Alpha-1 adrenergic receptor antagonists to prevent hyperinflammation and death from lower respiratory tract infection
Figures

Model of clinical progression of respiratory dysfunction from local infection to hyperinflammation.
The timing and relation of hyperinflammation to specific organ manifestations of severe acute respiratory distress syndrome (ARDS) are areas of uncertainty and investigation.
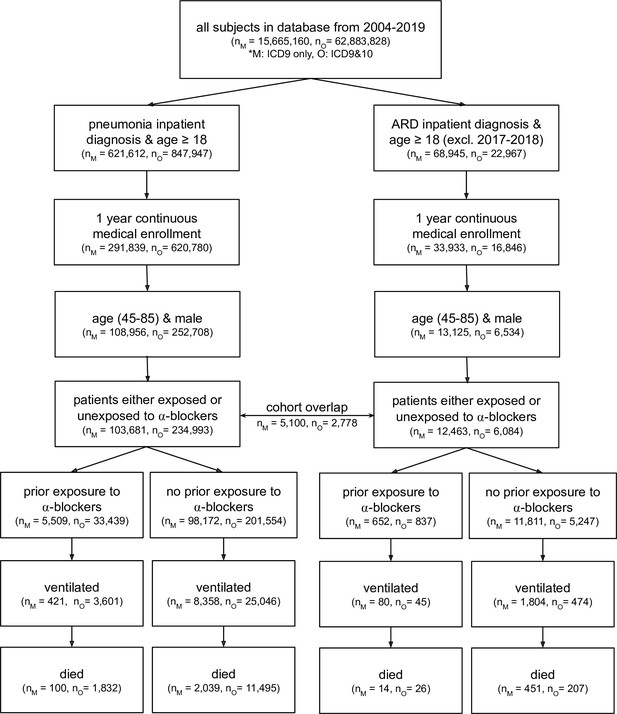
CONSORT flow diagram for four claims datasets where M represents MarketScan and O represents Optum; ARD represents acute respiratory distress.
Note that patients are considered exposed to ⍺1-AR antagonists if they have a medication possession ratio ≥50 % in the prior year, and are considered unexposed if they have not taken any amount of ⍺1-AR antagonists in the prior year. ARD inpatient visits are not considered between 2017–2018 as ARD ICD-9 codes were being phased out while ICD-10 codes for ARD were not yet commonly used. Within a single dataset (MarketScan or Optum), there exists some patient overlap for the two cohort diagnoses (pneumonia and ARD): 5,100 patients in MarketScan and 2,778 in Optum. This diagram only presents four of the five cohorts studied; the fifth cohort (the Swedish National Patient Register) uses a different set of inclusion/exclusion criteria (see section Sweden National Patient Register for criteria description, and Figure 3—figure supplement 1 for information on dataset characteristics).
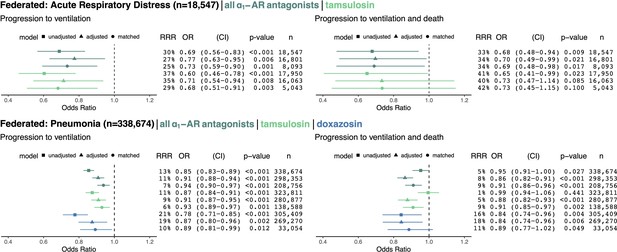
Cohorts across datasets (MarketScan and Optum) associated with the same disease (ARD in top row, pneumonia in bottom row) were pooled using federated causal learning techniques described in Materials and methods.
In each quadrant, we show: (left) plotted odds ratios (OR) with confidence intervals (CI), and (right) values for relative risk reductions (RRR), OR, CI, p-values (p), and sample sizes (n) for unadjusted, adjusted, and matched models, including any ⍺1-AR antagonists or specifically tamsulosin or doxazosin. We only study exposure to doxazosin in the pneumonia cohorts since there is insufficient statistical power to analyze the drug in ARD cohorts. Results are shown for outcomes of mechanical ventilation (left column) and mechanical ventilation leading to death (right column). In general, ⍺1-AR antagonists were associated with reducing risk of adverse events across exposures, outcomes, and modeling approaches. Each federated analysis yielded an OR point estimate below 1.
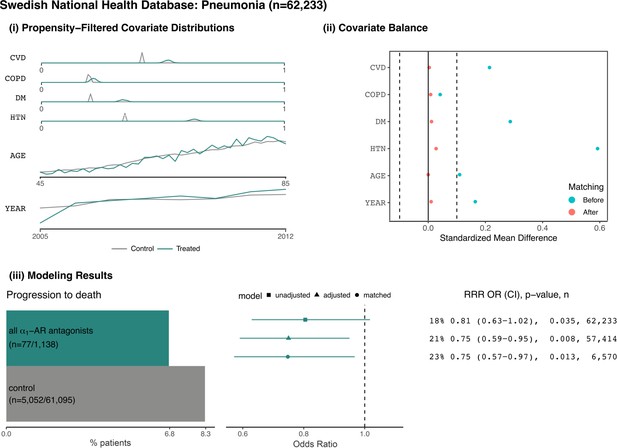
Patients from the Swedish National Patient Register with pneumonia.
(i) Distributions of sample proportion estimates for comorbidities identified from healthcare encounters in the year prior to a patient’s first pneumonia inpatient admission: cardiovascular disease (CVD), chronic obstructive pulmonary disorder (COPD), diabetes mellitus (DM), and hypertension (HTN). Data distributions for additional covariates within the area of propensity score overlap: age and year. (ii) Covariate balance plots before (cyan) and after (red) matching. (iii) Assessing the outcome of progression to death: (left) number and proportion of patients taking indicated medications who experience the outcome, (right) relative risk reductions (RRR), odds ratios (OR), confidence intervals (CI), p-values (p), and sample sizes (n) for unadjusted, adjusted, and matched models. Here, the exposed group includes any patients who have filled at least one prescription for an ⍺1-AR antagonist in the prior year; the unexposed group includes any patients who have never filled a prescription for an ⍺1-AR antagonist in the year prior to hospitalization.
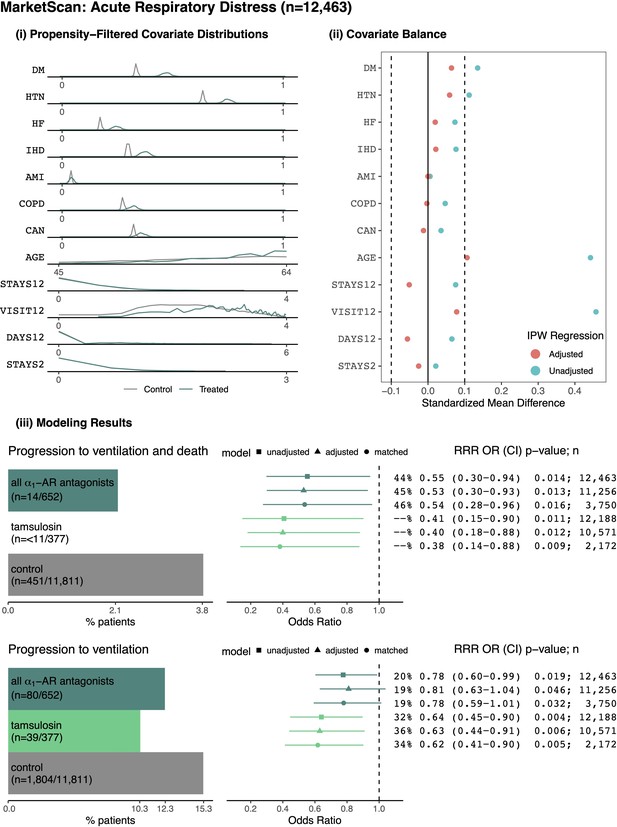
Patients from MarketScan Research Database with acute respiratory distress.
(i) Distributions of sample proportion estimates for comorbidities identified from healthcare encounters in the year prior to a patient’s first ARD inpatient admission: diabetes mellitus (DM), hypertension (HTN), heart failure (HF), ischemic heart disease (IHD), acute myocardial infarction (AMI), chronic obstructive pulmonary disorder (COPD), and cancer (CAN). Data distributions for additional covariates within the area of propensity score overlap: age, total weeks with inpatient admissions in the prior year (STAYS12), total outpatient visits in the prior year (VISIT12), total prior days as an inpatient in the prior year (DAYS12), total weeks with prior inpatient stays in the previous two months (STAYS2), and fiscal year (YEAR). (ii) Covariate balance plots before (cyan) and after (red) inverse propensity weighting. (iii) For the outcome of progressing to ventilation and death: (left) number and proportion of patients taking indicated medications who experienced the outcome, (right) relative risk reductions (RRR), odds ratios (OR), confidence intervals (CI), p-values (p), and sample sizes (n) for unadjusted, adjusted, and matched models, including any ⍺1-AR antagonists and specifically tamsulosin. Likewise for the secondary outcome of requiring ventilation. In general, ⍺1-AR antagonists are associated with reducing risk of adverse events across treatments, outcomes, and modeling approaches. The raw outcome count and corresponding RRR for tamsulosin in the ventilation and death outcome are redacted per MarketScan policy for displaying small counts.
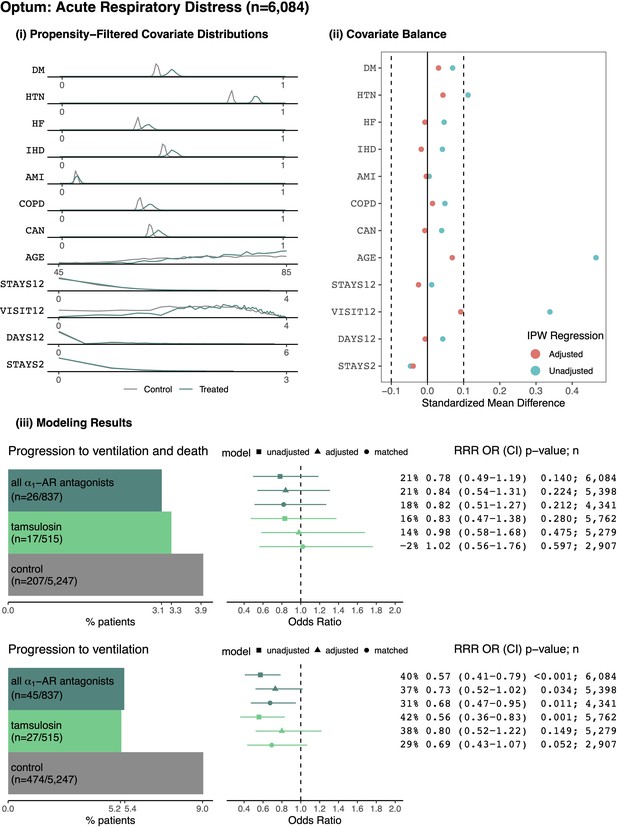
Patients from Optum with acute respiratory distress.
(i) Distributions of sample proportion estimates for comorbidities identified from healthcare encounters in the year prior to a patient’s first ARD inpatient admission: diabetes mellitus (DM), hypertension (HTN), heart failure (HF), ischemic heart disease (IHD), acute myocardial infarction (AMI), chronic obstructive pulmonary disorder (COPD), and cancer (CAN). Data distributions for additional covariates within the area of propensity score overlap: age, total weeks with inpatient admissions in the prior year (STAYS12), total outpatient visits in the prior year (VISIT12), total prior days as an inpatient in the prior year (DAYS12), total weeks with prior inpatient stays in the previous two months (STAYS2), and fiscal year (YEAR). (ii) Covariate balance plots before (cyan) and after (red) inverse propensity weighting. (iii) For the outcome of progressing to ventilation and death: (left) number and proportion of patients taking indicated medications who experienced the outcome, (right) relative risk reductions (RRR), odds ratios (OR), confidence intervals (CI), p-values (p), and sample sizes (n) for unadjusted, adjusted, and matched models, including any ⍺1-AR antagonists and specifically tamsulosin. Likewise for the secondary outcome of requiring ventilation. In general, ⍺1-AR antagonists are associated with reducing risk of adverse events across treatments, outcomes, and modeling approaches.
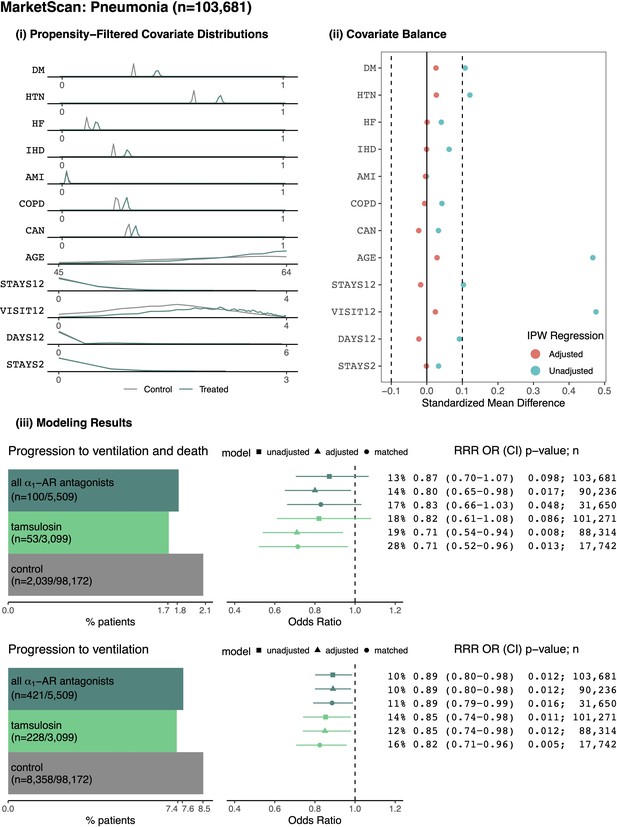
Patients from MarketScan Research Database with pneumonia.
(i) Distributions of sample proportion estimates for comorbidities identified from healthcare encounters in the year prior to a patient’s first ARD inpatient admission: diabetes mellitus (DM), hypertension (HTN), heart failure (HF), ischemic heart disease (IHD), acute myocardial infarction (AMI), chronic obstructive pulmonary disorder (COPD), and cancer (CAN). Data distributions for additional covariates within the area of propensity score overlap: age, total weeks with inpatient admissions in the prior year (STAYS12), total outpatient visits in the prior year (VISIT12), total prior days as an inpatient in the prior year (DAYS12), total weeks with prior inpatient stays in the previous two months (STAYS2), and fiscal year (YEAR). (ii) Covariate balance plots before (cyan) and after (red) inverse propensity weighting. (iii) For the outcome of progressing to ventilation and death: (left) number and proportion of patients taking indicated medications who experienced the outcome, (right) relative risk reductions (RRR), odds ratios (OR), confidence intervals (CI), p-values (p), and sample sizes (n) for unadjusted, adjusted, and matched models, including any ⍺1-AR antagonists and specifically tamsulosin. Likewise for the secondary outcome of requiring ventilation. In general, ⍺1-AR antagonists are associated with reducing risk of adverse events across treatments, outcomes, and modeling approaches.
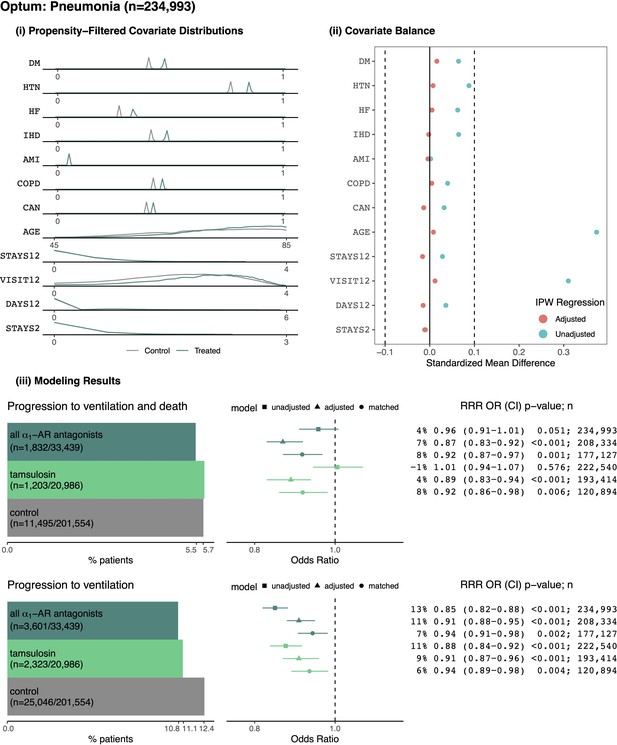
Patients from Optum with pneumonia.
(i) Distributions of sample proportion estimates for comorbidities identified from healthcare encounters in the year prior to a patient’s first ARD inpatient admission: diabetes mellitus (DM), hypertension (HTN), heart failure (HF), ischemic heart disease (IHD), acute myocardial infarction (AMI), chronic obstructive pulmonary disorder (COPD), and cancer (CAN). Data distributions for additional covariates within the area of propensity score overlap: age, total weeks with inpatient admissions in the prior year (STAYS12), total outpatient visits in the prior year (VISIT12), total prior days as an inpatient in the prior year (DAYS12), total weeks with prior inpatient stays in the previous two months (STAYS2), and fiscal year (YEAR). (ii) Covariate balance plots before (cyan) and after (red) inverse propensity weighting. (iii) For the outcome of progressing to ventilation and death: (left) number and proportion of patients taking indicated medications who experienced the outcome, (right) relative risk reductions (RRR), odds ratios (OR), confidence intervals (CI), p-values (p), and sample sizes (n) for unadjusted, adjusted, and matched models, including any ⍺1-AR antagonists and specifically tamsulosin. Likewise for the secondary outcome of requiring ventilation. In general, ⍺1-AR antagonists are associated with reducing risk of adverse events across treatments, outcomes, and modeling approaches.
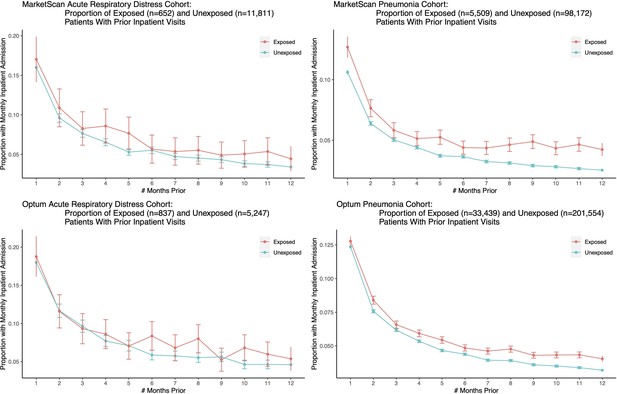
We plot the proportion of exposed and unexposed patients having any inpatient admissions a certain number of months prior to the first ARD or pneumonia admission date, and present corresponding confidence intervals.
Both exposed and unexposed groups had similar trends of declining health leading up to the target admission date, where health decline is defined as having more frequent inpatient visits.
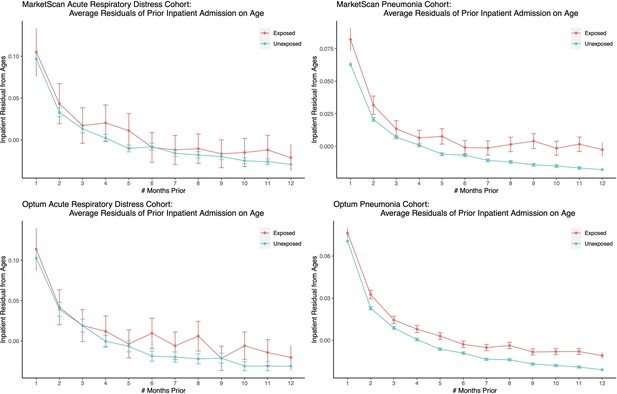
We plot the average residuals of inpatient visits after controlling for age effects (as well as age squared and age cubed) for exposed and unexposed patients having inpatient admissions a certain number of months prior to the first ARD or pneumonia admission date, and present corresponding confidence intervals.
Both exposed and unexposed groups had similar trends of declining health leading up to the target admission date, where health decline is defined as having more frequent inpatient visits after controlling for age effects.
Additional files
-
Source code 1
R Markdown code to run retrospective analysis and generate figures.
- https://cdn.elifesciences.org/articles/61700/elife-61700-supp1-v2.zip
-
Source code 2
R script to generate cohorts and perform propensity matching.
- https://cdn.elifesciences.org/articles/61700/elife-61700-supp2-v2.zip
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/61700/elife-61700-transrepform1-v2.docx





