Keratinocytes contribute to normal cold and heat sensation
Figures

Mammalian keratinocytes respond to temperature decreases.
In vitro calcium imaging was performed on primary cultured keratinocytes from the glabrous hindpaw skin of C57BL/6 mice, Sprague Dawley rats, and 13-lined ground squirrels and human breast skin. (A) Representative keratinocytes from all species exhibited a calcium transient upon extracellular buffer cooling (~23°C to 12°C). (B) The greatest proportion of cold-responsive cells was observed in hibernating 13-lined ground squirrel samples. The vast majority of mouse keratinocytes also responded to decreasing temperatures; fewer rat and human keratinocytes responded to cold (Chi-square p<0.0001; Fisher’s Exact tests: ***p<0.001 vs. mouse; ### p<0.001 vs. rat; &&& p<0.001 vs. squirrel; n = 28 mice, four rats, four squirrels, five humans). (C) The peak calcium response to decreasing temperatures varied between species; hibernating 13-lined ground squirrel keratinocytes exhibited the largest calcium transients (75% increase over baseline) and Sprague Dawley rats exhibited the smallest (44% increase over baseline; mouse: 56% increase; human: 69% increase; 1-way ANOVA p<0.0001; Bonferroni’s multiple comparisons: ****p<0.0001 vs. mouse; #### p<0.0001 vs. rat, && p=0.0084 vs. squirrel). (D) The temperature at which keratinocytes responded to cold (>30% increase in Δ340/380) differed between species. Mean temperature thresholds (°C) for mouse: 20.9, rat: 18.3, squirrel: 19.1, human: 19.8 (Kruskal-Wallis test p<0.0001; Dunn’s multiple comparisons: ****p<0.0001 vs. mouse, #### p<0.0001 vs. rat, &&&& p<0.0001 vs. squirrel).
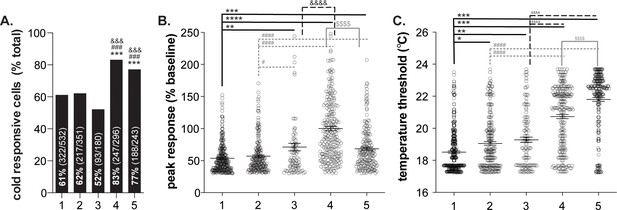
Variability in human keratinocyte responses to temperature decreases.
In vitro calcium imaging was performed on primary human breast skin keratinocytes isolated from females of the following ages (years): 1: 41, 2: 50, 3: 54, 4: 52, 5: 49. (A).). Samples 4 and 5 contained the most cold-responsive cells (Chi-square: p<0.0001; Fisher’s Exact tests: ***p<0.001 vs. sample 1, ### p<0.001 vs. sample 2, &&& p<0.001 vs. sample 3). (B).) Peak calcium responses differed between the samples; keratinocytes from sample four exhibited the largest calcium response during cold stimulation (100% increase over baseline. Mean increase for sample 1: 54%, 2: 57%, 3: 71%, 5: 68% (1-way ANOVA p<0.0001; Bonferroni’s multiple comparisons: **p<0.01, ***p<0.001, ****p<0.0001 vs. 1; # p<0.05, #### p<0.0001 vs. 2; &&&& p<0.0001 vs. 3; $$$$ p<0.0001 vs. 4). (C).) The temperature at which keratinocytes responded to cold (>30% increase in Δ340/380) differed between the samples. Mean temperature thresholds (°C) for sample 1: 18.5, 2: 19.1, 3: 19.2, 4: 20.7, 5: 21.8 (Kruskal-Wallis test p<0.0001; Dunn’s multiple comparisons: *p<0.05, **p<0.01, ***p<0.001 vs. 1; ### p<0.001 vs. 2; &&&& p<0.0001 vs. 3; $$$$ p<0.0001 vs. 4).

Characterizing murine cold sensor(s) in keratinocytes.
(A).) In order to characterize the proteins involved in cold transduction, cold-induced calcium transients were measured in keratinocytes exposed to extracellular buffer containing different ionic concentrations or pharmacological agents, or in keratinocytes from transgenic mice. (B).) Extracellular calcium chelation (EGTA, 0 µM Ca2+) decreases the proportion of keratinocytes that respond to cold; substituting NMDG for extracellular sodium did not further decrease the percentage of cold-responsive cells. Cold responses were only abolished when endoplasmic reticulum Ca2+ stores were depleted and unable to be refilled with extracellular sources (EGTA, 0 µM Ca2+, thapsigargin). CRAC channel inhibition (5J4) did not decrease the percentage of cold-responsive cells, and similar proportions of keratinocytes from C57BL/6, global TRPA1, and global TRPC5 knockout mice responded to decreasing buffer temperature (Chi square p<0.0001; Fisher’s Exact tests ***p<0.001 vs. vehicle; n ≥ 4). (C).) Peak calcium responses to cold were lower in the absence of extracellular calcium; responses were unaltered in other conditions (1-way ANOVA p<0.0001; Bonferroni’s multiple comparisons: ****p<0.0001 vs. vehicle, #### p<0.0001 vs. EGTA, 0 µM Ca2+, &&&& p<0.0001 vs. EGTA, 0 µM Ca2+, NMDG). (D).) Altering extracellular buffer contents increased or decreased the temperature at which keratinocytes responded to cold by ≤0.6°C. Mean temperature thresholds (°C) for vehicle: 20.9, EGTA, 0 µM Ca2+,±NMDG: 20.3, 20 µM 5J4: 20.5, TRPA1 KO: 21.5, TRPC5 KO: 20.7 (Kruskal-Wallis test p<0.0001; Dunn’s multiple comparisons: *p<0.05, ****p<0.0001 vs. vehicle).
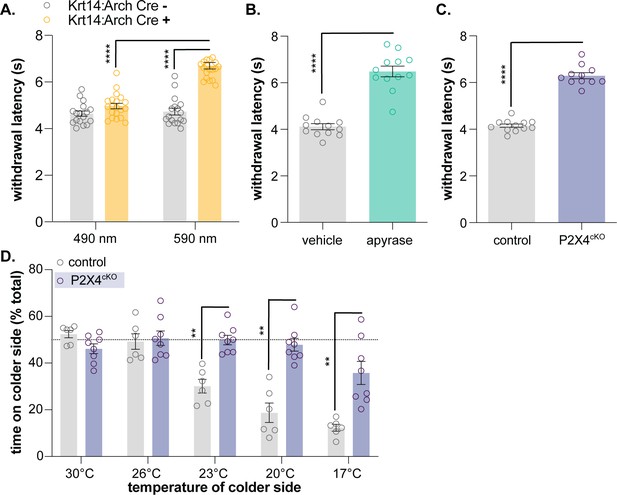
Keratinocyte-to-sensory neuron signaling is required for normal cold sensation.
(A) 590 nm light exposure increases the withdrawal latency of Krt14:Arch Cre+ mice during plantar dry ice stimulation (2-way ANOVA main effects of light, genotype, and light x genotype interaction p<0.0001; Bonferroni’s multiple comparisons: ****p<0.0001 Krt14:Arch Cre+ 490 vs. 590 nm, 590 nm Krt14:Arch Cre+ vs. Cre-; n = 17–20) (B) Intraplantar administration of apyrase (0.4 units; catalyzes ATP hydrolysis) increases the withdrawal latency of C57BL/6 mice during plantar dry ice stimulation (unpaired t-test ****p<0.0001; n = 12). (C) Animals lacking sensory neuron P2X4 receptors (P2X4cKO) exhibit increased withdrawal latencies to plantar dry ice stimulation (unpaired t-test ****p<0.0001; n = 11–12). (D) In a two-temperature preference test, P2X4cKO mice spend more time on innocuous cold surfaces than their P2X4 expressing littermates (2-way ANOVA main effects of temperature, genotype, and temperature x genotype interaction p<0.0001; Bonferroni’s multiple comparisons: **p<0.01 P2X4cKO vs. control; n = 6–8).
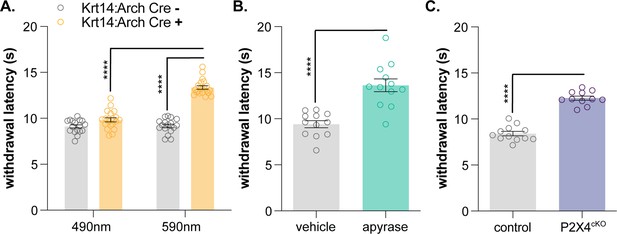
Keratinocyte-to-sensory neuron signaling is required for normal heat sensation.
(A) 590 nm light exposure increases the withdrawal latency of Krt14:Arch Cre+ mice during plantar radiant heat stimulation (2-way ANOVA main effects of light, genotype, and light x genotype interaction p<0.0001; Bonferroni’s multiple comparisons: ****p<0.0001 Krt14:Arch Cre+ 490 vs. 590 nm, 590 nm Krt14:Arch Cre+ vs. Cre-; n = 17–20). (B) Intraplantar administration of apyrase (0.4 units; catalyzes ATP hydrolysis) increases the withdrawal latency of C57BL/6 mice during plantar radiant stimulation (unpaired t-test ****p<0.0001; n = 10). (C) Animals lacking sensory neuron P2X4 receptors (P2X4cKO) exhibit increased withdrawal latencies to plantar radiant heat stimulation (unpaired t-test ****p<0.0001; n = 11–12).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background Rattus norvegicus | Sprague Dawley rat | Taconic Biosciences | NTac:SD; SD-F, SD-M | |
| Strain, strain background Ictidomys tridecemlineatus | 13-lined ground squirrel | University of Wisconsin Oshkosh Squirrel Colony | ||
| Strain, strain background Mus musculus | Krt14:Arch Cre+ Krt14:Arch Cre- | Moehring et al., 2018 | K14:Arch Cre+ K14:Arch Cre- | |
| Strain, strain background Mus musculus | P2X4cKO | Moehring et al., 2018 | AdvilCre+::P2rX4fl/f AdvilCre-::P2rX4fl/f | |
| Strain, strain background Mus musculus | TRPA1 KO | Kwan et al., 2006 | ||
| Strain, strain background Mus musculus | TRPC5 KO | The Jackson Laboratory | Trpc5tm1.1Lbi/Mmjax Jackson Stock: 37349-JAX | |
| Other | In-line heater/cooler | Warner Instruments | https://www.warneronline. com/in-line-heater-cooler-sc-20 | |
| Other | Refrigerated circulator | Julabo | https://www.julabo.com/en-us/products/refrigerated-circulators/refrigerated-heating-circulators/f25-he | |
| Other | USB thermocouple probe | https://www.omega.com/en-us/sensors-and-sensing-equipment/temperature/sensors/thermocouple-probes/p/TJ-USB |
Additional files
-
Source data 1
Figure individual data points.
- https://cdn.elifesciences.org/articles/58625/elife-58625-data1-v3.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/58625/elife-58625-transrepform-v3.pdf





