Obesity and diabetes as comorbidities for COVID-19: Underlying mechanisms and the role of viral–bacterial interactions
Figures
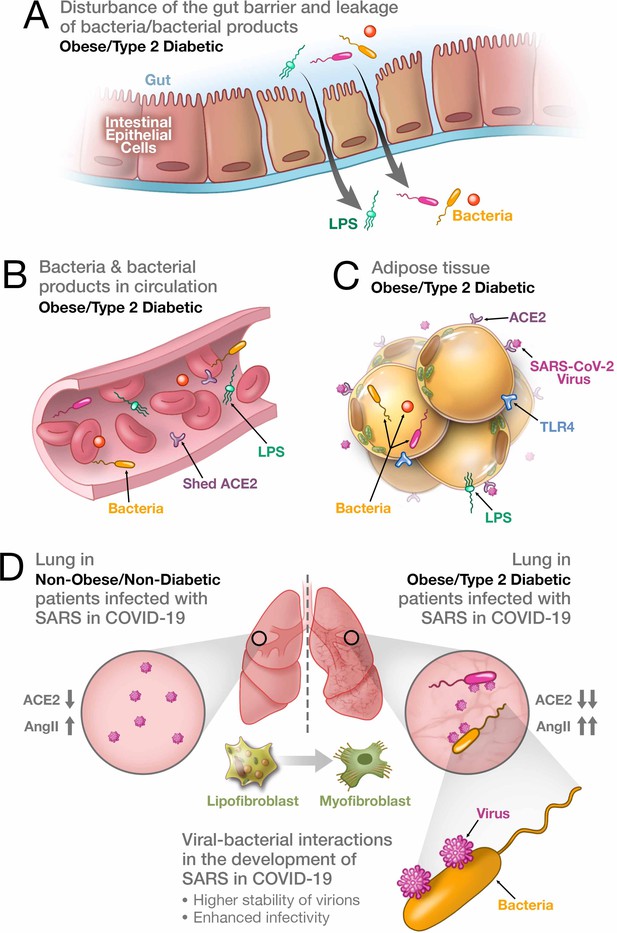
Synergistic interaction between SARS-CoV-2 and bacteria/bacterial products as a possible reason for more severe forms of COVID-19 in patients with obesity and T2D.
(A) Metabolic dysregulation in obese/T2D patients provides the conditions for disturbance of the gut barrier and leakage of bacteria/bacterial products into the circulation. This dysregulation can be additionally enhanced through viral-induced disbalance in a local renin-angiotensin system (RAS). (B) Leakage of bacteria and bacterial products into the circulation provides their system-wide dissemination. (C) In the setting of a synergistic effect of viral–bacterial interactions, some bacterial products can trigger an intense response in the adipose tissue. In obesity and T2D, bacteria and bacterial DNA have been found as long-term constituents in different fat depots. (D) Increase in circulating LPS will lead to the accumulation of endotoxins in the lung, causing progressive pulmonary inflammation and vascular complications. Viral–bacterial interactions lead to hypercytokinemia – the dysproportional increase in the expression of pro-inflammatory cytokines, which is much higher than what an individual exposure to either viral or bacterial agents can achieve. This also leads to higher stability of virions and enhanced infectivity of SARS-CoV-2. A local synergistic pulmonary ACE2 deficiency develops a disbalance between vasodilating and vasoconstricting RAS agents resulting in inflammation. This interaction can also cause enhanced trans-differentiation of lipofibroblasts into myofibroblasts in obese and T2D patients causing the pulmonary fibrosis.
Tables
Some possible pathophysiological pathways connecting obesity/T2D to severity of COVID-19.
| Nr. | Description | Comments |
|---|---|---|
| 1. | High integral viral load induced by the local up-regulation of ACE2 Expression of angiotensin-converting enzyme 2 (ACE2), which is the functional receptor that SARS-CoV/CoV-2 exploits to enter host cells, is strongly upregulated in different tissues of patients with obesity and T2D. This can lead to a high integral viral load of these tissues. | Pro Non-obese ACE2 KO mice manifest a mild form of SARS-CoV infection and strongly reduced pathological changes in the lungs compared to their wild-type counterparts. Contra Comorbidity of obesity/T2D with severity of COVID-19 was observed in viral infections other than SARS-CoV/CoV-2 and thus is not ACE2 specific. ACE inhibitors (ACEi) and Ang II receptor blockers (ARBs), both inducing the expression of ACE2, are thought to be beneficial in COVID-19. |
| 2. | Shedding of ACE2 Increased shedding of ACE2 from different tissues (including adipose tissue demonstrating high expression of this enzyme in obesity and T2D) induced by ADAM17 leads to re-distribution of ACE2 in the body and its accumulation in the lungs. | Pro Hyperglycemia, typical in obesity and T2D, induces increased ADAM17 protein expression. Application of ADAM17 siRNA reduces SARS-CoV infectivity. Contra Comorbidity of obesity and T2D with severity of COVID-19 was observed in viral infections other than SARS-CoV/CoV-2 and thus is not ACE2 specific. ADAM17 regulates the fusion of viral particles with cytoplasmic membranes involved in entering of SARS-CoV/CoV-2 into the host cells. Thus, the positive effect of ADAM17 suppression is not in an obvious way connected with a reduced ACE2 shedding. |
| 3. | Disturbance of the vasodilation-vasoconstriction balance in the RAS system The pulmonary renin-angiotensin system (RAS) is adapted to the conditions of increased ACE2 expression in obese individuals. Enhanced internalization of the virus/ACE2 complex leads to a quick production of a local pulmonary ACE2 deficiency, thereby disturbing the balance between vasodilating (Ang-(1-7)) and vasoconstricting (Ang II) agents in RAS and inducing the development of inflammation and fibrosis. | Pro ACE inhibitors (ACEi) and Ang II receptor blockers (ARBs), both inducing the expression of ACE2, are thought to be beneficial in COVID-19. Levels of vasoconstricting agent Ang II in circulation of COVID-19 patients are significantly elevated and linearly associated with viral load and lung injury. Contra Comorbidity of obesity and T2D with severity of COVID-19 was observed in viral infections other than SARS-CoV/CoV-2 and thus is not ACE2 specific. ACE2 deficiency seems to be not a single parameter influencing this effect. ACE2 KO mice exacerbate Ang II-mediated inflammation via overexpression of matrix metalloproteinases MMP2, −9 and −14. |
| 4. | Compromised endothelial function in obesity and diabetes The vasculature of obese and diabetic subjects has a reduced baseline ACE2 expression, which leads to a compromised endothelial function and increased vascular permeability. This dysfunction can be further increased through virally-mediated reduction of ACE2. Administration of insulin and other anti-diabetic drugs causes additional suppression of ACE2. | Pro ACE inhibitors (ACEi) and Ang II receptor blockers (ARBs), both inducing the expression of ACE2, are thought to be beneficial in COVID-19. Experiments with double mutant Akita (murine model for human diabetes)/ACE2 KO mice revealed that the loss of ACE2 leads to impaired vascular function. This effect was observed only in double mutant mice, whereby neither Akita mice nor ACE2 KO mice alone demonstrate such changes. Contra Comorbidity of obesity and T2D with severity of COVID-19 was observed in viral infections other than SARS-CoV/CoV-2 and thus is not ACE2 specific. |
| 5. | Synergistic viral–bacterial interaction Binding of viruses to lipopolysaccharides (LPS) can enhance their attachment to receptors on the surface of the host cells, thereby enhancing the infectivity. This effect can synergistically increase the integral viral load in the lungs. On the other hand, respiratory viruses can promote bacterial pneumonia, thereby altering the microbiota in the upper and promoting bacterial accumulation in the lower respiratory tract. | Pro Bacteria, bacterial DNA and LPS are present in circulation of obese and T2D individuals, and metabolic endotoxemia is causally connected with obesity, T2D, cardiovascular and pulmonary diseases. The spike protein of SARS-CoV2 binds to high density lipoprotein (HDL) cholesterol, and the severity of the SARS-CoV2 infection is inversely associated with plasma levels of HDL cholesterol. HDL and LPS bind to the scavenger receptor class B type I, which belongs to a cholesterol delivery system and is present in cells such as adipocytes and type two alveolar epithelial cells. Strong synergistic effects of combined coronavirus and bacterial infections on the severity of lung injury was demonstrated in the porcine respiratory coronavirus (PRCV) model: whereas pigs exposed to either PRCV or LPS demonstrated no or only mild symptoms, the combination of PRCV and LPS induced severe SARS and death in the majority of animals. Similar synergistic effects were observed in combined viral–bacterial infections. LPS induces lung injury through the suppression of ACE2 and the upregulation of Ang II, ACE, and AT1 receptors, thus dysregulating RAS before the viral infection. LPS binding protein (an enhancer of LPS endotoxicity) demonstrates a positive correlation with BMI and is significantly elevated in obesity and T2D. Synergistic viral–bacterial interactions seem to be involved in comorbidities of severe COVID-19 beyond obesity and T2D. |
| 6. | Cellular transformations in lungs Adipose tissue is generally compromised in obesity and T2D. SARS-CoV/CoV-2 virus additionally modifies adipocytes and adipocyte-like cells causing their differentiation state, which directly modifies the function of the tissue containing these cells. In severe forms of SARS, this may involve the trans-differentiation of pulmonary lipofibroblasts into myofibroblasts. | Pro Lipofibroblasts trans-differentiate into myofibroblasts under different conditions, including hyperoxia and infection. This transformation is connected with a deprivation of parathyroid hormone-related protein, secreted by type two alveolar epithelial cells, which is suppressed by LPS. LPS leads to the trans-differentiation of pericytes into myofibroblasts in renal fibrosis. LPS-stimulated pericytes undergo trans-differentiation, even upon TGF-β receptor-blocking, which suggests the involvement of TLR4 signaling. Modification of TLR4 signaling is involved in different viral infections including SARS-CoV. Application of the PPARγ agonist rosiglitazone in a rat model of bronchopulmonary dysplasia induced by LPS significantly attenuates the negative effects of LPS and leads to reduced lung injury. |





