The Warburg Effect and lactate signaling augment Fgf-MAPK to promote sensory-neural development in the otic vesicle
Figures
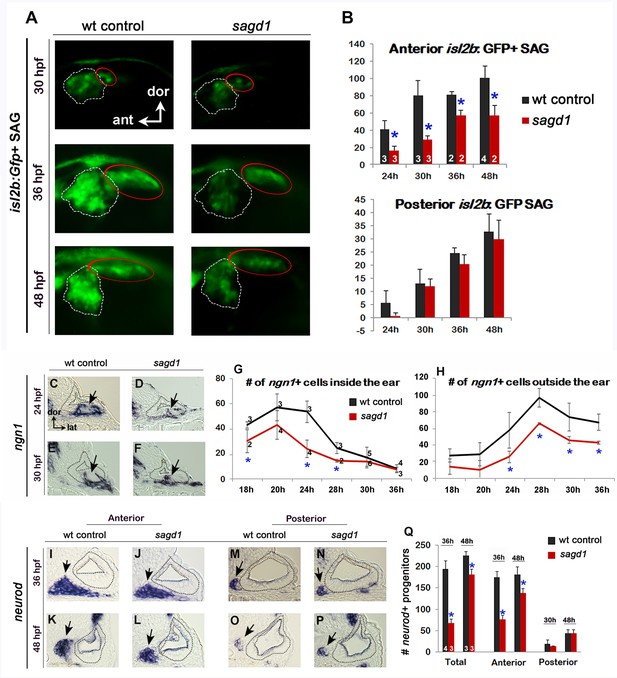
Initial analysis of sagd1.
(A) Lateral views of isl2b:Gfp+ SAG neurons in live wild-type (wt) embryos and sagd1 mutants at the indicated times. The anterior/vestibular portion of the SAG (outlined in white) is deficient in sagd1 mutants, whereas the posterior SAG (outlined in red) appears normal. (B) Number (mean and s.d.) of isl2b:Gfp+ anterior neurons and posterior neurons in wild-type and sagd1 embryos at the times indicated. Sample sizes are indicated. Asterisks, here and in subsequent figures, indicate significant differences (p<0.05) from wild-type controls. (C–F) Cross-sections through the anterior/vestibular portion of the otic vesicle (outlined) showing expression of ngn1 in wild-type embryos and sagd1 mutants at 24 and 30 hpf. (G, H) Mean and standard deviation of ngn1+ cells in the floor of the otic vesicle (G) and in recently delaminated SAG neuroblasts outside the otic vesicle (H), as counted from serial sections. (I–P) Cross-sections through the anterior/vestibular and posterior/auditory regions of the otic vesicle showing expression of neurod in transit-amplifying SAG neuroblasts at 36 and 48 hpf. (Q) Mean and standard deviation of neurod+ SAG neuroblasts at 30 and 48 hpf counted from serial sections. Sample sizes are indicated (B, G, Q).
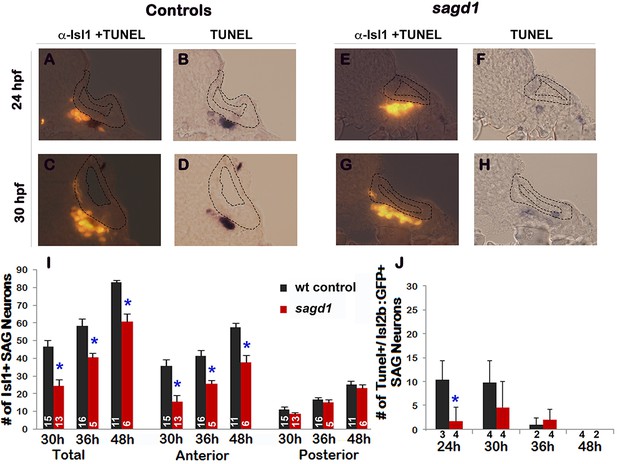
Quantitation of mature Isl1/2+ SAG neurons and TUNEL in sagd1 mutants.
(A–H) Cross sections (dorsal up, medial to the right) through the anterior/vestibular portion of the otic vesicle in control embryos (A–D) and sagd1 mutants (E–H) showing anti-Isl1/2 stained SAG neurons (A,C, E, G) and co-staining for TUNEL (B, D, F, H). (I) Number of Isl1/2+ SAG neurons at 30 hpf counted from whole mount preparations. Anterior/vestibular SAG neurons are under-produced in sagd1 mutants, whereas posterior/auditory neurons develop normally. (J) Number of TUNEL+ apoptotic cells at the indicated times. sagd1 mutants show fewer apoptotic cells than normal at 24 and 30 hpf, indicating that the deficiency in vestibular SAG neurons is not due to cell death. Sample sizes are indicated (I, J).
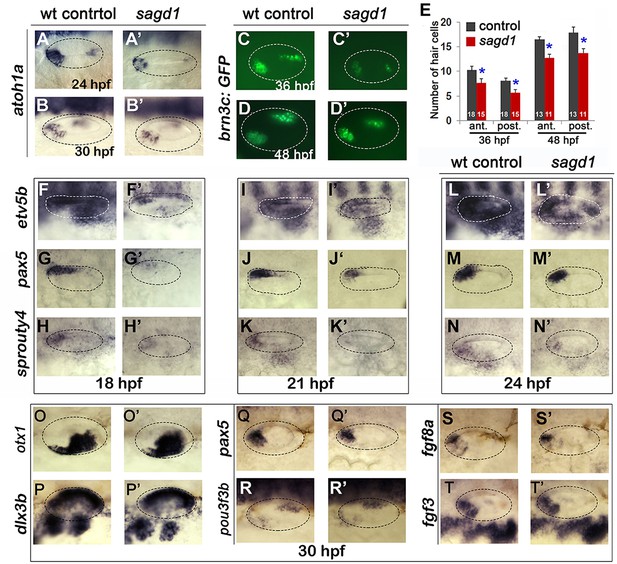
Sensory development and early Fgf signaling are impaired in sagd1 mutants.
(A–D”, F–T’) Dorsolateral views of whole mount specimens (anterior to the left) showing expression of the indicated genes in the otic vesicle (outlined) at the indicated times in wild-type embryos and sagd1 mutants. (E) Mean and standard deviation of hair cells in the anterior/utricular and posterior/auditory maculae at 36 and 48 hpf in wild-type embryos (black) and sagd1 mutants (red). Sample sizes are indicated.
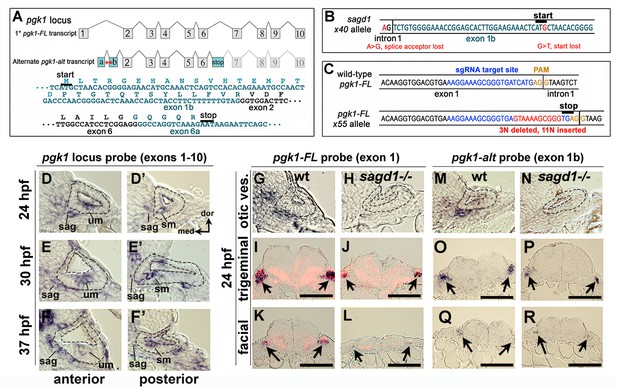
Two independent transcripts associated with the pgk1 locus.
(A) Exon-intron structure of the primary full-length pgk1 transcript (pgk1-FL) and alternate transcript (pgk1-alt) arising from an independent transcription start site containing two novel exons (1a and 1b) that splice in-frame to exon 2, and a third novel exon (6a) containing a stop codon. The nucleotide and peptide sequences of exons 1b and 6a are shown. Relative positions of the lesions in sagd1 affecting exon 1b are indicated (red asterisks). (B) Nucleotide sequence near the 5’ end of exon 1b showing the SNPs detected in sagd1 (red font). (C) Nucleotide sequence of pgk1-FL showing the sgRNA target site (blue font) and the altered sequence of the x55 mutant allele (red font), which introduces a premature stop codon. (D–F’) Cross sections through the otic vesicle (outlined) showing staining with ribo-probe for the entire pgk1 locus (covering both pgk1-FL and pgk1-alt) in wild-type embryos. Note elevated expression in the SAG, utricular macula (um), and saccular macula (sm). (G, H, M, N) Cross sections through the otic vesicle (outlined) in wild-type embryos and sagd1 mutants stained with ribo-probe for exon 1 (pgk1-FL alone) (G, H) or exon 1b (pgk1-alt alone) (M, N). Accumulation of both transcripts is dramatically reduced in sagd1 mutants. (I–L, O–R) Cross sections through the hindbrain (dorsal up) showing expression of pgk1-FL (black) plus neurod (red) (I–L) or pgk1-alt alone (O–R). Arrows indicate positions of the trigeminal and facial ganglia. Scale bar, 100 µm.

Mapping of SNPs linked to sagd1.
(A, B) Homozygosity mapping showing chromosomal distributions of homozygous (red) and heterozygous (black) SNPs identified by whole genome sequencing of bulked DNA from 50 homozygous sagd1- / - mutants. (A) Homozygous SNPs are most abundant on Chromosome 21. Blue lines show local regression (LOESS) curves of homozygous-to-heterozygous ratios. (B) SNP densities and best fit for maximum homozygosity and minimum heterozygosity were used to plot map scores along chromosome 21. The location of pgk1 (red arrow) lies near the peak map score.
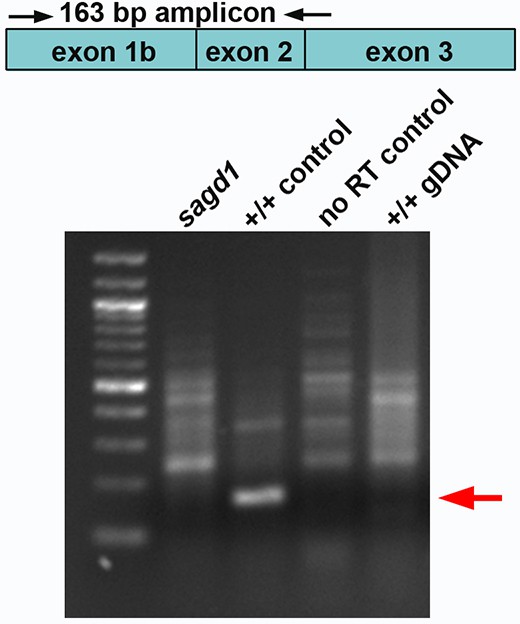
RT-PCR identification of pgk1-alt.
RT-PCR was conducted on mRNA harvested at 24 hpf using primers binding near the 5’ end of exon1b and at the exon2/3 junction. A 163 bp amplicon (red arrow) was obtained from wild-type embryos but not sagd1- / - mutants. As controls, reverse transcriptase was excluded from wild-type mRNA samples (no RT control), or genomic DNA was used as template (+/+ gDNA).
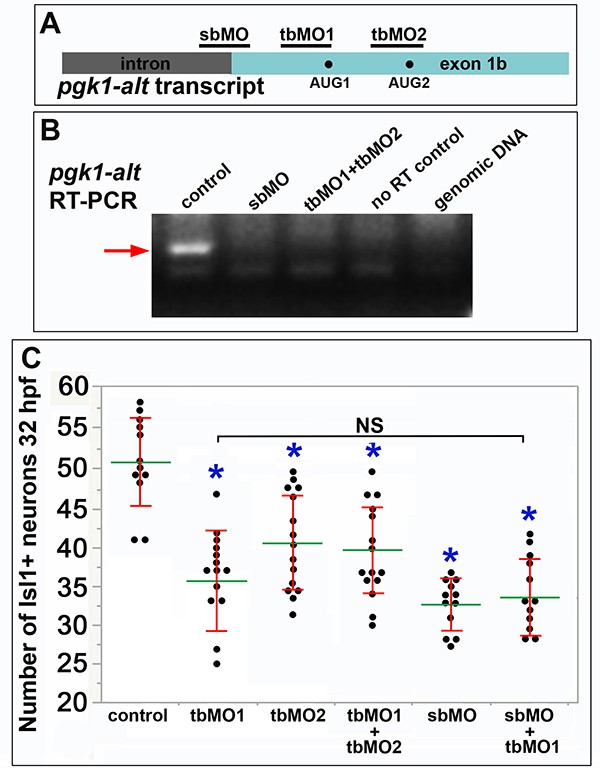
Morpholinos for pgk1-alt phenocopy sagd1.
(A) Diagram of nascent pgk1-alt transcript showing exon 1b and upstream intron. Binding sites for splice-blocking and translation blocking MOs, and potential translation start sites (AUG1 andAUG2) are shown. (B) RT-PCR for pgk1-alt using mRNA harvested at 24 hpf from wild-type control embryos, or wild-type embryos injected with sbMO (10 ng) or a mixture of tbMO1 + tbMO2 (5 ng each). Wild-type mRNA samples lacking reverse transcriptase (no RT control) or samples containing only wild-type genomic DNA served as controls. The expected pgk1-alt amplicon (red arrow) was obtained only from uninjected wild-type embryos. (C) The number of anti-Isl1+ SAG neurons at 32 hpf counted from whole-mount specimens. Injection of any of the MOs (10 ng total), or a combination of tbMO1+tbMO2 or sbMO+tbMO1 (5 ng each) significantly reduced accumulation of SAG neurons (asterisks), whereas there was no significant difference between morphants (NS).

Expression of pgk1-FL during development.
(A) RT-PCR for pgk1-FL and pgk1-alt conducted on mRNA harvested at the 2–4 cell stage (lane 1), mRNA samples excluding reverse transcriptase (lane 2), or genomic DNA (lane 3). The expected 211 bp amplicon was obtained for pgk1-FL (red arrow), but not the 163 bp amplicon (exp. cDNA) for pgk1-alt (black arrow). (B–H) Whole mount in situ hybridization for pgk1-FL at the indicated times. Images show dorsal views with anterior to the top (B–D, G,H) or lateral views with anterior to the left (E, F). Expression of pgk1-FL shows the first signs of upregulation in developing somites at 14 hpf (B), whereas diffuse upregulation becomes evident in the hindbrain region beginning at 18 hpf (C) and becomes more intense in developing reticulospinal neurons (rsn) by 20 hpf (D). The position of the otic vesicle (ov) is indicated. The specimen in (D) is depicted at lower magnification in (E) and shows that expression of pgk1-FL also increases in the midbrain-hindbrain border (mhb) and somites. (F–H) Expression of pgk1-FL at 24 hpf in wild-type embryos (F, G) and a pgk1- / - mutant (H). In wild-type embryos local upregulation increases near sites of Fgf expression, including the olfactory epithelium (olf), SAG, midbrain-hindbrain border (mhb), the trigeminal ganglion (tg) and reticulospinal neurons (rsn). Expression is strongly reduced in pgk1- / - mutants.
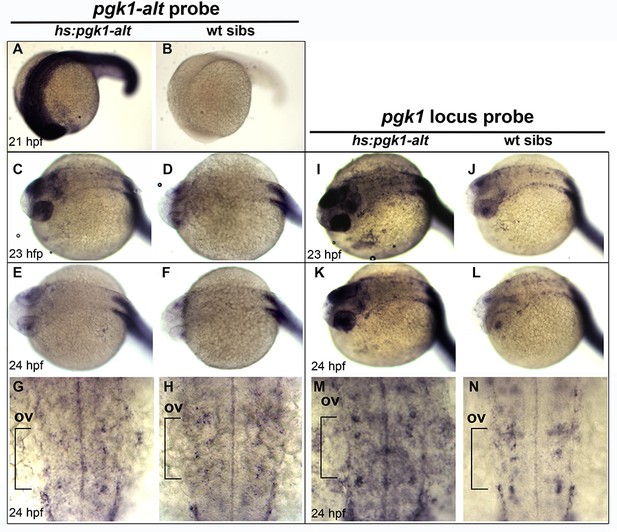
pgk1-alt misexpression increases pgk1 transcript abundance.
(A–N) Expression of pgk1-alt (A–H) and pgk1 (I–N) following heat shock (39°C for one hour) of transgenic and wild-type embryos at 20 hpf. Images show whole embryos (A–F, I–L) or close-ups of the hindbrain (anterior to the top) (G, H, M, N). The position of the otic vesicle (ov) is indicated. Expression of pgk1-alt peaks at 21 hpf in transgenic embryos but decays to normal levels by 24 hpf. In contrast, expression of pgk1 remains elevated in transgenic embryos through 24 hpf.
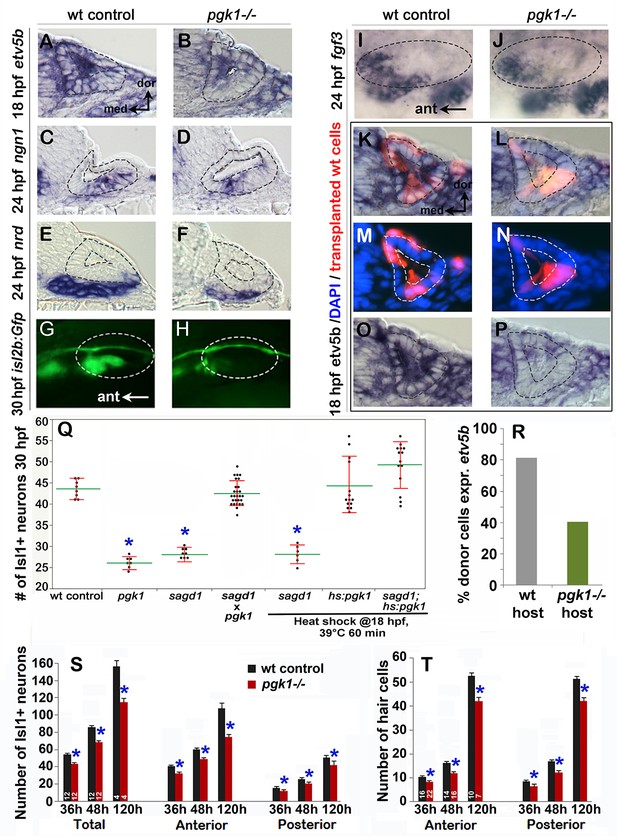
Initial characterization of pgk1- / - mutants and genetic mosaics.
(A–F) Cross sections through the anterior/vestibular region of the otic vesicle (outlined) showing expression of the indicated genes in wild-type embryos and pgk1- / - mutants at the indicated times. (G–J) Lateral views showing isl2b-Gfp expression in live embryos at 30 hpf (G, H) and fgf3 at 24 hpf (I, J). The otic vesicle is outlined. (K–P) Cross sections through the otic vesicle (outlined) showing positions of lineage labeled wild-type cells (red dye) transplanted into a wild-type host (K, M, O) or a pgk1- / - mutant host (L, N, P). Sections are co-stained with DAPI (M, N) and etv5b probe (O, P). Note the absence of etv5b expression in wild-type cells transplanted into the mutant host (P). (Q) Number of mature Isl1+ SAG neurons at 30 hpf in embryos with the indicated genotypes, except for sagd1 x pgk1 intercross expected to contain roughly 25% each of +/+, sagd1/+, pgk1/+ and sagd1/pgk1 embryos. (R) Percent of wild-type donor cells located in the ventral half of the otic vesicle expressing etv5b in wild-type or pgk1- / - hosts. A total of 151 wild-type donor cells were counted in eight otic vesicles of wild-type host embryos, and 247 wild-type donor cells were counted in eight otic vesicles of pgk1- / - host embryos. (S) Number of Isl1+ SAG neurons (total, anterior and posterior) in wild-type and pgk1- / - mutant embryos at 36 hpf (n = 12), 48 hpf (n = 12) and 120 hpf (n = 4). (T) Number of phalloidin-stained hair cells (anterior/utricular and posterior/saccular) in wild-type and pgk1- / - mutant embryos at 36 hpf (n = 16), 48 hpf (n = 16) and 120 hpf (n = 10). Sample sizes are indicated (S, T).
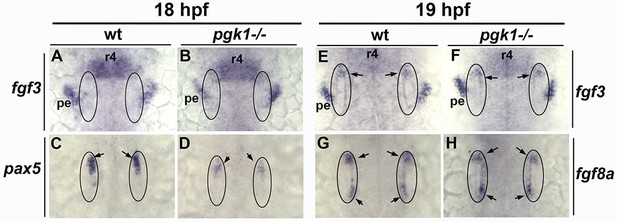
pgk1– / - mutants show normal onset of fgf3 and fgf8a, but not pax5, in the otic vesicle.
(A–D) Expression of fgf3 and pax5 in wild-type and pgk1- / - mutant embryos at 18 hpf. fgf3 (A, B) is expressed in rhombomere 4 (r4) and pharyngeal endoderm (pe) and appears normal in pgk1- / - mutants, while otic expression of pax5 (C, D, arrows) is reduced in mutant embryos. (E–H) At 19 hpf, expression of fgf3 (E, F) and fgf8a (G, H) is reliably detected in the otic vesicle (arrows) and appears normal in pgk1- / - mutants. Images show dorsal views with anterior to the top.

pgk1– / - mutants show normal expression of regional markers of the otic vesicle.
(A–H) Otic expression of the indicated genes at 24 hpf in wild-type embryos and pgk1- / - mutants. Images show dorsolateral views with anterior to the left.

Deficiencies in Fgf-dependent cell types in pgk1- / - mutants.
(A–D) Expression of neurod at 24 hpf showing lateral views of the olfactory epithelium (olf) (A, B) and dorsal views of the facial (fac) and glossopharyngeal (glos) ganglia (C, D) in wild-type and pgk1- / - embryos. Numbers indicate relative surface area of each expression domain (normalized to wild-type controls)± standard deviation and number of specimens. Expression domains are significantly smaller in pgk1- / - mutants (p<0.0001). (E, F) Dorsal view (anterior to the top) of anti-acetylated tubulin staining in the hindbrain region in a wild-type embryo (E) and pgk1- / - mutant (F). Positions of reticulospinal neurons (rsn) and trigeminal ganglia (tg) are indicated. Numbers indicate the mean number of neurons per side ± standard deviation and number of specimens. The number of neurons is significantly reduced in pgk1- / - mutants (p<0.0001).
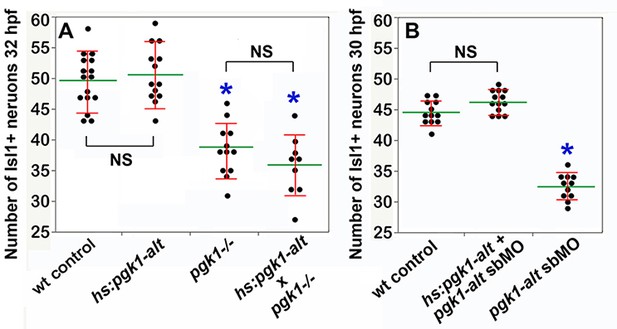
Misexpression of pgk1-alt does not rescue pgk1- / - mutants but does rescue pgk1-alt morphants.
(A, B) Mean (green) and standard deviation (red) of Isl1+ SAG neurons. (A) The number of SAG neurons at 32 hpf is reduced in pgk1- / - mutants but is not affected by activation of hs:pgk1-alt. (B) The number of SAG neurons at 30 hpf is reduced in pgk1-alt morphants but is restored to normal following activation of hs:pgk1-alt at 18 hpf.
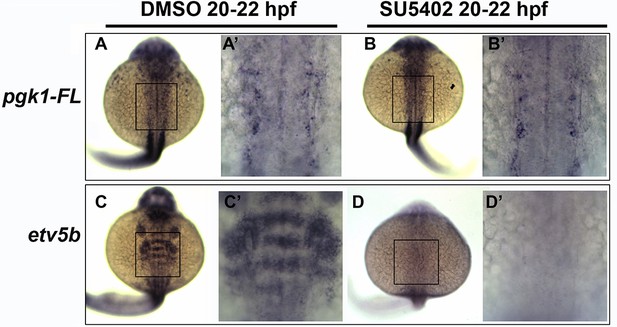
Fgf signaling is not required for normal pgk1-FL expression.
(A–D’) Dorsal views (anterior to the top) of expression of pgk1-FL and etv5b in wild-type embryos incubated with 0.5% DMSO or 50 uM SU5402 from 20 to 22 hpf. Images show whole embryos next to enlargements (primed letters) of the indicated regions of the hindbrain. SU5402 had no effect on expression of pgk1-FL but completely eliminated expression of etv5b.
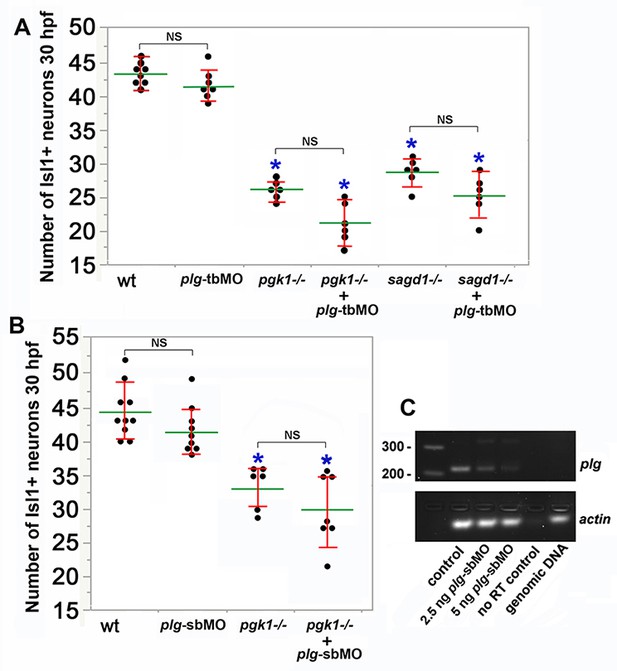
Knockdown of plasminogen does not alter pgk1- / - or sagd1- / - phenotypes.
(A, B) Mean (green) ± standard deviation (red) of the number of Isl1+ SAG neurons at 30 hpf in embryos with the indicated genotypes and/or following injection of 5 ng plg translation-blocker (plg-tbMO) (A) or splice-blocker (plg-sbMO) (B). Asterisks indicate significant differences from wild-type controls. NS, no significant difference between groups indicated in brackets. (C) RT-PCR of plg transcript harvested at 24 hpf from wild-type controls or embryos injected with 2.5 or 5 ng plg-sbMO. As controls, reverse transcriptase was excluded from wild-type mRNA samples (no RT control), or genomic DNA was used as template.
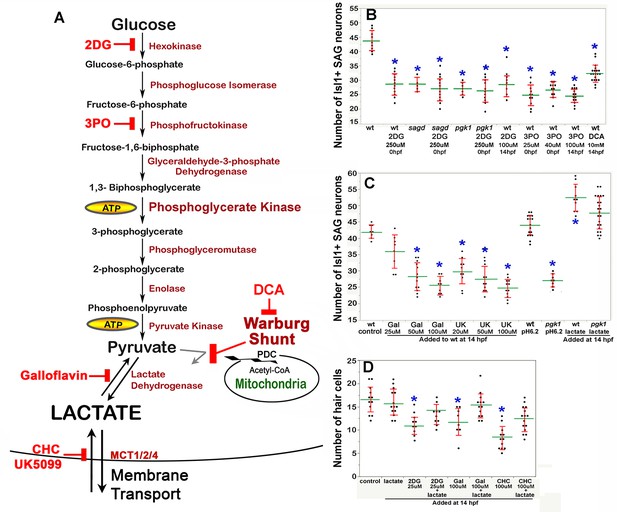
Drugs blocking aerobic glycolysis mimic pgk1- / - mutants.
(A) Diagram of the glycolytic pathway and changes associated with the Warburg Effect in which pyruvate is shunted from mitochondria in favor of synthesis and secretion of lactate. The indicated inhibitors were used to block discrete steps in the pathway. In the Warburg Shunt, mitochondrial Pyruvate Dehydrogenase Complex (PDC) is inhibited by elevated Pyruvate Dehydrogenase Kinase (PDK), the activity of which is blocked by DCA. (B, C) Scatter plots showing the mean (green) and standard deviation (red) of the number of Isl1/2+ SAG neurons at 30 hpf in embryos with the indicated genotypes and/or drug treatments. Lactate-treatments and some controls were buffered with 10 mM MES at pH 6.2. (D) Scatter plats showing the mean and standard deviation of the total number of hair cells at 36 hpf in embryos treated with the indicated drugs. Asterisks show significant differences (p ≤. 05) from wild-type control embryos.
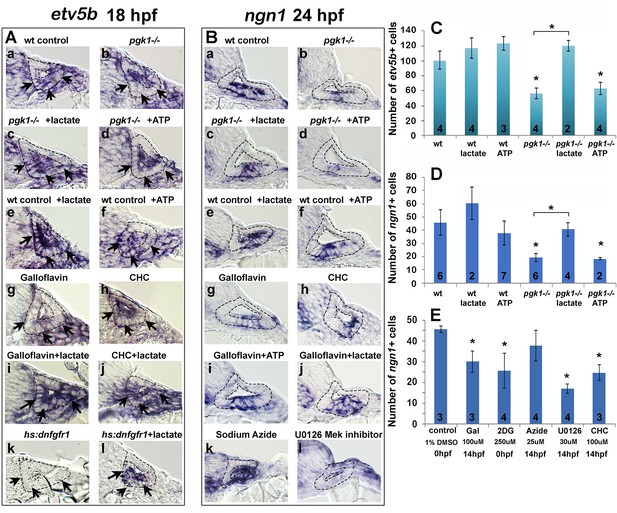
Exogenous lactate reverses the effects of disrupting aerobic glycolysis.
(A, B) Cross sections through the anterior/vestibular portion of the otic vesicle (outlined) showing expression of etv5b at 18 hpf (A) and ngn1 at 24 hpf (B) in embryos with the indicated genotype or drug treatment. Arrows in (A) highlight expression in the ventromedial otic epithelium. (C) Mean and standard deviation of the number of etv5b+ cells in the ventral half of the otic vesicle counted from serial sections of embryos with the indicated genotype or drug treatment. (D, E) Mean and standard deviation of the number of ngn1+ cells in the floor of the otic vesicle counted from serial sections of embryos with the indicated genotype or drug treatment. Asterisks indicate significant differences (p<0.05) from wild-type controls, or between groups indicated by brackets.
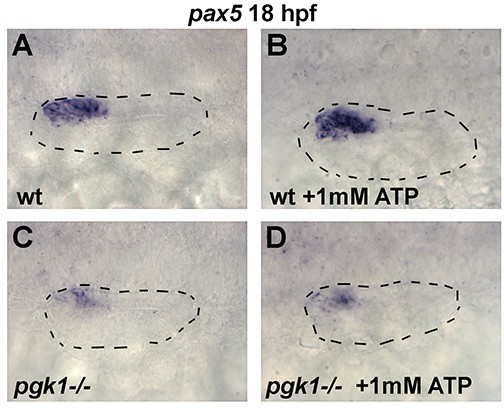
Exogenous ATP does not rescue pgk1- / - mutants.
Dorsolateral view (anterior to the left) of the otic vesicle (outlined) showing expression of etv5b (A–D) or pax5 (E–H) at 18 hpf in wild-type embryos or pgk1- / - mutants with or without exposure to exogenous 1 mM ATP. NS, no significant difference between groups indicated in brackets.
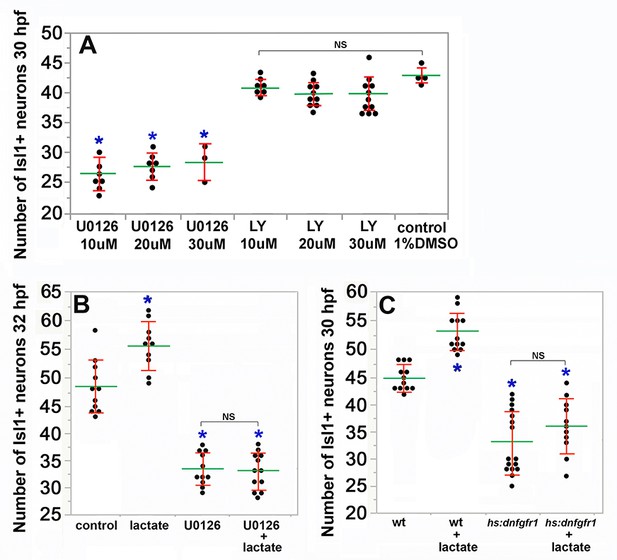
Effects of altering MAPK/Erk, lactate and Fgf on SAG development.
(A–C) Mean and standard deviation of the number of Isl1+ SAG neurons. (A) The number of Isl1+ SAG neurons at 30 hpf in embryos treated with MEK inhibitor U0126 or PI3K inhibitor LY294002 (LY) at the indicated concentrations. (B). The number of Isl1+ SAG neurons at 32 hpf in embryos treated with 6.7 mM lactate and/or 20 uM U0126 from 14 hpf. All embryos were incubated at pH 6.2. (C) The number of Isl1+ SAG neurons at 30 hpf in embryos treated with 6.7 mM lactate beginning at 14 hpf as indicated. All embryos were incubated at pH6.2 and heat shocked at 37°C for 30 min at 16 hpf. NS, no significant difference between groups in brackets. .
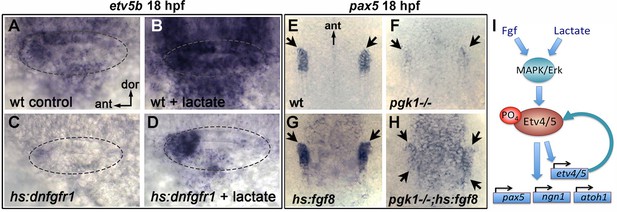
Fgf and lactate are required together for full activation of early otic genes.
(A–D) Dorsolateral view of the otic vesicle (outlined) showing expression of etv5b at 18 hpf in wild-type embryos or hs:dnfgfr1/+ transgenic embryos with or without exogenous 6.7 mM lactate added at 14 hpf, as indicated. Embryos were heat shocked at 39°C for 30 min beginning at 16 hpf. (E–H) Dorsal view (anterior to the top) of the hindbrain and otic region showing expression of the otic domain of pax5 (black arrows) in embryos with the indicated genotypes. Embryos were heat shocked at 37°C for 30 min beginning at 16 hpf. (I) A model for how lactate and Fgf signaling converge on MAPK/Erk to increase the pool of phosphorylated activated Etv4/5, enabling cells to respond more quickly and robustly to dynamic changes in Fgf.
Additional files
-
Source data 1
Raw data for cell counts.
- https://cdn.elifesciences.org/articles/56301/elife-56301-data1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/56301/elife-56301-transrepform-v2.pdf





