Defective memory engram reactivation underlies impaired fear memory recall in Fragile X syndrome
Figures
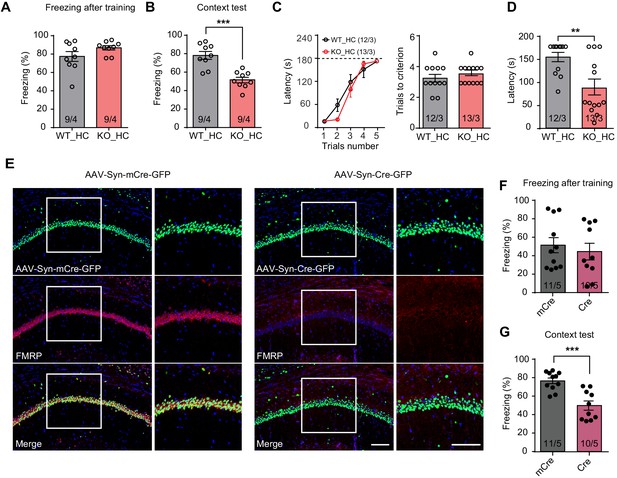
Impaired hippocampus-dependent contextual fear memory in constitutive Fmr1 KO and CA1-Fmr1 KO mice.
(A and B) Contextual fear memory test in constitutive Fmr1 KO mice. Freezing levels were measured immediately after fear conditioning training (A) and again in training context three days after (B) (***p<0.001, two-tailed unpaired t test). (C–D) Passive avoidance test in constitutive Fmr1 KO mice. (C) Learning curves (left) and trials number to criterion (right) were measured during training, and contextual fear memory in passive avoidance was measured 1 day later (D) (**p<0.01, two-tailed Mann-Whitney U test). (E) Representative images of hippocampal CA1 regions from Fmr1 conditional KO mice with bilateral injections of AAVs expressing Cre-GFP or mCre-GFP (green). FMRP expression was evaluated with immunostaining (red). Scale bars: 100 μm. (F and G) Contextual fear memory test in CA1-Fmr1 KO mice. Freezing levels were measured immediately after fear conditioning training (F) and again in training context 1 day after (G) (***p<0.001, two-tailed unpaired t test). n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
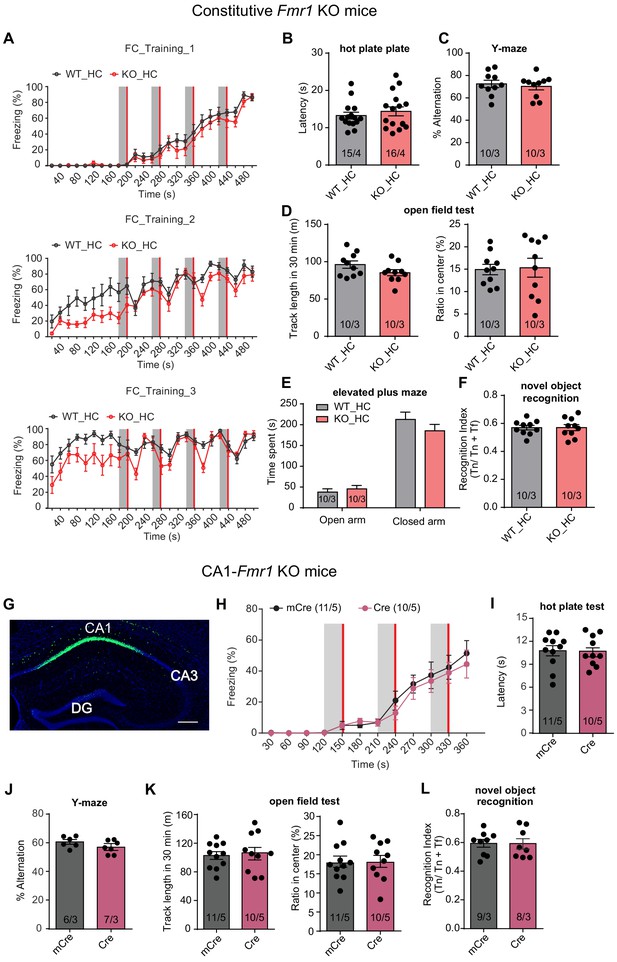
Behavioral characterization of constitutive Fmr1 KO mice and CA1-Fmr1 KO mice reared in home cage.
(A) Learning curves of constitutive Fmr1 KO mice in contextual fear conditioning. The intensive fear conditioning training paradigm has three sessions, each consists of four pairs of co-terminating tone (20 s, gray bar) and foot shock (2 s, red bar). Freezing levels before, during and after each tone-shock pair were quantified (Two-way repeated measure ANOVA: training 1: group factor, F(1, 16)=0.8305, p=0.3756; interaction, F(24, 384)=0.4943, p=0.9797; training 2: group factor, F(1, 16)=9.416, **p<0.01; interaction, F(24, 384)=1.170, p=0.2658; training 3: group factor, F(1, 16)=5.896, *p<0.05; interaction, F(24, 384)=1.959, **p<0.01). (B) Hot plate test. Latencies to escape were quantified (p=0.82, two-tailed Mann Whitney U test). (C) Y maze test. Percentage of spontaneous alterations was quantified (p=0.637, two-tailed unpaired t test). (D) Open field test. Left, quantification of track length in 30 min (p=0.1095, two-tailed unpaired t test); Right, quantification of percent track length in center (p=0.8692, two-tailed unpaired t test). (E) Elevated plus maze. Time spent on the open vs. closed arms was measured (Two-way ANOVA: group factor, F(1, 36)=0.5884, p=0.4481; interaction, F(1, 36)=1.853, p=0.1819). (F) Novel object recognition. Recognition index is defined as the ratio between time spent on novel object and time spent on both objects (p=0.9856, two-tailed unpaired t test). (G) A representative image of a hippocampal tissue section showing CA1-specific AAV-mediated expression of Syn-Cre-GFP. Scale bar: 200 μm. (H) Learning curves of CA1-Fmr1 KO mice in conventional contextual fear conditioning test. Three pairs of tone (30 s, gray bar) and foot shock (2 s, red bar) were delivered with a 60 s interval. Two-way repeated measure ANOVA: group factor, F(1, 19)=0.2128, p=0.6498; interaction, F(11, 209)=0.3191, p=0.9812. (I) Hot plate test. Quantification of latencies to escape (p=0.9602, two-tailed unpaired t test). (J) Y maze test. Quantification of percent spontaneous alterations (p=0.1707, two-tailed Mann Whitney U test). (K) Open field test. Left, quantification of track length in 30 min (p=0.7037, two-tailed unpaired t test); Right, quantification of percent track length in center (p=0.9375, two-tailed unpaired t test). (L) Novel object recognition. Recognition index defined as the ration between time spent on novel object and time spent on both objects (p=0.973, two-tailed unpaired t test). n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
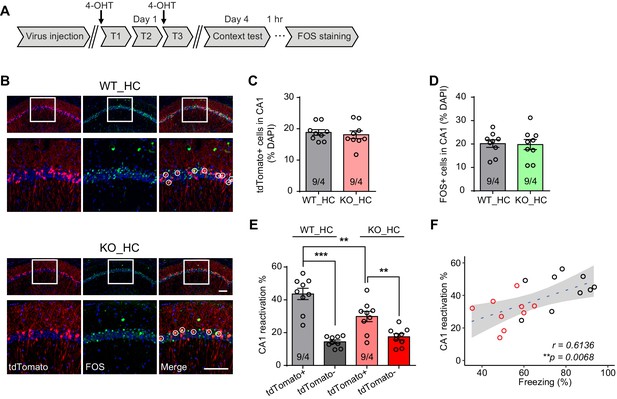
Reduced engram reactivation efficacy is correlated with impaired contextual fear memory in Fmr1 KO mice.
(A) Experimental protocol for activity-dependent genetic labeling of neural ensembles. Intensive fear conditioning training was used in constitutive Fmr1 KO mice to facilitate labeling. (B) Representative images showing memory-encoding neural ensembles (engram cells) labeled with tdTomato (red) and memory recall-activated neurons labeled with FOS immunostaining (green). The circles in zoomed in images highlight reactivated neurons (yellow). Scale bar: 100 μm. (C) Quantification of percentage of neurons activated during learning. (D) Quantification of percentage of neurons activated during memory recall. (E) Quantification of engram reactivation efficacy in CA1 as percent FOS-positive neurons in tdTomato-positive and tdTomato-negative populations [one-way ANOVA with Tukey’s multiple comparison test: F (3, 32)=26.57, p<0.0001, ***p<0.001; **p<0.01]. (F) Positive correlation between neural ensemble reactivation efficacy and behavioral perform during context memory test (**p<0.01, Pearson correlation coefficient). n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
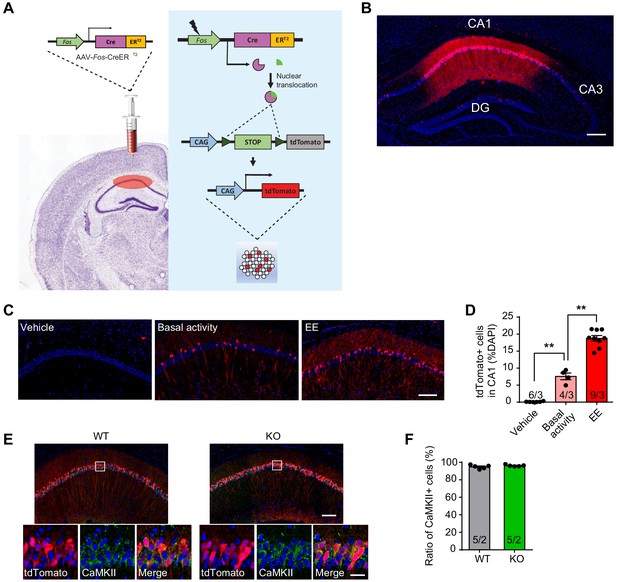
Validation of activity-dependent genetic labeling method and characterization of Fos-TRAP-labeled neurons.
(A) Schematics of AAV-Fos-TRAP application. Ai9 reporter mice were injected with AAV-Fos-ERT2-Cre-ERT2-PEST at hippocampal CA1. Behavioral experience (fear conditioning training or enriched environment) activates Fos expression, which drives Cre expression and Cre-mediated recombination in the presence of tamoxifen, labeling a population of activated cells (tdTomato+) in the CA1. Non-active cells do not undergo recombination due to lack of Cre expression. (B) A representative image of a hippocampal tissue section showing intensive fear conditioning training-activated neurons in CA1. Scale bar: 200 μm. (C) Representative images showing the expression of tdTomato in CA1 after mice underwent 4 hr enriched environment. Scale bar: 100 μm. Mice with vehicle injection (Vehicle) or maintained in home cage with basal activity were used as controls. (D) Quantification of activated cells (tdTomato+) in CA1 [Kruskal-Wallis test with Dunn’s multiple comparison test: H (2)=15.41, p<0.001, **p<0.01]. (E) Representative images showing Fos-TRAP-tdTomato expression and CaMKII immunostaining in wild type and Fmr1 KO mice. Ai9 Mice expressing tdTomato reporter were injected with Fos-TRAP viruses in hippocampal CA1 region and trained with intensive fear conditioning. Learning activated neurons were labeled by tdTomato expression. Excitatory principle neurons in the hippocampus were identified with CaMKII immunostaining. Scale bars: 100 μm (upper) and 20 μm (lower). (F) Quantification of CaMKII-positive neurons in the tdTomato-expressing population. n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
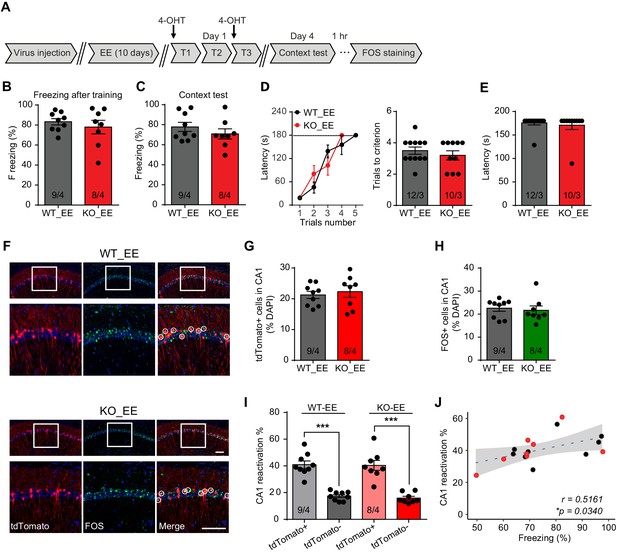
Enriched environment experience rescues fear memory deficits in Fmr1 KO mice by improving engram reactivation efficacy during memory recall.
(A) Experimental protocol for EE, memory-encoding neural ensemble and reactivation labeling. (B and C) Contextual fear conditioning test. Freezing levels were measured immediately after fear conditioning training (B) and again in training context 3 days after (C). (D and E) Passive avoidance test. (D) Learning curves (left) and trials number to criterion (right) were measured during training, and contextual fear memory in passive avoidance was measured 1 day later (E). (F) Representative images showing memory-encoding neural ensembles (engram cells) labeled with tdTomato (red) and memory recall-activated neurons labeled with FOS immunostaining (green). The circles in zoomed in images highlight reactivated neurons (yellow). Scale bar: 100 μm. (G) Quantification of percentage of neurons activated during learning. (H) Quantification of percentage of neurons activated during memory recall. (I) Quantification of engram reactivation in CA1. Percent FOS-positive neurons in both tdTomato+ and tdTomato- populations were measured [one-way ANOVA with Tukey’s multiple comparison test: F (3, 30)=32.51, p<0.0001, ***p<0.001]. (J) Positive correlation between engram reactivation efficacy (percent FOS+ neurons in tdTomato+ population) and behavioral performance during contextual memory test (*p<0.05, Pearson correlation coefficient). n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
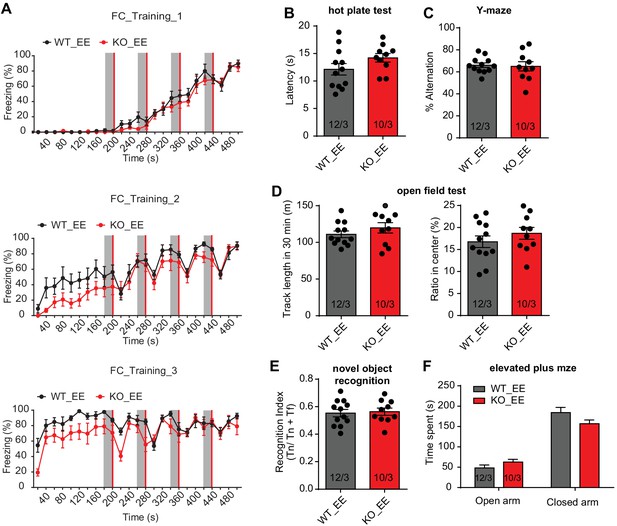
Behavioral characterization of constitutive Fmr1 KO mice with enriched environment experience (EE).
(A) Learning curve of contextual fear conditioning. The intensive fear conditioning training paradigm has three sessions, each consists of four pairs of co-terminating tone (20 s, gray bar) and foot shock (2 s, red bar). Freezing levels before, during, and after each tone-shock pair were quantified (two-way repeated measure ANOVA: training 1: group factor, F(1, 15)=0.6939, p=0.4179; interaction, F(24, 360)=0.6893, p=0.8627; training 2: group factor, F(1, 15)=4.353, p=0.0544; interaction, F(24, 360)=0.9350, p=0.5538; training 3: group factor, F(1, 15)=8.723, **p<0.01; interaction, F(24, 360)=1.557, *p<0.05). (B) Hot plate test. Quantification of latencies to escape (p=0.1379, two-tailed unpaired t test). (C) Y maze test. Quantification of percent spontaneous alterations (p=0.8186, two-tailed unpaired t test). (D) Open field test. Left, quantification of track length in 30 min (p=0.2913, two-tailed unpaired t test); Right, quantification of percent track length in center (p=0.3298, two-tailed unpaired t test). (E) Novel object recognition. Recognition index is defined as the ratio between time spent on novel object and time spent on both objects (p=0.7874, two-tailed unpaired t test). (F) Elevated plus maze. Quantification of time spent on the open vs. closed arms (two-way ANOVA: group factor, F(1, 40)=0.5001, p=0.5001; interaction, F(1, 40)=4.710, *p<0.05). n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
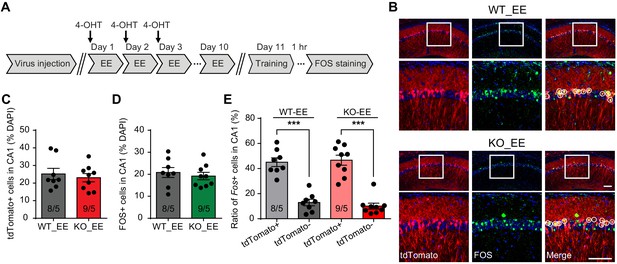
Neurons activated during enriched environment experience are more likely to become engram cells in subsequent learning.
(A) Experimental protocol for enriched environment (EE) and neural ensemble labeling. (B) Representative images of EE-activated neural ensembles labeled with tdTomato (red) and fear conditioning training-activated neurons labeled with FOS immunostaining (green). The circles in zoomed in images highlight reactivated EE-engaged cells during fear conditioning learning (yellow). Scale bar: 100 μm. (C) Quantification of percentage of neurons activated during EE. (D) Quantification of percentage of neurons activated during fear conditioning learning. (E) Quantification of neuronal activation during subsequent learning (FOS+) in EE-engaged (tdTomato+) and non-EE engaged (tdTomato-) populations [Kruskal-Wallis test with Dunn’s multiple comparison test: H (3)=24.75, p<0.001, ***p<0.001]. n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
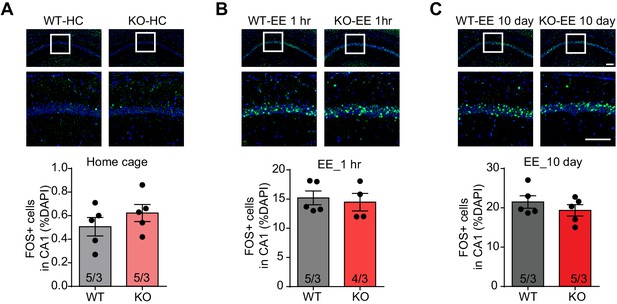
Histological characterization of constitutive Fmr1 KO mice with enriched environment experience (EE).
(A) Representative images (top) and quantification (bottom) of activated cells (FOS+, green) in CA1 of WT and Fmr1 KO mice maintained in home cages (p=0.4127, two-tailed Mann Whitney U test). (B) Representative images (top) and quantification (bottom) of activated cells (FOS+, green) in CA1 of WT and Fmr1 KO mice after 1 hr EE experience (p=0.5238, two-tailed Mann Whitney U test). (C) Representative images (top) and quantification (bottom) of activated cells (FOS+, green) in CA1 of WT and Fmr1 KO mice after 10 days of EE experience (p=0.5317, two-tailed Mann Whitney U test). Scale bar: 100 μm. n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
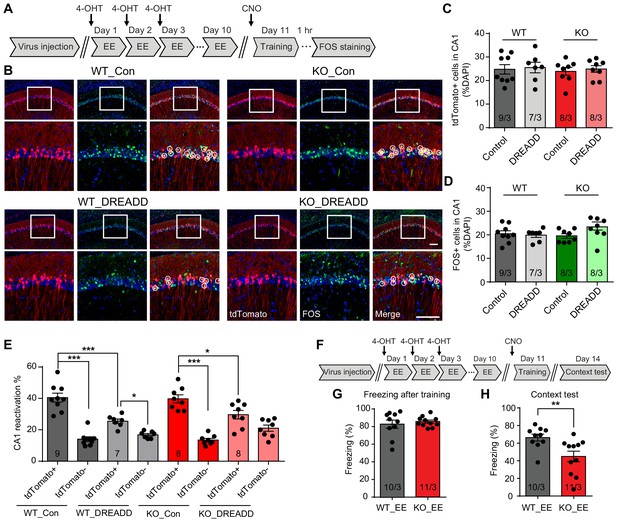
Inhibiting CA1 neurons activated by the enriched environment experience prevents memory improvement by EE in Fmr1 KO mice.
(A) Experimental protocol for chemogenetic inhibition of EE-activated neurons before fear conditioning training. (B) Representative images showing EE-activated neural ensembles labeled with tdTomato (red) and fear conditioning learning-activated neurons labeled with FOS immunostaining (green) in control and inhibitory DREADD-expressing mice CA1. The circles highlight reactivated EE-engaged cells during fear conditioning learning (yellow). Scale bar: 100 μm. (C) Quantification of neuronal activation by EE (Kruskal-Wallis test: H (3)=0.1518, p=0.9850). (D) Quantification of neuronal activation by fear conditioning learning (Kruskal-Wallis test: H (3)=5.669, p=0.1289). (E) Quantification of neuronal activation during subsequent learning (FOS+) in EE-engaged (tdTomato+) and non-EE engaged (tdTomato-) populations [WT group: two-way ANOVA: group factor, F(1, 28)=9.608, **p<0.01; interaction, F(1, 28)=20.22, ***p<0.001; Tukey post hoc test: ***p<0.001; *p<0.05. KO group: two-way ANOVA: group factor, F(1, 28)=0.3268, p=0.5721; interaction, F(1, 28)=16.29, ***p<0.001; Tukey post hoc test: ***p<0.001; *p<0.05]. (F) Experimental protocol for EE-activated neural ensemble inhibition and contextual fear memory test. (G and H) Contextual fear conditioning test. Freezing levels were measured immediately after fear conditioning training (G) and again in training context 3 days after (H) (**p<0.01, two-tailed Mann-Whitney U test). n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
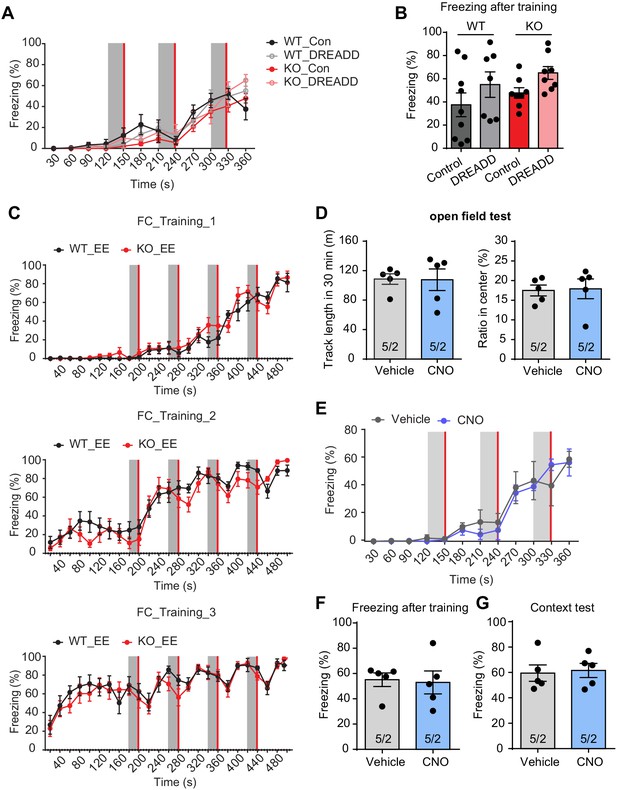
Behavioral characterization of Fmr1 KO mice after inhibition of EE-activated neurons in CA1.
(A and B) Conventional contextual fear conditioning test. Three pairs of tone (30 s, gray bar) and foot shock (2 s, red bar) were delivered with a 60 s interval. Learning curves (A) (two-way repeated measure ANOVA: group factor, F(3, 28)=0.5147, p=0.6755; interaction, F(33, 308)=1.176, p=0.2392) and freezing levels measured immediately after fear conditioning training (B) (p=0.1602, Kruskal-Wallis test). (C) Learning curve of contextual fear conditioning. The intensive fear conditioning training paradigm has three sessions, each consists of four pairs of co-terminating tone (20 s, gray bar) and foot shock (2 s, red bar). Freezing levels before, during, and after each tone-shock pair were quantified (Two-way repeated measure ANOVA: training 1: group factor, F(1, 19)=0.3120, p=0.5830; interaction, F(24, 456)=1.084, p=0.3575; training 2: group factor, F(1, 19)=0.7560, p=0.3954; interaction, F(24, 456)=1.304, p=0.1540; training 3: group factor, F(1, 19)=0.4480, p=0.5113; interaction, F(24, 456)=0.6499, p=0.8984). (D–G) Characterization of CNO’s effect on locomotion and freezing behavior. (D) Open field test. Left, quantification of track length in 30 min (p=0.5317, Mann-Whitney test); Right, quantification of percent track length in center (p=0.3217, Mann-Whitney test). (E–G) Conventional contextual fear conditioning learning. Three pairs of tone (30 s, gray bar) and foot shock (2 s, red bar) were delivered with a 60 s interval. Learning curves (E) (two-way repeated measure ANOVA: group factor, F(1, 8)=0.06812, p=0.8007; interaction, F(11, 88)=0.5860, p=0.8355) and freezing levels measured immediately after fear conditioning training (F) (p=0.4444, Mann-Whitney test). (G) Freezing level in training context measured 1 day after training (p=0.8016, Mann-Whitney test).

Cued fear memory and contextual fear memory are both impaired in CA1-Fmr1 KO mice and constitutive Fmr1 KO mice.
(A and B) Altered context test and cued fear memory test in CA1-Fmr1 KO mice. Freezing levels in an altered context (A) and altered context with tone CS (B) were measured three days after training (**, p < 0.01, two-tailed Mann-Whitney U test). (C-F) Cued fear memory in constitutive Fmr1 KO mice. (C) Learning curves of constitutive Fmr1 KO mice in cued fear conditioning. The intensive fear conditioning training paradigm has three sessions, each consists of four pairs of co-terminating tone (20 s, gray bar) and foot shock (2 s, red bar). Freezing levels before, during and after each tone-shock pair were quantified (Two-way repeated measure ANOVA: training 1: group factor, F(1, 11) = 3.497, p = 0.0883; interaction, F(24, 264) = 1.776, *, p < 0.05; training 2: group factor, F(1, 11) = 14.90, **, p < 0.01; interaction, F(24, 264) = 1.150, p = 0.2899; training 3: group factor, F(1, 11) = 2.745, p = 0.1258; interaction, F(24, 264) = 1.632, *, p < 0.05). (D) Freezing levels measured immediately after fear conditioning training. (E) Freezing levels in an altered context and (F) altered context with tone CS were measured three days after training (**, p < 0.01, two-tailed Mann-Whitney U test). (G-I) Contextual fear memory in constitutive Fmr1 KO mice. (G) Learning curves of constitutive Fmr1 KO mice in contextual fear conditioning. The intensive fear conditioning training paradigm has three sessions, each consists of four foot shock (2 s, red bar) delivered at 198 s, 278 s 358 s and 438 s. Freezing levels before, during and after each shock were quantified (Two-way repeated measure ANOVA: training 1: group factor, F(1, 12) = 1.497, p = 0.2446; interaction, F(24, 288) = 0.8571, p = 0.6612; training 2: group factor, F(1, 12) = 0.4586, p = 0.5111; interaction, F(24, 288) = 0.7814, p = 0.7597; training 3: group factor, F(1, 12) = 0.1740, p = 0.6839; interaction, F(24, 288) = 0.8450, p = 0.6775). Freezing levels were measured immediately after fear conditioning training (H) and again in training context three days after (I) (**, p < 0.01, two-tailed Mann-Whitney U test). n/N, number of mice/number of independent litters. All graphs represent mean ± SEM.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Mus musculus) | Fmr1 | PubMed | 14265 | |
| Strain, strain background (Mus musculus, male) | Fmr1 KO: FVB.129P2-Pde6b+ Tyrc-chFmr1tm1Cgr/J | The Jackson Laboratory | JAX: 004624 RRID:IMSR_JAX:004624 | |
| Strain, strain background (Mus musculus, male) | Ai9: B6.Cg-Gt(ROSA) 26Sortm9(CAG-tdTomato)Hze/J | The Jackson Laboratory | JAX: 007909 RRID:IMSR_JAX:007909 | |
| Strain, strain background (Mus musculus, male) | conditional Fmr1 KO | Zhong et al., 2018 | N/A | |
| Antibody | Mouse monoclonal anti-FMRP | DSHB | Cat#2F5-1, RRID:AB_10805421 | (1:2.5) |
| Antibody | Rabbit polyclonal anti-c-FOS | Millipore | Cat#ABE457, RRID:AB_2631318 | (1:500) |
| Antibody | Mouse monoclonal anti-CaMKII | Abcam | Cat# ab22609, RRID:AB_447192 | (1:500) |
| Antibody | Goat polyclonal anti-rabbit IgG secondary antibody | Thermo Fisher Scientific | Cat#A-11034, RRID:AB_2576217 | (1:200) |
| Antibody | Goat polyclonal anti-mouse secondary antibody (Cy3-conjugated) | Jackson ImmunoResearch Laboratories | Cat#115-165-146, RRID:AB_2338690 | (1:100) |
| Antibody | Goat polyclonal anti-mouse secondary antibody (Cy2-conjugated) | Jackson ImmunoResearch Laboratories | Cat#115-225-146, RRID:AB_2307343 | (1:200) |
| Other | AAV-DJ-Syn-mCre-GFP | Hsu et al., 2019 | N/A | AAV virus expressing mCre-GFP |
| Other | AAV-DJ-Syn-Cre-GFP | Hsu et al., 2019 | N/A | AAV virus expressing Cre-GFP |
| Other | AAV8-Fos-ERT2- Cre-ERT2-PEST | Gift from Karl Deisseroth lab Ye et al., 2016 | N/A | AAV-Fos-TRAP |
| Other | AAV-DJ-EF1a-DIO- hM4D(Gi)-mCherry | Stanford Virus Core | Stock ID: AAV-129, Lot#5289 | AAV virus expressing Cre-dependent hM4D(Gi)-mCherry |
| Other | AAV-DJ-EF1a-DIO-mCherry | Stanford Virus Core | Stock ID: AAV-14, Lot#5020 | AAV virus expressing Cre-dependent mCherry |
| Chemical compound, drug | 4-Hydroxytamoxifen (4-OHT) | Sigma-Aldrich | Cat#H6278 | 10 mg/ml |
| Chemical compound, drug | Clozapine N-oxide (CNO) | Tocris | Cat#4936 | 5 mg/kg |
| Chemical compound, drug | Corn oil | Sigma-Aldrich | Cat#C8267 | |
| Software, algorithm | Freezeview | Coulbourn Instruments | https://www.coulbourn.com/ | |
| Software, algorithm | FreezeFrame 4 | Coulbourn Instruments | RRID:SCR_014429 https://www.coulbourn.com/ product_p/act-100a.htm | |
| Software, algorithm | Viewer III tracking system | Biobserve | RRID:SCR_014337 http://www.biobserve.com/ behavioralresearch/ products/viewer/ | |
| Software, algorithm | MED-PC IV | Med Associates | RRID:SCR_014296 https://www.med-associates.com/ product/deluxe-shuttle-box- avoidance-chamber- package-for-mouse/ | |
| Software, algorithm | Nikon Elements | Nikon | RRID:SCR_014329 https://www.microscope. healthcare.nikon.com/products/ software/nis-elements/nis- elements-advanced-research | |
| Software, algorithm | Prism 6 | GraphPad | RRID:SCR_002798 https://www.graphpad.com/ scientific-software/prism/ |





