Canonical goal-selective representations are absent from prefrontal cortex in a spatial working memory task requiring behavioral flexibility
Figures
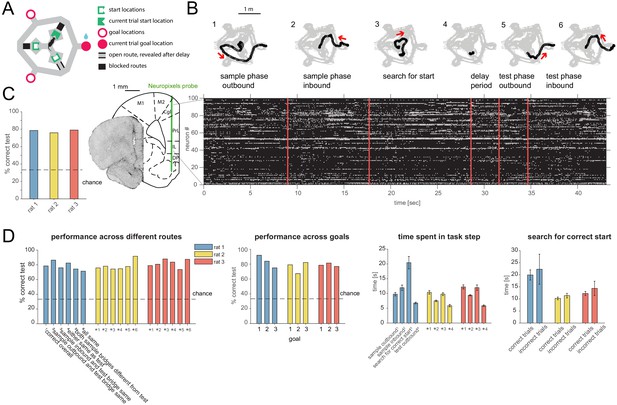
Task design, behavior and recording.
(A) Schematic of ‘multi-start/multi-goal/multi-route’ (MSMGMR) task environment. (B) Top: Each trial consisted of the following steps: (1) the current goal is randomly assigned and cued with lights; a single, randomly assigned route (bridge) is available; rat gets small reward upon arrival at cued goal; (2) animal returns to center via any route; all routes are blocked upon arrival at center; (3) animal searches for randomly assigned start position, indicated by a tone once animal pokes nose into correct port; (4) animal must maintain nose poke for 3 s (3.2 s in one animal); (5) a randomly assigned route becomes available and animal can navigate to goal; and (6) animal returns to center via any route to initiate next trial (see Video 1). Bottom left: Neuropixels probe is chronically implanted in mPFC, and 384 channels spanning multiple subareas are recorded from simultaneously. Bottom right: Spiking activity during task. (C) Task performance (test phase). (D) Left: Task performance in subsets of trials in which different routes were taken. Again, a single outbound route was randomly assigned for both test and sample phases; inbound routes could be freely chosen by the animal. Center left: Performance for each goal location. Center right: Time spent for each of the task steps (see also B) (mean and 95% CI). Right: Search time for the start location for correct and incorrect trials (mean and 95% CI).
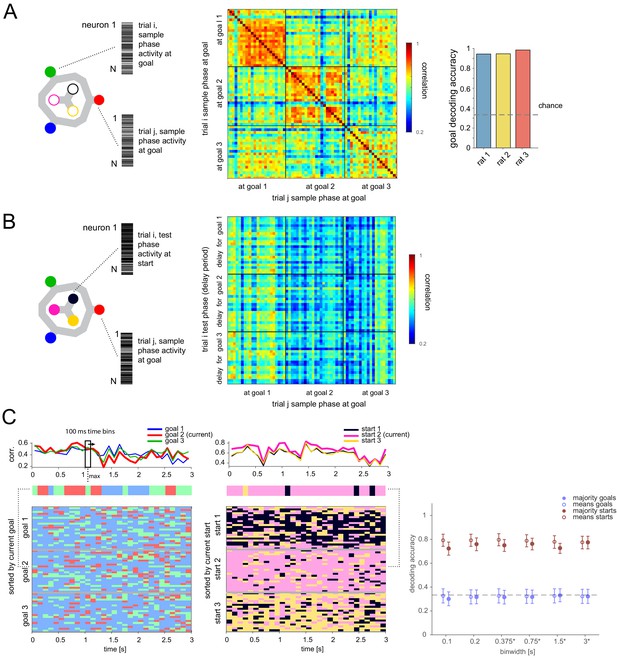
Memory is not maintained by goal-location-specific activity in the delay period.
(A) Population vectors (PVs) of activity while animal at each goal during sample phase (left) are distinct and stable (middle, correlation matrix of single trials in one animal; right, decoding accuracy, logistic regression classifier, mean over all trials and animals: 94.48%, 95% bootstrap CI: [90.87, 96.80]). (B) Correlation of PV while at goal during sample phase and PV during delay period while animal must maintain that goal-specific information. (C) Left top: Example single-trial PV over time during delay period correlated with each goal PV. Left middle: Goal with maximum correlation at each time bin above. Left bottom: Same for all correct trials in this animal, sorted by current goal. Middle: Analogous to left but correlated with each start location PV (excluding contribution from current trial). Right: Correlation-based classification for range of binwidths (mean and 95% CI). Class per trial determined by highest mean correlation over entire delay (unfilled) or majority vote of class with highest correlation at each time point (filled). *Binwidths were 0.4, 0.8, 1.6, and 3.2 s for one animal that had 3.2 (versus 3) s delay (also for Figures 3C, 6B).
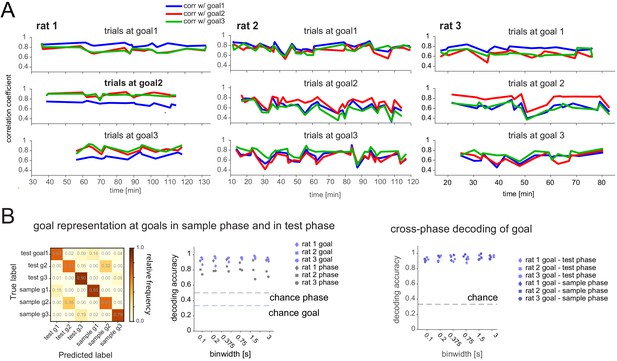
Representations are distinct and stable at goals.
(A) The average firing rate PV at each goal (first 3 s after arrival) over the first five visits in the sample phase was calculated and each was correlated with the single visit PVs of all following trials during the sample phase. The correlation coefficient was generally higher and at a similar level over the duration of recording sessions (90–135 min) for the current goal location. (B) Sample and test goal decoding at goal. Left: SVM decoding confusion matrix for one animal (binwidth: 750 ms); Middle: decoding performance for goal when at goal (regardless of phase, light blue) and trial phase (regardless of which goal, gray) for all animals at a range of binwidths. Note that both the identity of the goal as well as the trial phase in which it is visited can be decoded. Reward amount is smaller in sample than in test phase, which may contribute to the difference between test and sample phase activity at the goal. However, activity exhibits similarities at each goal across sample and test phases; right: Decoding of goal identity as in middle, but here the ability to predict the goal the animal is at is based on a classifier trained on data from the respective other task phase, thus assessing the overlap of goal representation during sample and test phases. Decoding accuracy was just as high as in the task phase-mixed classifier (middle), suggesting no interference between task phase representation and spatial location code.
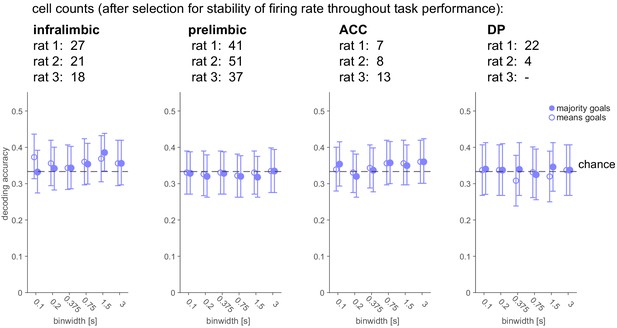
Goal-location-specific representations are not maintained in the delay period for any subarea.
Same analysis as in Figure 2C, right, but for subareas of prefrontal cortex separately: correlation scores for remembered goal location at different temporal binwidths (mean and 95% CI). Remembered goal location could not be decoded in any subarea.
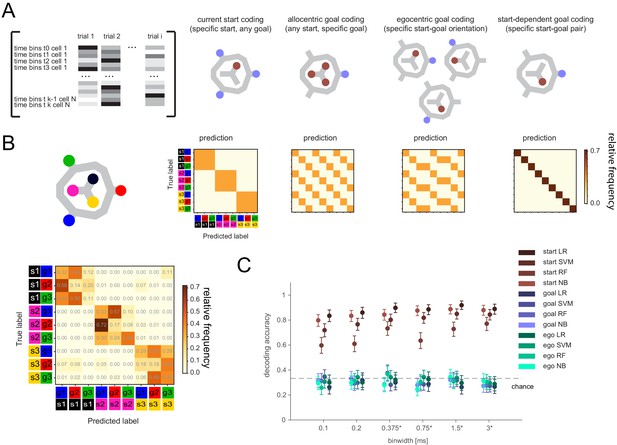
Lack of differential activity patterns corresponding to the current, remembered goal in the delay period.
(A) Leftmost: Differential patterns considered corresponded to activity across cells and time bins during delay period. Potential encoding schemes (left to right): start location represented independently of current, remembered goal; current goal represented independently of current (start) location; current goal represented in egocentric coordinates, that is direction to current goal with respect to current (start) location; current goal represented distinctly in different start locations. (B) Population activity analysis of potential encoding schemes during delay period using supervised classification. Top: Confusion matrices expected for each scheme. Bottom, left: Confusion matrix using support vector machine (SVM) classification (0.75 s bins) for one animal. (C) Three-class delay period activity classification using logistic regression (LR), SVM, random forest (RF), or Naïve Bayes after feature selection (NB) over range of time resolutions (mean and 95% CI).
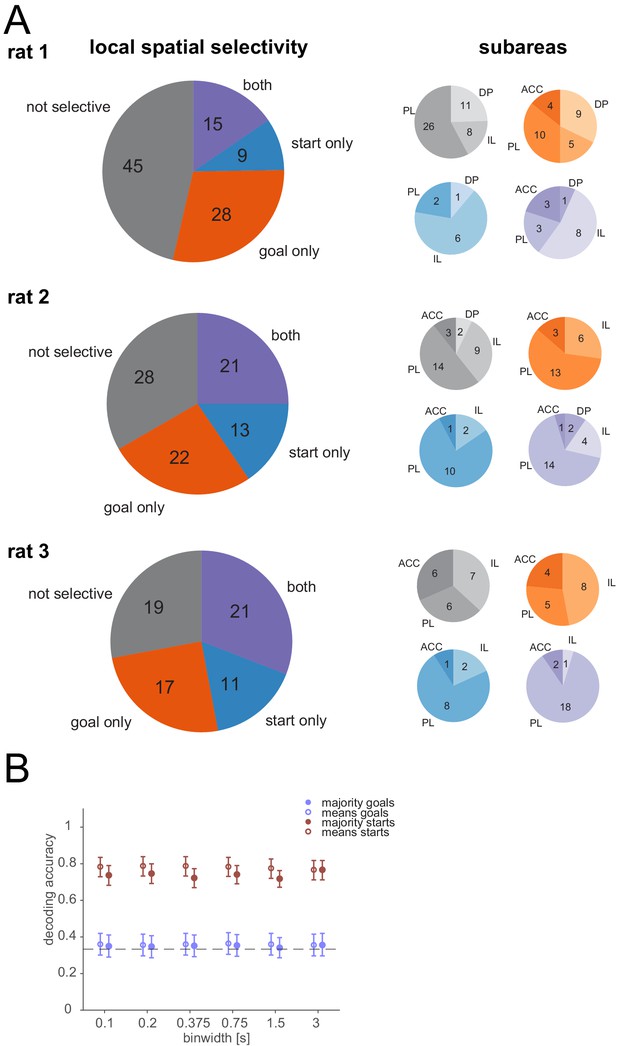
Local spatial selectivity of individual cells at goal and/or start locations.
(A) Left: Fraction of cells that were selective for at least one goal (while there), start, both, or neither for each animal. Right: Distribution of selectivity across subareas. (B) Same as Figure 2C but using only cells that showed a significant modulation at the goals.
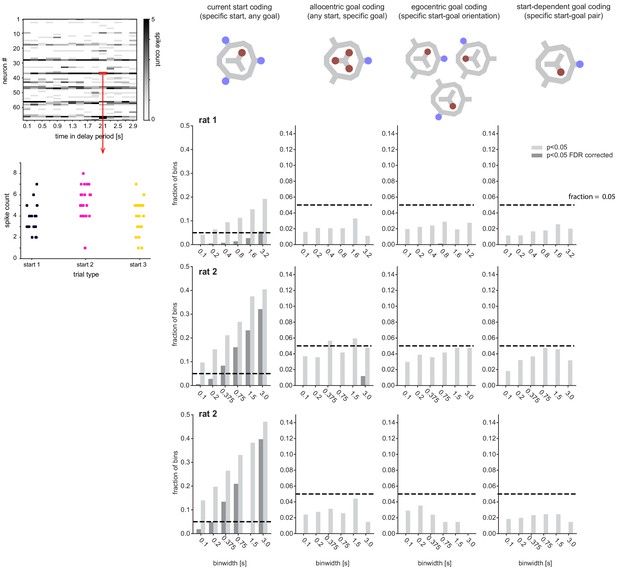
Single-cell firing rate analysis for individual animals.
Left top: Example 200 ms binned activity and test of significant encoding by single cell × bin (bottom). Middle top: List of potential encoding schemes (analogous to Figure 3). Bottom: Corresponding fraction of cell × bins with Kruskal–Wallis p-value<0.05 (light gray, dark gray: false discovery rate corrected). Note that for all animals a fraction of cell × bins encoded the current start position but not the maintained goal location.
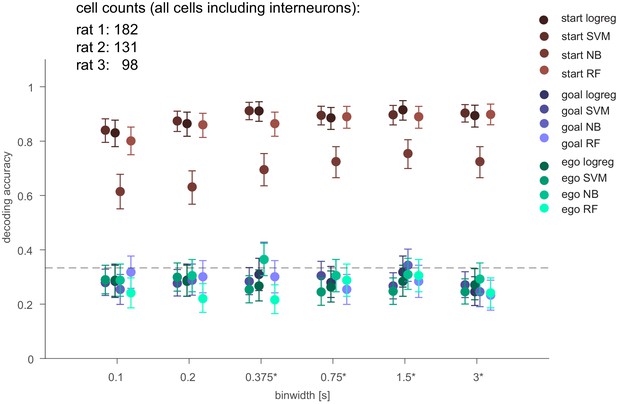
Population decoding for all cells independent of selection criteria.
Summary of three-class classification of delay period activity using different methods using all cells recorded (same as Figure 3C but without stability selection criteria [see Materials and methods] and including interneurons; logistic regression [LR], SVM, random forest [RF], Naïve Bayes after feature selection [NB]). Means across all animals and 95% CIs are shown.
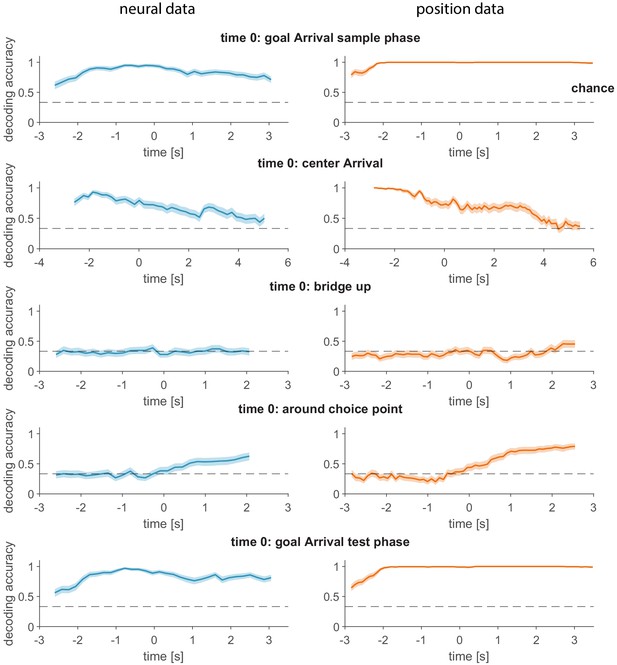
Decoding of current goal during task progression.
Both neural data and position tracking data (location of the two LEDs on the head, giving head direction as well as location) were aligned to the indicated key reference time points. The current goal was decoded (line: mean, shaded region: 95% CI) using a support vector machine in overlapping windows of 800 ms for the neural data (200 ms bins, 200 ms steps, left) and 330 ms for the position data (100 ms steps, right) were used. Note the similar time course of decoding accuracy using neural data and position data. Note the lack of above-chance decoding from −3 to 0 s with respect to the time of ‘bridge up’ (row 3), which corresponds to the nose poke fixation delay period.
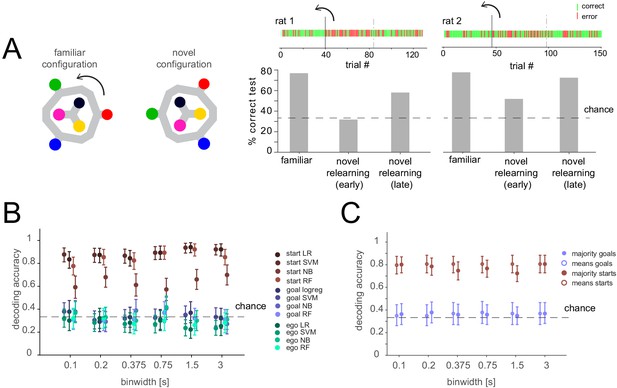
Lack of differential activity patterns corresponding to the current, remembered goal in the delay period during relearning.
(A) Left: Task layout in familiar and novel configuration. The maze was turned by 60 degrees after 40 or 46 trials for the two animals, respectively. Right: Performance before the rotation (familiar), after the rotation (novel relearning – early) and in the last ~1/3 of the session (novel relearning – late). Above, outcomes of single trials are shown (green: correct, red: error, solid line indicates time of rotation, dashed line indicates division of trials in early and late relearning periods). (B) Three-class delay period activity classification using logistic regression (LR), SVM, random forest (RF), or Naïve Bayes after feature selection (NB) over range of time resolutions (mean and 95% CI). Same analysis as Figure 3C, but for relearning trials (all trials after the rotation). (C) Correlation-based classification for range of binwidths (mean and 95% CI). Same analysis as Figure 2C, right for relearning trials.
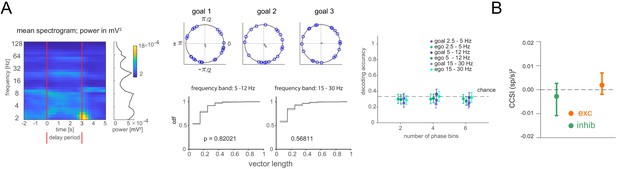
Lack of differential phase or covariance of firing corresponding to the current, remembered goal in the delay period.
(A) Phase analysis. Left: Spectrogram of delay period local field potential (LFP) with average power during the delay period at right (LFP was notch-filtered at 60 Hz, then power was computed within each of 31 logarithmically spaced bins). Middle: Example cell phase preference of delay period spikes and resultant vector length (r, gray) across all trials for each current goal (top). Cumulative distribution of r for all cells from one animal compared to shuffle of trials for two frequency bands (bottom). Right: Decoding accuracy (mean and 95% CI) using spike counts at specific phases. Phases for each frequency band were divided into 2, 4, or 6 phase bins. (B) cross-correlation selectivity index for the delay period (CCSI, after Barbosa et al., 2020) is a measure of the difference in covariance between trials where the current goal is the one where a given pair of neurons preferentially fires at during the sample period and trials where the current goal is either of the other two goals for cell pairs determined to have excitatory or inhibitory interactions (mean and 95% CI, see Materials and methods).
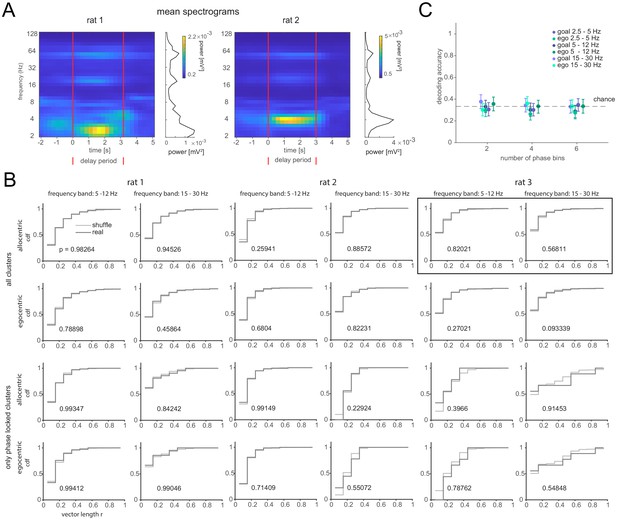
Phase analysis for individual animals.
(A) Same as Figure 5A, left but for rat 1 and rat 2. (B) Cumulative distribution of vector length for real and goal label-shuffled data when using all clusters (two top rows) or clusters that were significantly phase-locked to the indicated frequency. The plots in the black rectangle are shown in Figure 5A and included for completeness here. Number in each plot denotes p-value for the probability that real and shuffled data come from the same distribution (Kruskal–Wallis test). The same analysis was conducted for the frequency band 2.5–5 Hz with similar results. (C) Same as Figure 5A, right but only for cells that are phase locked to at least one of the target classes (allocentric or egocentric goal position).
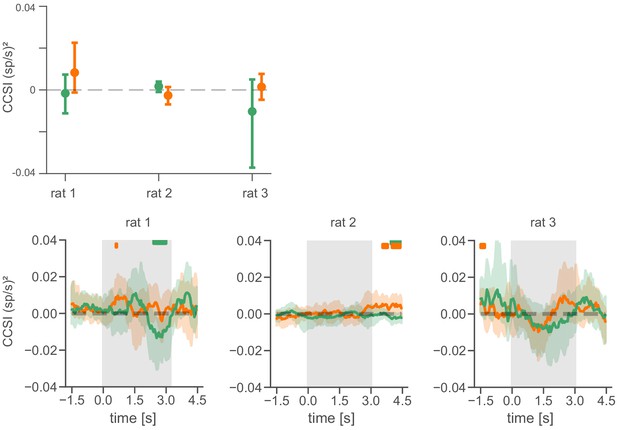
Covariance analysis for individual animals.
Top: CCSI for the full delay period for individual animals (mean and 95% CI). Bottom: Time course of CCSI (in sliding 1 s windows) for individual animals. Gray shaded area depicts the delay period. Green: inhibitory pairs. Orange: excitatory pairs. Plotted are means and 95% CIs. Green and orange horizontal segments represent centers of individual windows where the mean covariance differed for preferred and non-preferred trials; however, the CCSIs for the full delay period above (and pooled across animals in Figure 5B) show that there is no overall significant relationship between excitatory or inhibitory neuron pair covariances and the current goal in the delay period.
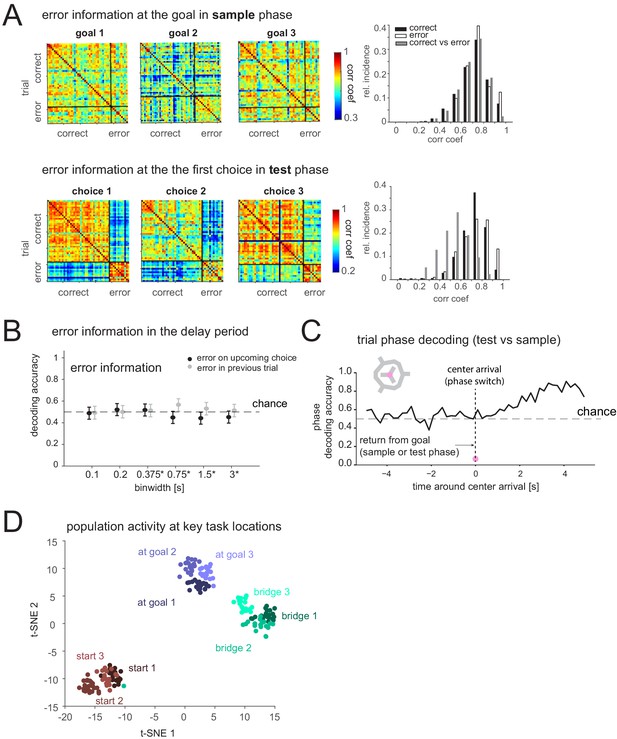
Prefrontal cortex encodes task-relevant information and forms groups of behavioral equivalence.
(A) Top: Correlation matrices between population vectors of activity at each goal in sample phase of trials where the animal is correct or incorrect in the subsequent test phase. Distribution of correlation coefficients from these matrices (right). Bottom: Correlation matrices of population vectors at each first choice goal location in test phase for correct and incorrect choices. Distribution of correlation coefficients (right). (B) Decodability of whether goal error occurred in upcoming or previous test phase based on population activity during delay period (mean and 95% CI). (C) Decodability of task phase from population activity (200 ms bins) while animal is moving inbound from goal to center in sample or test phase (pre-0 s) and after it arrives at center, for one animal. Similar results in another animal (not shown). (D)
t-distributed stochastic neighbor embedding (t-SNE) of population vectors of activity while animal is at key task locations: individual starts, goals, and bridges/routes.
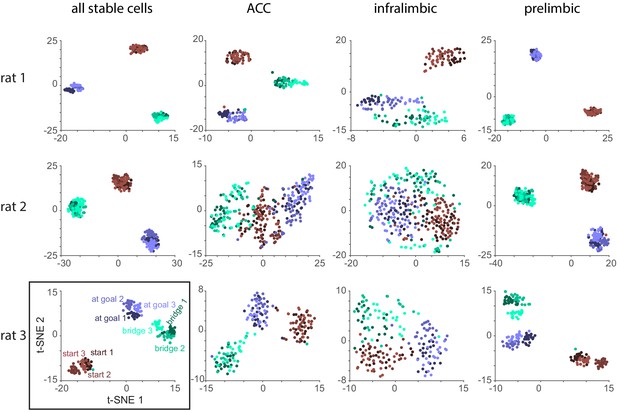
t-SNE analysis for each animal and subarea.
Same as Figure 6C but for cells estimated to belong to anterior cingulate cortex (ACC), prelimbic cortex, and infralimbic cortex separately. Only cells with stable firing rates were considered (see Materials and methods). Numbers of cells for each subarea as in Figure 2—figure supplement 2. Black rectangle: same as shown in Figure 6C.





