Cancer-associated fibroblasts and their influence on tumor immunity and immunotherapy
Figures
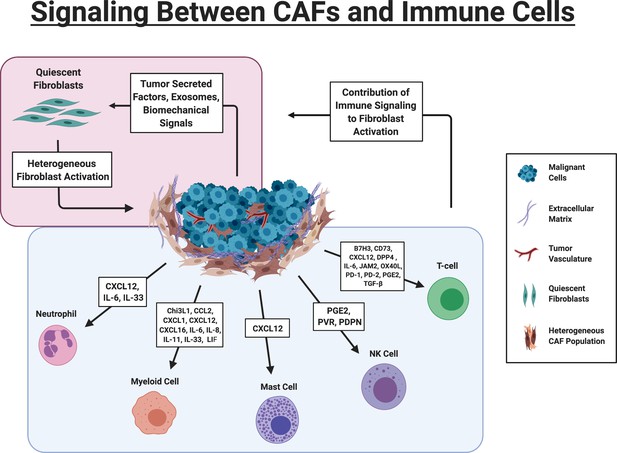
Signaling Between CAFs and Immune Cells.
Red Box: Tumor cells drive activation of fibroblasts via multiple mechanisms. Fibroblasts are heterogeneously activated into different sub-populations based on the biochemical and biomechanical cues in their immediate environment. Blue Box. Key signaling pathways involved in CAF-immune cell signaling. B7H3 (CD276); 5′-nucleotidase (CD73); Chitinase 3-like 1 (Chi3L1); chemokine (C-C motif) ligand 2 (CCL2; chemokine (C-X-C motif) ligand 1 (CXCL1)); chemokine (C-X-C motif) ligand 12 (CXCL12), chemokine (C-X-C motif) ligand 16 (CXCL16), Dipeptidyl peptidase 4 (DPP4); Junctional adhesion molecule B (JAM2); Interleukin 6 (IL-6); Interleukin 8 (IL-8); Interleukin 11 (IL-11); Leukemia Inhibitory Factor (LIF), tumor necrosis factor receptor superfamily member four ligand (OX40L); Programmed cell death protein 1 (PD-1), Programmed cell death protein 2 (PD-2), Prostaglandin E2 (PGE2), Transforming growth factor beta (TGF-β), Polio Virus Receptor (PVR), CAF) (Figure created with BioRender.com).
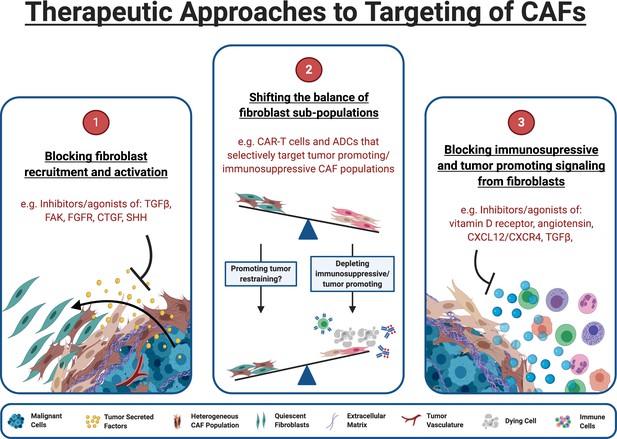
Therapeutic approaches to targeting of CAFs.
(1) Inhibitors of pathways known to drive fibroblast activation can block tumor cells ability to manipulate fibroblasts for their own survival. (2) The functional heterogeneity between CAF populations in the TME means that targeting specific subpopulations can be an effective strategy. Targeted therapeutics such as chimeric antigen receptor (CAR) expressing T cells (CAR-T) and antibody-drug conjugates (ADCs) can target the fibroblast sub-populations responsible for tumor protection and immunosuppression while leaving quiescent and tumor restraining populations intact. (3) Blocking CAFs ability to exert immunosuppressive/tumor promoting influence within the TME may alleviate immunosuppression and allow immunotherapies to be effective within this space. Some targets, such as TGF-β, can act both upstream and downstream, blocking CAF formation and attenuating downstream signaling in CAFs that are already established. (Figure created with BioRender.com).
Tables
Common markers recently used to study CAF populations that influence tumor immunity and progression.
Many potential markers have been described throughout the literature; however, this table has been limited to the markers most relevant to the topics outlined in this review.
| Phenotypic marker | Features of expressing populations | Reported tumor types | Subcellular localization | Refs |
|---|---|---|---|---|
| aSMA (ACTA2) | Myofibroblasts/myCAFs Context dependent tumor promotion and/or tumor restraint Preferentially located tumor adjacent Contractile | Most | Cytoplasmic | Özdemir et al., 2015; Costa et al., 2018; Zhou et al., 2018; Kato et al., 2018 |
| FAP (FAP) | Tumor promoting through immune-dependent and immune-independent mechanisms Major producers of immunosuppressive cytokines like CXCL12 and CCL2 in the TME | Most | Membrane | Feig et al., 2013; Yang et al., 2016; Lo et al., 2015; Lee et al., 2011 |
| FSP1 (S100A4) | Commonly used fibroblast marker Marks both quiescent and activated fibroblasts Also present on macrophages | Most | Cytoplasmic, Nuclear | Strutz et al., 1995; Österreicher et al., 2011 |
| Gli1 (GLI1) | Fibroblast sub-population that closely associates with vasculature and ducts in pancreas Preferentially expands over other fibroblast populations during pancreatic tumor progression | Pancreatic | Cytoplasmic, Nuclear | Garcia et al., 2020 |
| Hoxb6 (HOXB6) | Fibroblast sub-population present dispersed throughout healthy pancreas Minimal contribution to desmoplasia in pancreatic tumor progression | Pancreatic | Nuclear | Garcia et al., 2020 |
| LRRC15 (LRRC15) | TGF-β-driven gene expression signature Correlate with poor tumor immunity | Pancreatic | Membrane | Dominguez et al., 2020 |
| Ly6C (Ly6c1) | Defines iCAF population in mouse, in combination with other CAF markers Common on myeloid cells | Pancreatic | Membrane | Elyada et al., 2019; Biffi et al., 2019 |
| Meflin (ISLR) | Suppress PDAC progression Expression correlates with CD8+ T cells, macrophages and dendritic cells in gastric cancer | Pancreatic, gastric | GPI-linked Membrane Protein | Maeda et al., 2016; Mizutani et al., 2019; Li et al., 2020 |
| PDGFRα (PDGFRA) | Pan-fibroblast marker Marks both quiescent fibroblasts and CAFs | Most | Membrane | Farahani and Xaymardan, 2015; Nurmik et al., 2020 |
| PDPN (PDPN) | Associated with poor tumor immunity Marker of follicular RCs and some macrophages | Most | Membrane | Kitano, 2010; Astarita et al., 2012; Shindo et al., 2013; Kerrigan et al., 2012 |





