Fine-scale computations for adaptive processing in the human brain
Figures
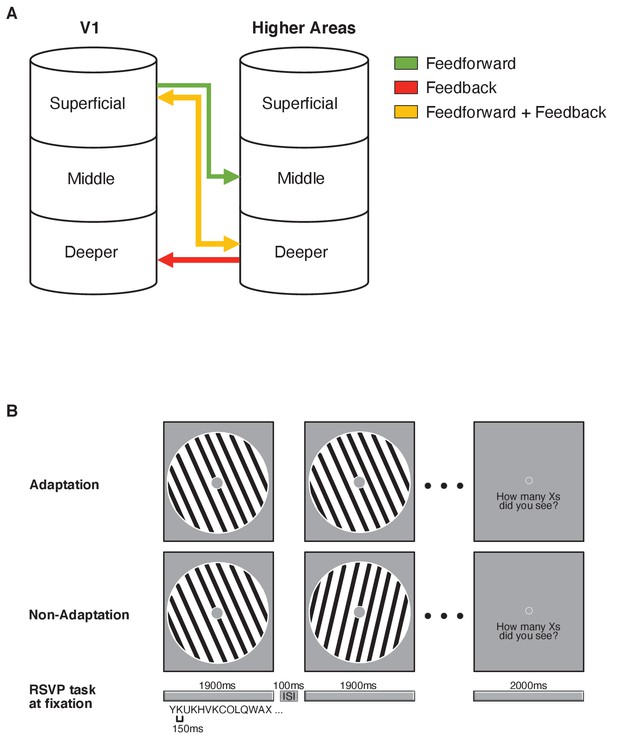
Functional magnetic resonance imaging (fMRI) laminar circuits and fMRI design.
(A) Schematic representation of feedforward (superficial – middle layers; green), feedback (deeper – deeper layers; red), and feedforward plus feedback (superficial – deeper layers; yellow) anatomical connectivity between V1 and higher cortical regions. Here, we focussed on feedforward vs. feedback connections. (B) fMRI design. Adaptation blocks comprised 16 sinewave gratings presented at the same orientation. Non-adaptation blocks comprised 16 gratings presented at different orientations. During stimulus presentation (1900 ms stimulus on, 100 ms stimulus off), participants were asked to perform an Rapid Serial Visual Presentation (RSVP) task; that is, count the number of times a target letter (e.g. X) was displayed in the stream of distracters and report it at the end of each stimulus block. Each letter was displayed for 150 ms and participants had 2000 ms to give their response.
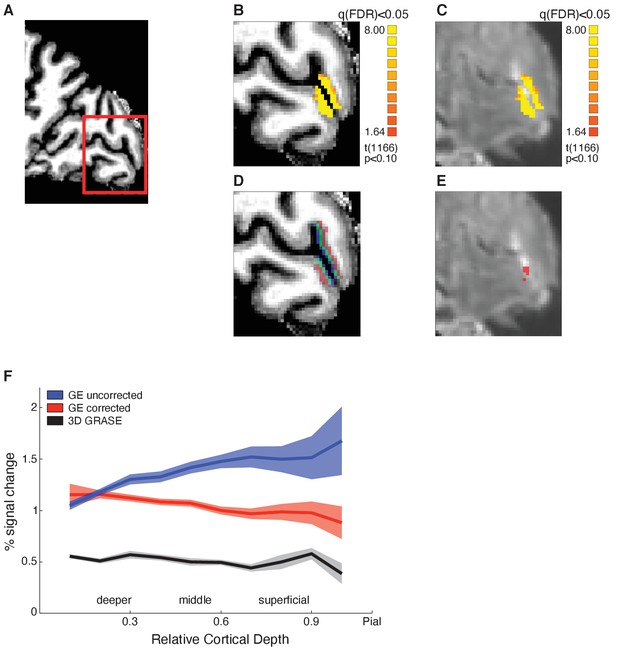
Functional magnetic resonance imaging (fMRI) data analysis, layer segmentation, and vascular contribution correction.
(A) Sagittal brain view of representative participant: red insert highlights region of interest (ROI, early visual cortex). Structural (B) and functional (C) images of the ROI showing activation maps for stimulus vs. fixation. Activation is well confined within the grey matter borders. (D) Mapping of cortical layers within the ROI: deeper layers shown in red, middle layers in green, superficial layers in blue. (E) Voxels confounded by vasculature effects (in red) overlaid on mean functional image. (F) Mean BOLD (per cent signal change from fixation baseline) across participants for V1 across cortical depth. Comparison between BOLD signal before (blue) and after temporal signal-to-noise ratio (tSNR) and t-value correction (red), and 3D GRASE BOLD signal (black). The superficial bias observed in the BOLD signal is reduced after correction and matches closely the laminar profile of the 3D GRASE data.
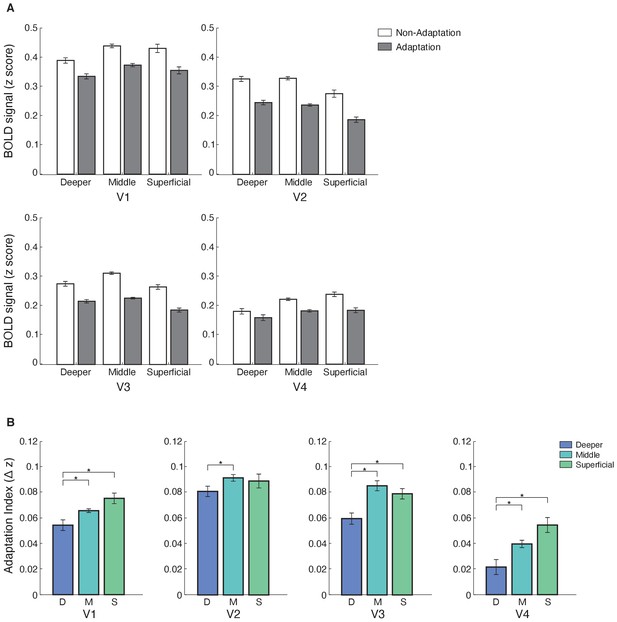
Laminar BOLD and fMRI adaptation index for V1, V2, V3, and V4.
(A) Mean BOLD across V1, V2, V3, and V4 cortical layers. Bar-plot shows z-scored BOLD signal from fixation baseline for adaptation (grey) and non-adaptation (white) across cortical layers of V1, V2, V3, and V4. Error bars indicate within-subject confidence intervals (Cousineau, 2005) (N = 15 for V1, V2, and V3; N = 14 for V4). (B) fMRI adaptation index across cortical layers (D: deeper, M: middle, S: superficial) for V1, V2, V3, and V4. Bar-plots show difference in z-scored BOLD signal between the non-adaptation and the adaptation conditions. Error bars indicate within-subject confidence intervals (Cousineau, 2005) (N = 15 for V1, V2, and V3; N = 14 for V4). Stars indicate statistically significant comparisons for p<0.05.
-
Figure 3—source data 1
Tables for mean BOLD responses to adaptation, non-adaptation, and adaptation index across cortical layers of areas V1, V2, V3, and V4.
- https://cdn.elifesciences.org/articles/57637/elife-57637-fig3-data1-v2.xlsx
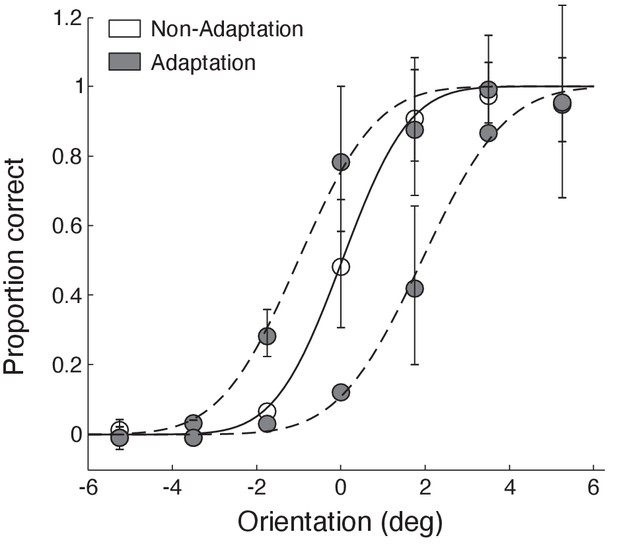
Behavioural tilt-aftereffect.
We investigated whether the layer-specific fMRI adaptation we observed relates to perceptual bias in orientation discrimination due to adaptation. We tested a subset of participants that took part in the fMRI study (N = 12) in an additional behavioural session using a tilt-aftereffect paradigm (e.g. Gibson and Radner, 1937). We presented participants with the same stimuli (oriented gratings) as in the fMRI experiment in adaptation vs. non-adaptation blocks. Participants were asked to perform the same RSVP task as in the fMRI experiment. Each block was followed by a test stimulus (a single grating with orientation close to vertical). Participants were then asked to judge whether the test stimulus was tilted clockwise or counterclockwise relative to vertical. We fitted psychometric functions to the participant responses (N = 12). Open circles indicate non-adaptation data, grey circles indicate adaptation for clockwise and counter-clockwise orientations from vertical. Solid line indicates fitting of the non-adaptation condition data, dashed lines of the adaptation condition data. Participant responses showed a perceptual bias, that is, a significant shift in the perceived orientation of the test stimulus for adaptation compared to non-adaptation (t(11)=3.197, p=0.0085).
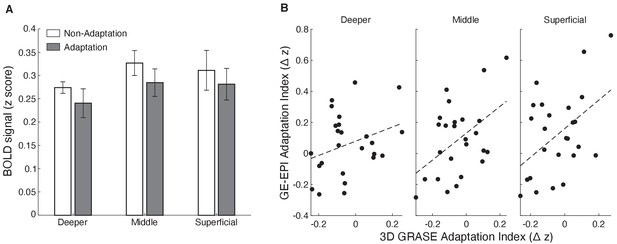
3D GRASE fMRI.
(A) Mean z-scored BOLD (signal change from fixation baseline) measured with 3D GRASE for adaptation (grey) and non-adaptation (white) across cortical layers in V1. Decreased BOLD for the adaptation compared to the non-adaptation condition is shown across cortical layers. The difference between conditions was not statistically significant (condition × cortical depth: F(2,6)=0.875, p=0.432) due to the small sample size (N = 4). (B) Correlation of fMRI adaptation index for participants scanned with both GE-EPI and 3D GRASE sequences (N = 4) across cortical layers. Individual dots indicate fMRI adaptation index (z-scored BOLD for non-adaptation minus z-scored BOLD for adaptation), for each participant and run (Nruns = 5 for one participant; Nruns = 6 for one participant; Nruns = 8 for two participants) for GE-EPI (y-axis) and 3D GRASE (x-axis) sequences. Black dashed lines indicate correlation between GE-EPI and 3D GRASE fMRI adaptation index. Correlations were stronger for superficial (r = 0.465, p=0.019) and middle (r = 0.467, p=0.019) than deeper (r = 0.315, p=0.125) layers. This correspondence of results across sequences suggests that our results showing stronger fMRI adaptation in superficial and middle than deeper layers could not be simply due to vasculature confounds.
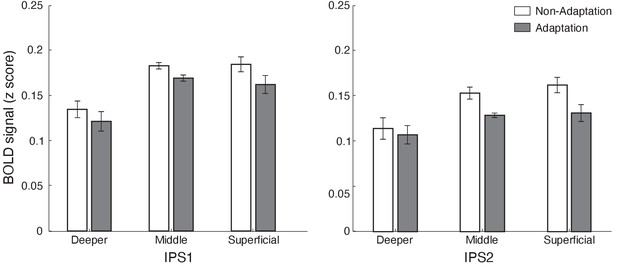
Laminar BOLD for IPS1 and IPS2.
Mean BOLD in IPS1 and IPS2 across cortical layers. Bar-plots show z-scored BOLD signal for adaptation (grey) and non-adaptation (white) across cortical layers of IPS1 and IPS2. Error bars indicate within-subject confidence intervals (Cousineau, 2005) (N = 15).
-
Figure 4—source data 1
Tables for mean BOLD responses to adaptation and non-adaptation across cortical layers of IPS1 and IPS2.
- https://cdn.elifesciences.org/articles/57637/elife-57637-fig4-data1-v2.xlsx
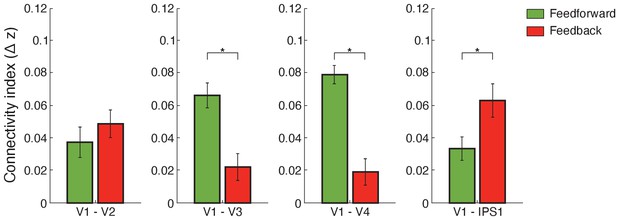
Functional connectivity.
Bar-plots show difference (adaptation minus non-adaptation) in Fisher z-transformed r values for connectivity between V1 and V2, V1 and V3, V1 and V4, and V1 and IPS1. Feedforward connections were tested between: (a) V1 superficial and V2, V3, V4 middle layers (b) V1 superficial and IPS1. Feedback connections were tested between: (a) V1 deeper and V2, V3, V4 deeper layers (b) V1 deeper layers and IPS1. Error bars indicate within-subject confidence intervals (Cousineau, 2005) (N = 15 for V1, V2, V3, and IPS1; N = 14 for V4). Stars indicate statistically significant comparisons for p<0.05.
-
Figure 5—source data 1
Tables for cortical depth dependent values of feedforward and feedback functional connectivity.
- https://cdn.elifesciences.org/articles/57637/elife-57637-fig5-data1-v2.xlsx





