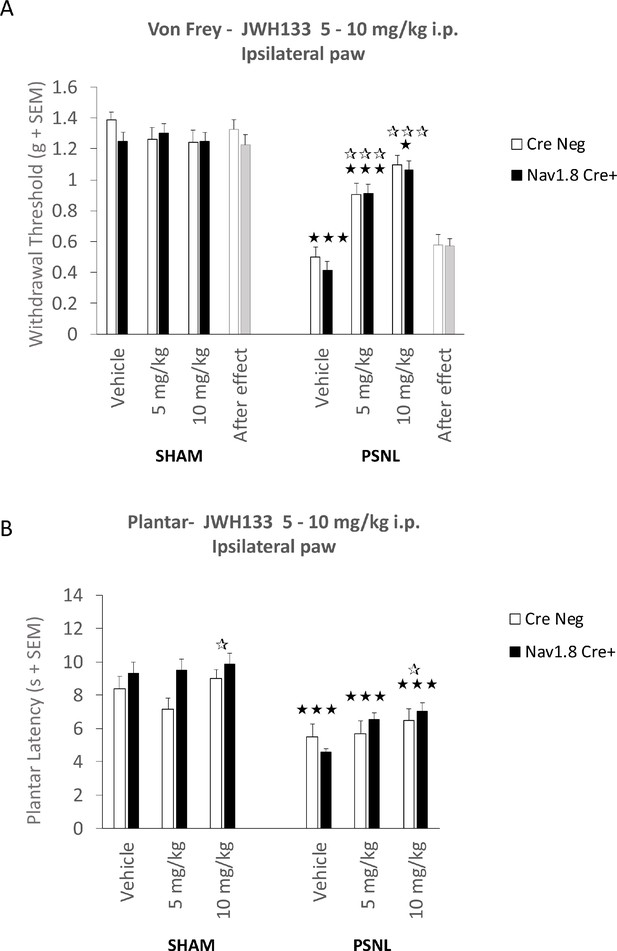
Mice lacking CB2 in Nav1.8+ peripheral neurons show unaltered JWH133 antinociceptive effects.
Intraperitoneal doses (i.p.) of 5 and 10 mg/kg JWH133 had similar effects on mechanical (A) and thermal (B) hypernociception of nerve-injured mice lacking CB2 in peripheral neurons (Nav1.8-Cre+) and their wild-type littermates (Cre Neg). Mechanical and thermal thresholds before (Vehicle, 0 mg/kg, 7 days after surgery) and after the end of the treatments (After effect) also revealed similar sensitivity in both strains. White stars represent comparisons vs. Vehicle, Black stars vs. Sham. *p<0.05, ***p<0.001. N = 8–18 mice per group. Means and error bars represent SEM.
-
Figure 3—figure supplement 2—source data 1
Antinociceptive effect of JWH133 in CB2 Nav1.8 Cre+ mice lacking CB2 in primary afferent neurons.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig3-figsupp2-data1-v3.xlsx
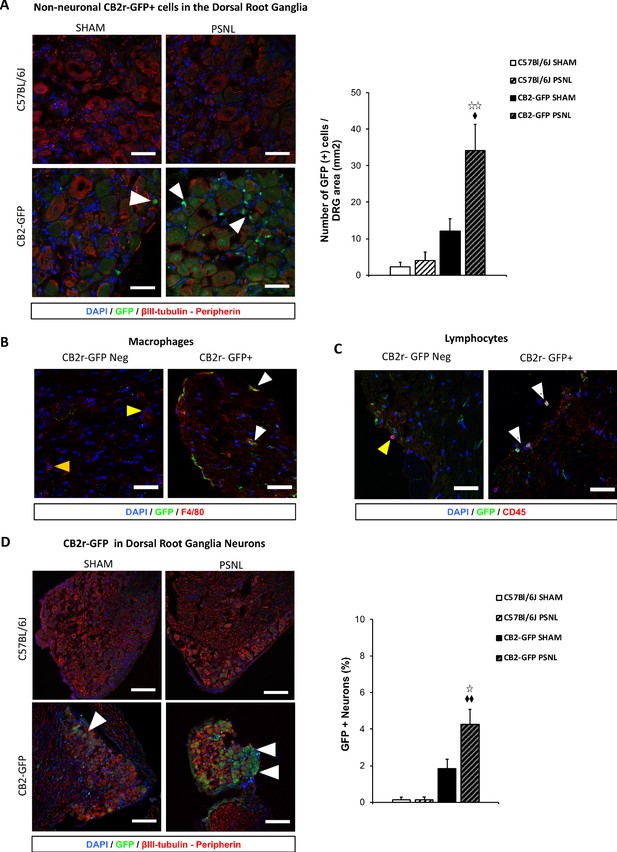
CB2 receptor-GFP immune cells infiltrate the dorsal root ganglia of the injured nerve and GFP from bone-marrow-derived cells is also found inside peripheral neurons.
The figure shows images of L3-L5 dorsal root ganglia from sham (SHAM) or nerve-injured mice (PSNL) transplanted with bone marrow cells from CB2 GFP BAC mice (CB2-GFP) or C57BL6/J mice (C57BL6/J). (A, D) Dorsal root ganglia sections stained with the nuclear marker DAPI (Blue), anti-GFP (Green), and neuronal markers anti-β-III tubulin and anti-peripherin (Red). (A) CB2-GFP mice showed significant infiltration of GFP+ bone-marrow-derived cells after the nerve injury, whereas sham or nerve-injured C57BL6/J mice did not show significant GFP immunorreactivity. Split channels in Figure 4—figure supplement 2. (B) Co-localization of CB2-GFP and the macrophage marker anti-F4/80. Co-staining with anti-GFP and anti-F4/80 revealed GFP+ (~60%) and GFP negative macrophages infiltrating the injured nerve. Split channels in Figure 4—figure supplement 3A. (C) Co-staining with anti-GFP and anti-CD45 revealed GFP+ (~40%) and GFP-negative lymphocytes infiltrating the injured nerve. Split channels in Figure 4—figure supplement 3B. (D) CB2-GFP mice showed a percentage of GFP+ neurons that was enhanced with the nerve injury. Scale bar, 140 μm. Split channels in Figure 4—figure supplement 4. Scale bar for B), C), D), 45 μm. Yellow arrows point to GFP negative cells and white arrows to GFP+ cells. A certain degree of image processing has been applied equally across the entire merged images for optimal visualization. N = 2–3 mice per group. Means and error bars representing SEM are shown. Stars represent comparisons vs. sham; diamonds vs. C57BL6/J. *p<0.05, **p<0.01, ***p<0.001. Flow cytometry of blood from CB2-GFP and C57BL6/J mice in Figure 4—figure supplement 1. Additional images of Sham and nerve-injured CB2-GFP mice in Figure 4—figure supplements 5 and 6. Specificity tests for Tyramide Signal Amplification in Figure 4—figure supplement 7. Controls for antibody specificity in Figure 4—figure supplement 8.
-
Figure 4—source data 1
CB2 GFP cells in dorsal root ganglia of C57BL6/J nerve-injured mice after bone-marrow transplants from CB2 GFP BAC mice.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig4-data1-v3.xlsx
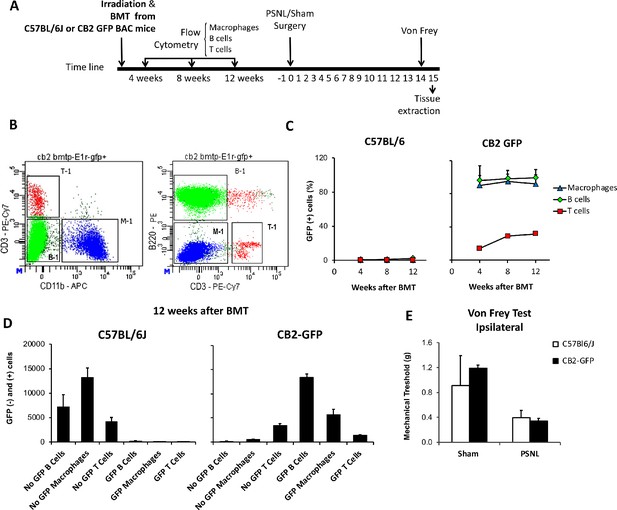
Bone marrow transplantation from CB2 GFP BAC to C57BL6/J mice yields mice with peripheral blood cells expressing GFP.
(A) C57BL6/J mice were irradiated and immediately transplanted with bone marrow cells from CB2 GFP BAC or C57BL6/J mice, yielding CB2-GFP or C57BL6/J mice. Repeated flow cytometry assays were conducted 4, 8 and 12 weeks after the bone marrow transplantation to assess reconstitution of the immune system. Afterwards, a nerve injury or sham surgery was conducted in mice with successful reconstitution (day 0). 14 days later, mechanical nociception was assessed with the von Frey test and dorsal root ganglia samples were collected the following day. (B) Representative dot plot of flow cytometry showing the labeling of peripheral blood cells from a CB2-GFP bone marrow-transplanted mouse. Cells were pre-gated as single live cells using DAPI staining. T cells (T-1), B cells (B-1) and macrophages (M-1) were gated. PE/Cy7-labeled CD3+ T lymphocytes, APC CD11b+ myeloid cells and PE-B220-labeled B lymphocytes. (C) Percentage of GFP+ immune cells in C57Bl/6J and CB2-GFP mice from 4 to 12 weeks after transplantation. (D) 12 weeks after transplantation CB2-GFP mice showed 98% of macrophages GFP+, 86% of B cells GFP+ and 30% of T cells GFP+, whereas C57BL/6J mice did not show significant GFP signal in the different cell populations. n = 4–6 mice per group. (E) Mechanical thresholds measured before sample collection showed ipsilateral paw sensitization in C57BL/6J and CB2-GFP mice with the nerve injury. n = 2–3 mice per group. Means and error bars representing SEM are shown.
-
Figure 4—figure supplement 1—source data 1
Flow cytometry of blood from C57BL6/J mice transplanted with bone marrow from CB2 GFP BAC mice.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig4-figsupp1-data1-v3.xlsx
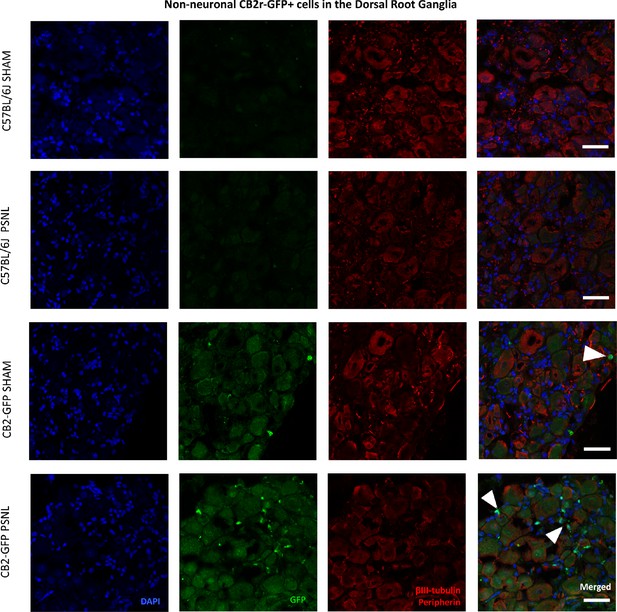
Non-neuronal CB2 -GFP+ cells in the Dorsal Root Ganglia.
Split and merged channels of L3-L5 dorsal root ganglia images from sham or nerve-injured mice transplanted with bone marrow cells from CB2 GFP BAC mice (CB2-GFP) or C57BL6/J mice (C57BL6/J). Dorsal root ganglia sections were stained with the nuclear marker DAPI, anti-GFP, and neuronal markers anti-β-III tubulin and anti-peripherin. Sham or nerve-injured C57BL6/J mice did not show significant GFP immunorreactivity. CB2-GFP mice showed infiltration of bone-marrow=derived cells enhanced with the nerve injury. Scale bar, 45 μm. White arrows point to GFP+ cells. A certain degree of image processing has been applied equally across merged images to allow optimal visualization.
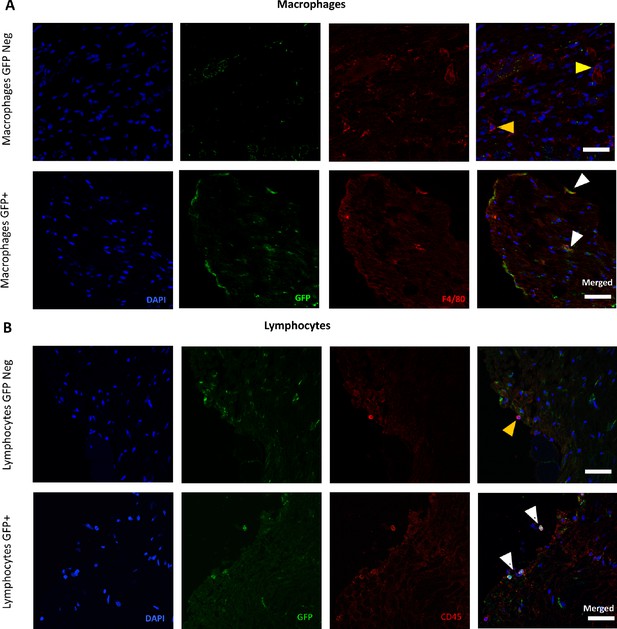
Presence of CB2 receptor-GFP in immune cells in the Dorsal Root Ganglia.
Split and merged channels showing (A) Co-localization of CB2-GFP and the macrophage marker anti-F4/80. (B) Co-staining of anti-GFP and lymphocyte marker anti-CD45. Scale bar, 45 μm. Yellow arrows point to GFP-negative cells and white arrows to GFP+ cells. Certain degree of image processing has been applied equally across merged images for optimal visualization.
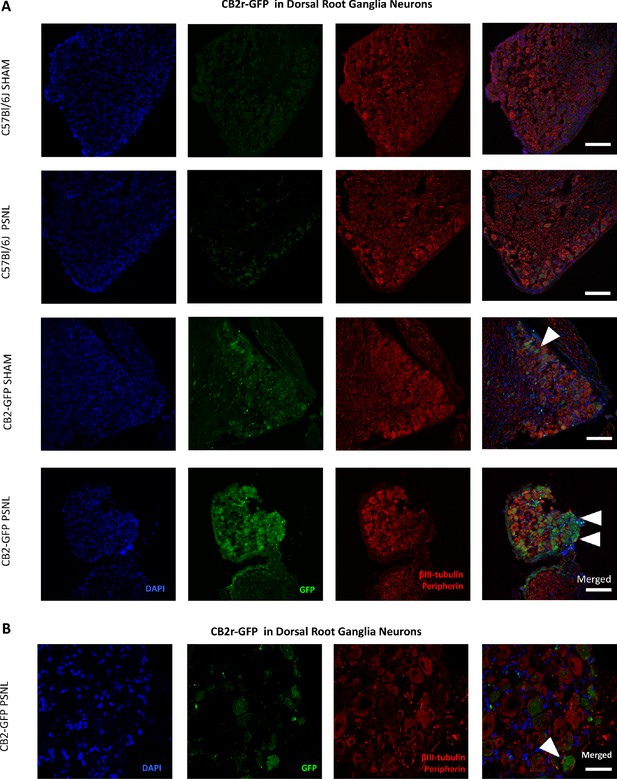
CB2-GFP in Dorsal Root Ganglia Neurons.
Images of L3-L5 dorsal root ganglia from sham or nerve-injured mice transplanted with bone marrow cells from CB2 GFP BAC mice (CB2-GFP) or C57BL6/J mice (C57BL6/J). (A), (B) Split and merged channels of dorsal root ganglia sections stained with the nuclear marker DAPI, anti-GFP, and neuronal markers anti-β-III tubulin and anti-peripherin. (A) Sham or nerve-injured C57BL6/J mice did not show significant GFP immunorreactivity. CB2-GFP mice showed a percentage of GFP+ neurons that was enhanced with the nerve injury. Scale bar, 140 μm. (B) Amplified section of dorsal root ganglia from CB2-GFP PSNL mice showing neuronal GFP. Scale bar, 45 μm. Certain degree of image processing has been applied equally across the merged images for optimal visualization.
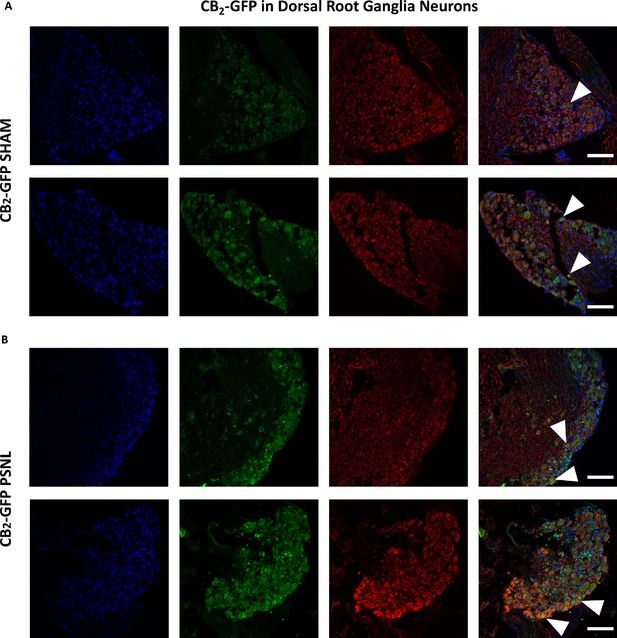
Additional images of CB2-GFP in Dorsal Root Ganglia Neurons.
Images of dorsal root ganglia from sham or nerve-injured mice transplanted with bone marrow cells from CB2 GFP BAC mice (CB2-GFP). (A), (B) Split and merged channels of dorsal root ganglia sections stained with the nuclear marker DAPI (Blue), anti-GFP (Green), and neuronal markers anti-β-III tubulin and anti-peripherin (Red). (A) Sham C57BL6/J mice showing neuronal GFP. (B) CB2-GFP PSNL mice showing neuronal GFP. CB2-GFP mice showed higher percentage of GFP+ neurons after the nerve injury. White arrows point to GFP+ cells. Scale bar, 45 μm. Certain degree of image processing has been applied equally across the merged images for optimal visualization.
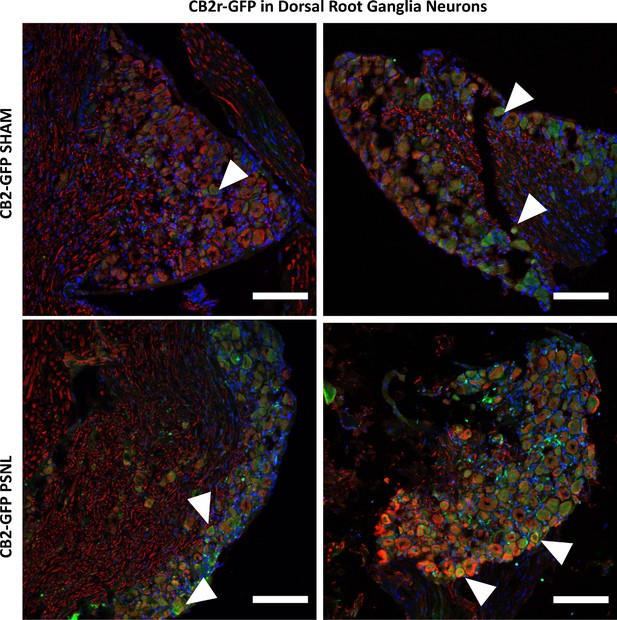
Higher magnification of merged images shown in Figure 4—figure supplement 5.
Merged channels of dorsal root ganglia sections stained with the nuclear marker DAPI (Blue), anti-GFP (Green), and neuronal markers anti-β-III tubulin and anti-peripherin (Red). Scale bar, 45 μm. White arrows point to GFP+ cells. Scale bar, 45 μm.
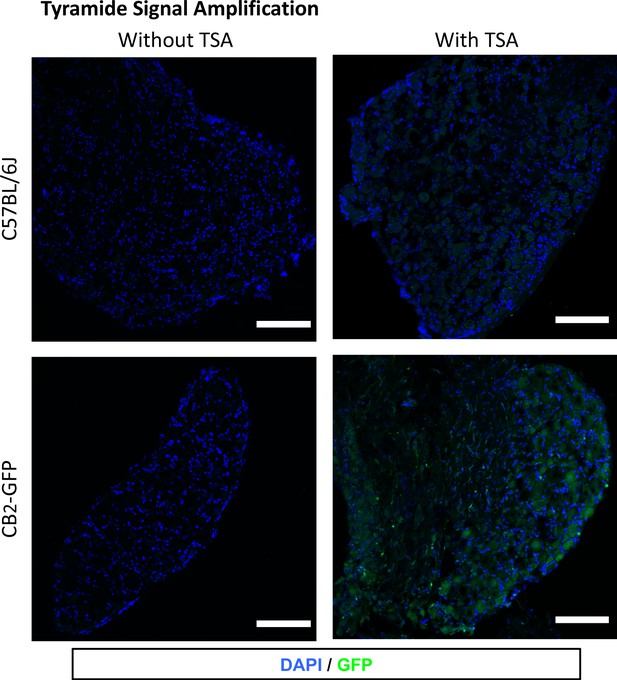
Tyramide signal amplification (TSA) for optimal visualization of GFP in the Dorsal Root Ganglia.
Images show dorsal root ganglia sections from mice transplanted with bone marrow cells from CB2 GFP BAC mice (CB2-GFP) or C57BL6/J mice (C57BL6/J), with or without TSA. Blue, nuclear marker DAPI; Green, anti-GFP. CB2-GFP, but not C57BL6/J mice showed GFP immunorreactivity after application of TSA. Scale bar 45 μm.
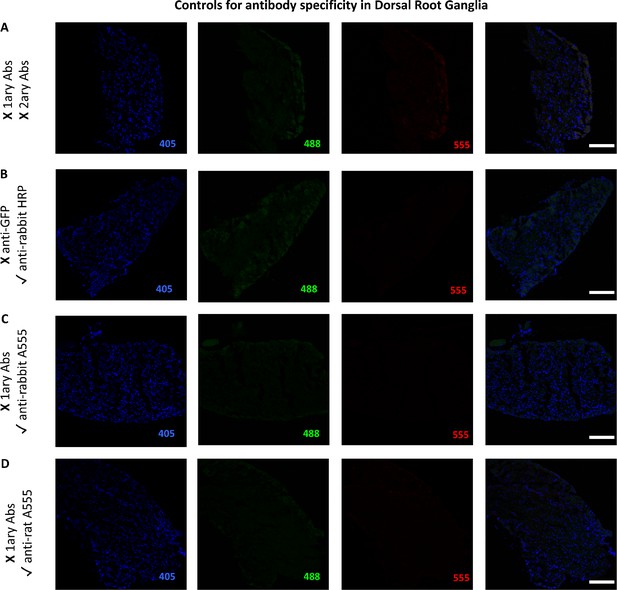
Controls for antibody specificity in the Dorsal Root Ganglia.
The figure shows images of L3-L5 dorsal root ganglia from mice transplanted with bone marrow cells from CB2 GFP BAC mice (CB2-GFP) or C57BL6/J mice (C57BL6/J). (A) No primary or secondary antibodies control revealed no endogenous staining artifacts arising from the sample itself. (B) Anti-rabbit HRP secondary antibody without primary anti-GFP antibody. (C) Anti-rabbit A555 secondary antibody without primary rabbit antibodies. (D) Anti-rat A555 secondary antibody without primary rat antibodies. Scale bar 45 μm. A: C57BL6/J Sham. B-D: GFP Sham.
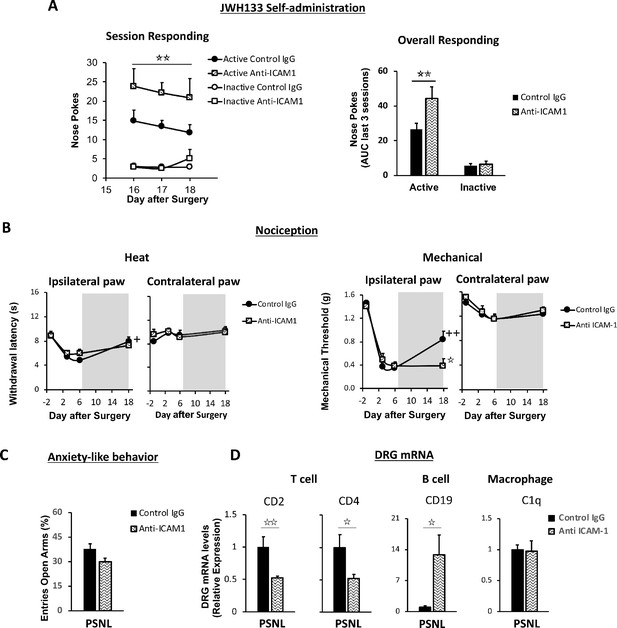
Lymphocytes modulate the effects of JWH133 on spontaneous pain and mechanical nociception.
C57BL/6J mice were food-trained in Skinner boxes (Food training, 5 days), subjected to partial sciatic nerve ligation (PSNL, day 0), catheterized and exposed to high doses of the CB2 agonist JWH133 (0.3 mg/kg/inf., days 7 to 18). Treatments with Anti-ICAM1 (an antibody that inhibits lymphocyte extravasation) or control IgG were given intraperitoneally once a day from day 0 until the end of self-administration. Nociceptive sensitivity to heat (Plantar) and mechanical (von Frey) stimulation was measured before and after nerve injury (−1,3,6,18), and anxiety-like behavior was evaluated at the end (day 19). Dorsal root ganglia were collected for mRNA analysis (A) Mice treated with anti-ICAM1 showed increased active responding for JWH133. (B) Thermal nociception after JWH133 self-administration was similar in mice treated with anti-ICAM1 or control IgG. Conversely, JWH133 effects on mechanical nociception were abolished by anti-ICAM1. (C) Anxiety-like behavior was similar in anti-ICAM1 and control IgG mice. (D) Levels of mRNA from T cell markers CD2 and CD4 were decreased in the dorsal root ganglia of anti-ICAM1 mice. Conversely, levels of B cell marker CD19 increased. Macrophage marker C1q was unaffected. N = 6–7 mice per group. Shaded areas represent drug self-administration. Mean and error bars representing SEM are shown. Stars represent comparisons vs. control IgG group; crosses indicate day effect. *p<0.05; **p<0.01; ***p<0.001.
-
Figure 5—source data 1
JWH133 self-administration, antinociception and anxiolytic-like effects in nerve-injured C57BL6/J mice treated with anti-ICAM1.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig5-data1-v3.xlsx
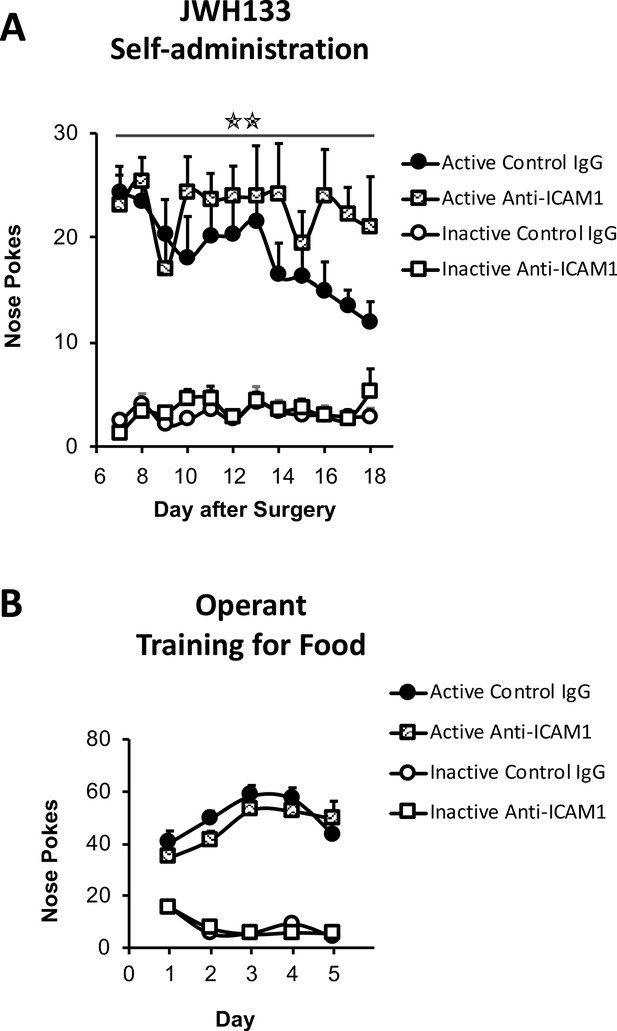
JWH133 self-administration of nerve-injured mice treated with anti-ICAM1 or control IgG and food-maintained operant training before nerve injury and drug self-administration.
(A) Anti-ICAM1-treated mice showed increased active operant responding directed to obtain high doses of the CB2 agonist JWH133 (0.3 mg/kg/inf.) after the nerve injury. (B) C57BL6/J mice of both groups anti-ICAM1 and control IgG developed similar operant behavior for food before the partial sciatic nerve ligation. N = 6–7 mice per group. Stars represent p<0.01 vs. control IgG group.
-
Figure 5—figure supplement 1—source data 1
Operant training and full JWH133 self-administration in nerve-injured C57BL6/J mice treated with anti-ICAM1.
- https://cdn.elifesciences.org/articles/55582/elife-55582-fig5-figsupp1-data1-v3.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus, male) | C57BL/6J | Charles Rivers, France | RRID:IMSR_JAX:000664 | |
| Genetic reagent (M. musculus) | CB2 KO | Institute of Molecular Psychiatry, University of Bonn, Germany | RRID:MGI:2663848 | Buckley et al., 2000 PMID:10822068 (male) |
| Genetic reagent (M. musculus) | SynCre+-Cnr2fl/fl:: Cnr2fl/fl | Institute of Molecular Psychiatry, University of Bonn, Germany | (C57BL/6J background, male) | |
| Genetic reagent (M. musculus) | LysMCre+-Cnr2fl/fl:: Cnr2fl/fl | Institute of Molecular Psychiatry, University of Bonn, Germany | (C57BL/6J background, male) | |
| Genetic reagent (M. musculus) | Nav1.8Cre+-Cnr2fl/fl:: Cnr2fl/fl | Institute of Molecular Psychiatry, University of Bonn, Germany | (C57BL/6J background, male) | |
| Genetic reagent (M. musculus) | TRPA1 KO | Universidad Miguel Hernández, Spain | RRID:MGI:3625358 | Kwan et al., 2006. PMID:16630838 (male) |
| Strain, strain background (M. musculus, male) | C57BL/6JRccHsd | Universidad Miguel Hernández, Spain | Envigo | |
| Antibody | Anti-mouse ICAM-1 (Hamster, monoclonal, clone 3e2) | BD Biosciences, USA | 550287 | (150 μg/day i.p.) |
| Antibody | IgG from rabbit serum (Unconjugated) | Sigma-Aldrich, Germany | I5006 | (150 μg/day i.p.) |
| Antibody | Allophycocyanin-conjugated anti-mouse CD11b (Monoclonal) | eBioscience, USA | cn.17–0112 | Flow cytometry (1:300) |
| Antibody | Phycoerythrin -conjugated anti-mouse B220 (Monoclonal) | eBioscience, USA | cn.12–0452 | Flow cytometry (1:100) |
| Antibody | Phycoerythrin/cyanine-conjugated anti-mouse CD3 (Monoclonal) | BioLegend, USA | cn.100320 | Flow cytometry (1:100) |
| Antibody | Rabbit anti-peripherin (Polyclonal) | Thermo Fisher, USA | PA3-16723 | IHC (1:200) |
| Antibody | Rabbit anti-GFP antibody (Polyclonal) | Thermo Fisher, USA | A11122 | IHC (1:2000) |
| Antibody | Rabbit anti-β-III tubulin (Polyclonal) | Abcam, UK | Ab18207 | IHC (1:1000) |
| Antibody | Rat anti-CD45R/B220 APC antibody (Monoclonal, Clone RA3- 6B2) | Biolegend, USA | 103229 | IHC (1:500) |
| Antibody | Rat anti-F4/80 antibody (Monoclonal, Clone A3-1) | Biorad, USA | MCA497GA | IHC (1:500) |
| Antibody | Anti-rabbit poly-HRP-conjugated (Polyclonal) | Thermo Fisher, USA | Tyramide Superboost Kit, B40922 | IHC (1X) |
| Antibody | Goat anti-rabbit Alexa Fluor A555 (Polyclonal) | Abcam, UK | Ab150078 | IHC (1:1000) |
| Antibody | Goat anti-rat Alexa Fluor A555 (Polyclonal) | Abcam, UK | Ab150158 | IHC (1:1000) |
| Chemical compound, drug | JWH133 | Tocris, UK | TO-1343 | CB2 receptor agonist |
| Chemical compound, drug | Sodium thiopental | Braun medical, Spain | 635573 | |
| Chemical compound, drug | Isoflurane | Virbac, Spain | 575837–4 | |
| Chemical compound, drug | Paraformaldehyde | Merck Millipore, Germany | 104005 | |
| Chemical compound, drug | DAPI Fluoromount-G mounting media | SouthernBiotech, USA | 0100–20 | |
| Commercial assay or kit | RNeasy Micro kit | Qiagen, Germany | 74004 | |
| Commercial assay or kit | Omniscript reverse transcriptase | Qiagen, Germany | 205111 | |
| Commercial assay or kit | Tyramide Superboost Kit | Thermo Fisher, USA | B40922 | |
| Software, algorithm | FACSDiva version 6.2 | BD biosciences, USA | RRID:SCR_001456 | |
| Software, algorithm | Fiji | Wayne Rasband, USA | RRID:SCR_002285 | |
| Software, algorithm | IBM SPSS 19 | IBM Corporation, USA | RRID:SCR_002865 | |
| Software, algorithm | STATISTICA 6.0 | StatSoft, USA | RRID:SCR_014213 |