Retrograde ERK activation waves drive base-to-apex multicellular flow in murine cochlear duct morphogenesis
Figures
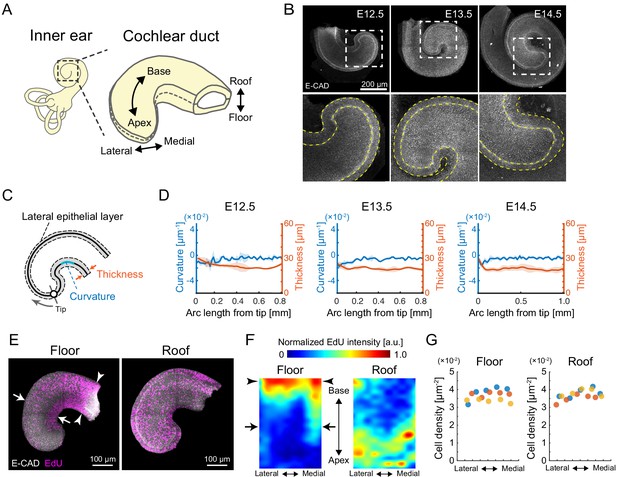
Quantification of morphology and cell proliferation in developing murine cochlear duct.
(A) Schematic diagrams showing the tissue axis and labels of the cochlear duct. (B) Immunofluorescence images of anti-E-cadherin staining in the murine developing cochlea from E12.5 to E14.5. The lower rows are magnified images of the dotted squares in the upper rows. Yellow dotted lines represent the edges of the epithelial layer. Scale bar, 200 µm. (C) Schematic diagram showing regions used for morphological quantification. (D) Curvature and thickness as a function of the arc length from the apex tip along the lateral epithelial layer from E12.5 to E14.5. Mean ± standard deviation (s.d.) N = 3. (E) Maximum projection images of stained anti-E-cadherin (white) and EdU (magenta) in the roof and floor region of cochlear duct at E12.5. Representative images showing EdU signals in the cochlear duct are shown. Arrows indicate the region of EdU signal gradient from the medial to the lateral side of the duct. Arrowheads indicate the base region where the EdU intensity is concentrated. Scale bar, 100 µm. (F) Heatmap of the sample-mean of maximum EdU intensity projection in the roof and floor region of the duct. Arrows and arrowheads are the same as in (E). N = 3. Heatmaps of summed and mean intensity projection are shown in Figure 1—figure supplement 1. (G) Cell density distribution along the mediolateral axis in the floor and roof region, respectively. Color indicates the different samples. N = 3.
-
Figure 1—source data 1
Curvature and thickness from E12.5 to E14.5.
- https://cdn.elifesciences.org/articles/61092/elife-61092-fig1-data1-v2.xlsx
-
Figure 1—source data 2
EdU intensity profiles.
- https://cdn.elifesciences.org/articles/61092/elife-61092-fig1-data2-v2.xlsx
-
Figure 1—source data 3
Cell density along the medial–lateral axis.
- https://cdn.elifesciences.org/articles/61092/elife-61092-fig1-data3-v2.xlsx
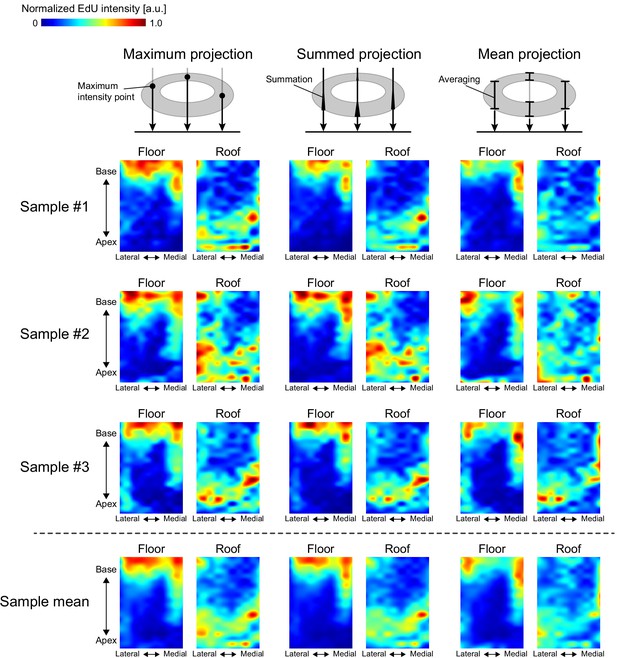
Heatmaps of EdU (EdU: 5-ethynyl-2′-deoxyuridine, DMSO: dimethyl sulfoxide, PFA: paraformaldehyde, PBS: phosphate-buffered saline) intensity.
Heatmaps of EdU intensity normalized to the maximum value of each graph using various projection methods: maximum intensity projection (left); summed intensity projection (center); and mean intensity projection (right). Note that, in the mean intensity projection, the EdU intensity is subject to increase toward the edge of the cochlear duct because the thickness of the epithelial layer along the projected axis is lesser in the edge than in the middle of the cochlear duct.
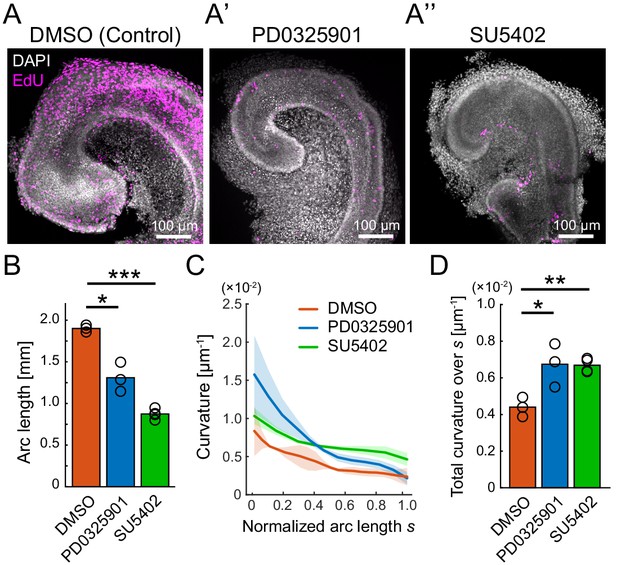
Pharmacological inhibition of FGFR–ERK signaling axis resulted in the impairment of cochlear duct growth.
(A–A’’) Maximum projection images of EdU labeling (magenta) with DAPI (white) nuclear counterstaining over the entire cochleae after 2 days of ex vivo culture from E12.5 in the treatment with DMSO (A), PD0325901 at 1 µM (A’), and SU5402 at 30 µM (A’’). Scale bar, 100 µm. (B) The arc length of the cochlear duct in the treatment with DMSO (red), PD0325901 (blue), and SU5402 (green). N = 3. Two-sample t-test without assuming equal population variances. p=0.023 for DMSO-PD0325901 and p<0.001 for DMSO-SU5402. (C) The curvature of the cochlear duct along the normalized arc length s. Mean ± s.d. N = 3. (D) Total curvature over the normalized arc length in the treatment with DMSO (red), PD0325901 (blue), and SU5402 (green). Two-sample t-test without assuming equal population variances. p=0.021 for DMSO-PD0325901 and p<0.0033 for DMSO-SU5402. ERK: extracellular signal-regulated kinase.
-
Figure 2—source data 1
Longitudinal length for treatments with DMSO, PD0325901, and SU5402.
- https://cdn.elifesciences.org/articles/61092/elife-61092-fig2-data1-v2.xlsx
-
Figure 2—source data 2
Curvature over arc length from the tip for treatments with DMSO, PD0325901, and SU5402.
- https://cdn.elifesciences.org/articles/61092/elife-61092-fig2-data2-v2.xlsx
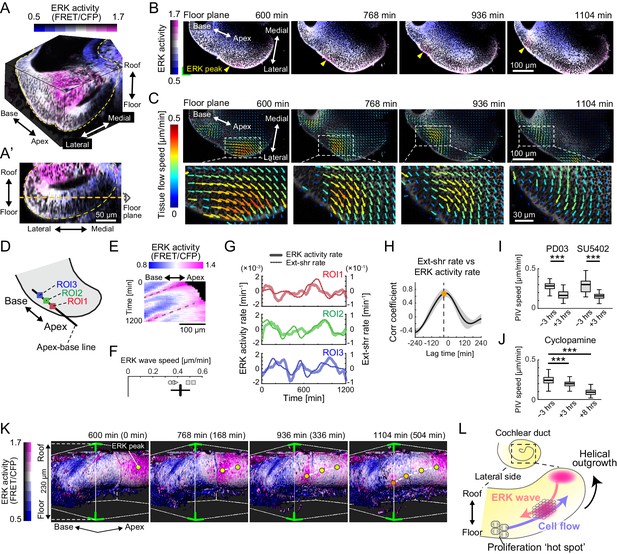
Retrograde helical extracellular signal-regulated kinase (ERK) activation waves drive base-to-apex multicellular flow.
(A) 3D ERK activity map in the cochlear duct cultured ex vivo for 1 day from E12.5. (A’) Cross-sectional view (medial–lateral and roof–floor plane) of (A). Orange dotted line indicates the floor plane shown in (B) and (C). Scale bar, 50 µm. (B) Time-lapse snapshots of ERK activity maps in the floor plane. Time indicates the elapsed time of live imaging. Yellow arrowheads indicate the ERK activity peak. Scale bar, 100 µm. (C) Time-lapse snapshots of tissue flow speed obtained by particle image velocimetry in the floor plane. Scale bar, 100 µm. (D) Schematic diagram showing the axis, the apex–base line for kymography, and regions of interests (ROIs). (E) Representative kymograph of ERK activity. The horizontal axis indicates the position on the apex–base line shown in (D), and the vertical axis indicates the elapsed time of live imaging. Dotted lines represent oscillatory waves from the apex to the base. Scale bar, 100 µm. (F) ERK wave speed with mean and s.d. n = 5 from N = 3. (G) Time-series ERK activity rate and extension-shrinkage rate in representative three different ROIs. (H) Cross-correlation between the extension-shrinkage rate and ERK activity rate. n = 12. Mean ± s.d. (I, J) Tissue flow speed before and after the PD0325901 treatment at 1 µM, the SU5402 treatment at 30 µM (I), and the cyclopamine treatment at 30 µM (J). n = 285. Confirmed by N = 2. Two-sample t-test, p<0.001. (K) Time-lapse snapshots of surface-rendered ERK activity maps in the cochlear duct at E12.5. The green corners correspond to the green corner on the images shown in (B) and viewed from the left-bottom corner of (B). Circles indicate the position of ERK activity peaks, and the connecting dotted lines indicate a trace of the peak shift. The timescale is the same as in (B). (L) Schematics for the ERK activity waves and cell flow.
-
Figure 3—source data 1
Extracellular signal-regulated kinase activity rate and extension-shrinkage rate.
- https://cdn.elifesciences.org/articles/61092/elife-61092-fig3-data1-v2.xlsx
-
Figure 3—source data 2
Particle image velocimetry speed before and after the treatments with PD0325901, SU5402, and cyclopamine.
- https://cdn.elifesciences.org/articles/61092/elife-61092-fig3-data2-v2.xlsx
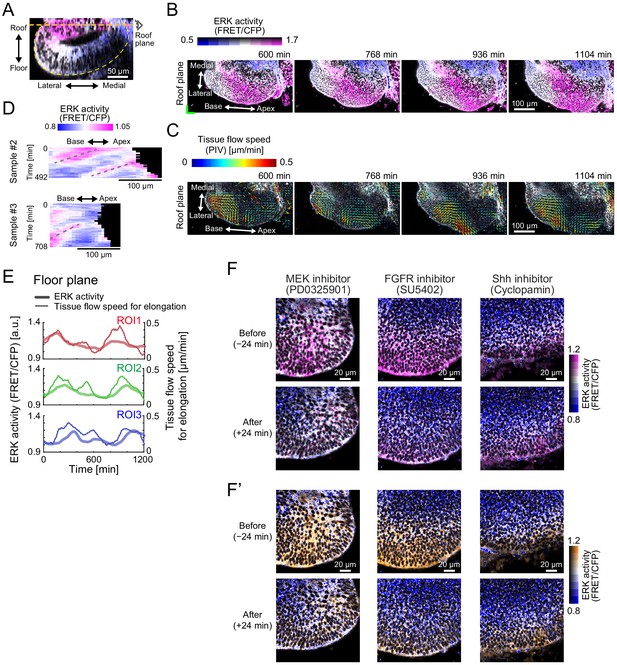
Extracellular signal-regulated kinase (ERK) activity waves and cell flows.
(A) Roof plane (orange dotted line) in the cross-sectional view of 3D ERK activity map in the cochlea at E12.5. Scale bar, 50 µm. (B) Time-lapse snapshots of ERK activity map in the roof plane. Time indicates the elapsed time of live imaging. Scale bar, 100 µm. (C) Time-lapse snapshots of tissue flow speed obtained by the particle image velocimetry in the roof plane. The multicellular flows direct toward the elongation direction around the apex tip despite winding in the region away from the tip in the roof side. Scale bar, 100 µm. (D) Kymograph of ERK activity for the two samples. The horizontal axis indicates the position on the apex–base line, and the vertical axis indicates the elapsed time of live imaging. Dotted lines represent the oscillatory wave trains from the apex to the base. Scale bar, 100 µm. (E) Time-series data of ERK activity and the tissue flow speed for elongation in the three different regions of interests. (F, F’) ERK activity before and after the MEK inhibitor PD0325901 treatment at 1 µM. Scale bar, 20 µm.
3D extracellular signal-regulated kinase (ERK) activity map of the cochlear at E12.5, related to Figure 3.
Color indicates the ERK activity level shown in Figure 3A. Views from the roof side at initial and ones from the apex side at last.
Time-lapse movie of the extracellular signal-regulated kinase (ERK) activity and multicellular tissue flow, related to Figure 3.
Color in the left panel indicates the ERK activity level shown in Figure 3B and that in the right panel indicates the particle image velocimetry speed shown in Figure 3C. Views at the floor plane indicated in Figure 3A’. The time interval is 12 min.
Time-lapse movie of extracellular signal-regulated kinase (ERK) activity upon treatment with cyclopamine, related to Figure 3.
Exactly 30 µM of cyclopamine was added immediately before time zero. Color indicates the ERK activity level. Views at the floor plane indicated in Figure 3A’. The time interval is 12 min.
3D time-lapse imaging of extracellular signal-regulated kinase (ERK) activity in the lateral side of the cochlear duct at E12.5, related to Figure 3.
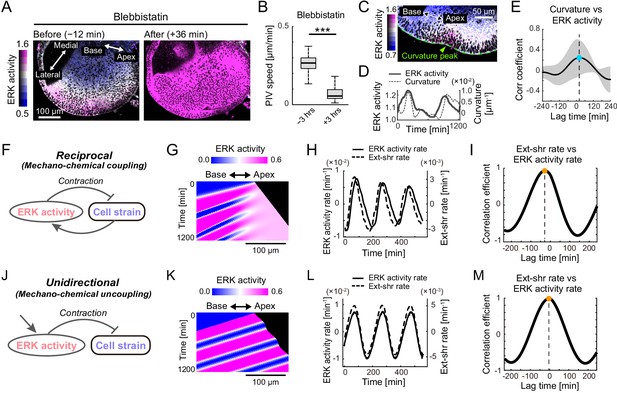
Extracellular signal-regulated kinase (ERK)-mediated mechanochemical feedback explains cell flow and ERK waves.
(A) ERK activity maps in the floor plane before (−12 min) and after (+36 min) the treatment with 30 µM of blebbistatin. Time indicates the timing of blebbistatin treatment. Scale bar, 100 µm. (B) Tissue flow speed before (−3 hr) and after (+3 hr) blebbistatin treatment. N = 258. Two-sample t-test, p<0.001. (C) Snapshot of the time-lapsed ERK activity map on the lateral side of the cochlear duct (green dotted line), showing coexistence of the ERK activity peak and the tissue curvature peak (green arrowhead). Scale bar, 50 µm. (D) Time-series ERK activity and the tissue curvature in the representative regions of interest. (E) Cross-correlation between the tissue curvature and ERK activity. n = 9. Mean ± s.d. (F) Schematics for the model mechanochemical coupling, in which ERK activity and cell deformation are reciprocally regulated. (G) Kymograph of the ERK activity in the model simulation. Scale bar, 100 µm. (H) Simulated time series of ERK activity rate and extension-shrinkage rate in the mechanochemical coupling regime. (I) Cross-correlation between the extension-shrinkage rate and the ERK activity rate. The lag time is −28 min. (J) Schematics for a counterpart regime of the mechanochemical coupling, in which the ERK activity unidirectionally regulates the cell deformation without closed feedback. (K) Kymograph of the ERK activity in the uncoupling model simulation. Scale bar, 100 µm. (L) Simulated time series of ERK activity rate and extension-shrinkage rate in the uncoupling regime. (D) Cross-correlation between the extension-shrinkage rate and the ERK activity rate. The lag time is −2 min.
-
Figure 4—source data 1
Particle image velocimetry speed before and after the treatments with blebbistatin.
- https://cdn.elifesciences.org/articles/61092/elife-61092-fig4-data1-v2.xlsx
-
Figure 4—source data 2
Extracellular signal-regulated kinase activity and tissue curvature.
- https://cdn.elifesciences.org/articles/61092/elife-61092-fig4-data2-v2.xlsx
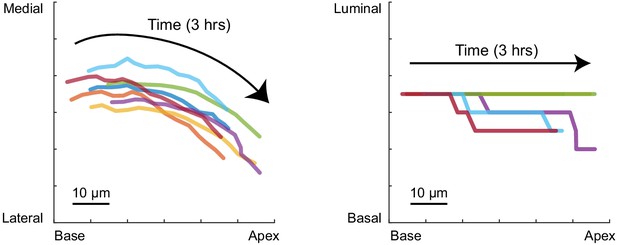
Multicellular tracking of the time-lapse images.
A representative trace line for seven neighboring cells around the future prosensory region at E12.5 on the apex–base and medial–lateral plane (left), and the apex–base and luminal–basal plane (right). Color represents different cells. The trace lines do not intersect each other within 3 hr. Scale bar, 10 µm.
Time-lapse movie of extracellular signal-regulated kinase (ERK) activity upon treatment with blebbistatin, related to Figure 4.
Exactly 30 µM of blebbistatin was added immediately before time zero. Color indicates the ERK activity shown in Figure 4A. Views at the floor plane indicated in Figure 3A’. The time interval is 12 min.





