Permeabilization-free en bloc immunohistochemistry for correlative microscopy
Figures
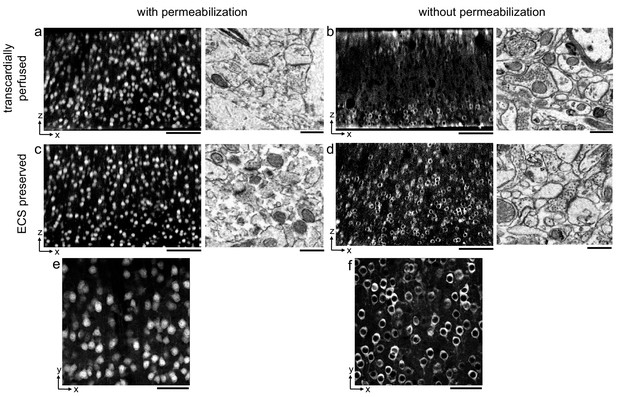
Permeabilization-free labeling of NeuN in extracellular space (ECS)-preserved cerebral cortex.
(a, b) 300-µm-thick sections from the cerebral cortex of a transcardially perfused mouse. Incubation with Alexa Fluor-488 conjugated anti-NeuN was performed with (a) or without (b) 0.3% Triton to label neuronal somata. X–Z reslices of two-photon image volumes (left panels) are 10-µm-average intensity projections from the center of the sections. Sections were then stained for electron microscopy, and a region from the center of the section was examined for ultrastructural integrity (right panels). (c, d) Same procedure as in (a, b) but for 300-µm-thick acute ECS-preserved sections from the cerebral cortex of a mouse. (e, f) 10 µm average intensity projections of X–Y slices from center of the image volumes highlighting nuclear exclusion of anti-NeuN labeling when Triton is omitted (f). Scale bars: (a–d), left panels: 100 µm; (a–d), right panels: 1 µm; (e, f): 50 µm.
-
Figure 1—source data 1
Table of tested primary and secondary antibodies.
Primary and secondary antibodies that successfully labeled protein targets throughout the depth of 300-μm-thick acute extracellular space-preserved brain slices.
- https://cdn.elifesciences.org/articles/63392/elife-63392-fig1-data1-v1.pdf
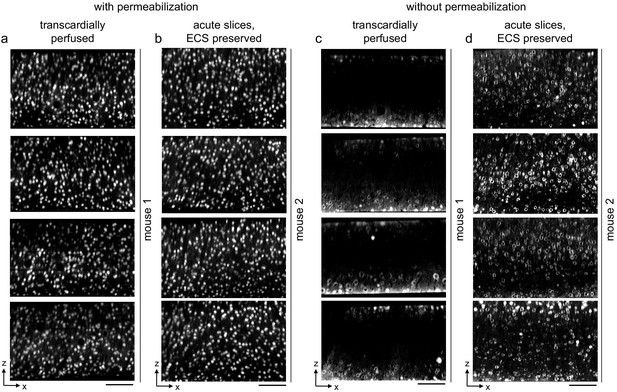
Replication of permeabilization-free labeling of NeuN across multiple tissue sections.
(a, b) 300-μm-thick sections from a transcardially perfused mouse (a, n = 4 sections) and a mouse from which acute extracellular space (ECS)-preserved sections were collected (b, n = 4 sections). Sections were labeled with Alexa Fluor-488 conjugated anti-NeuN with 0.3% Triton. Two-photon (2P) image volumes were acquired. X–Z reslices are 10 µm average intensity projections from the center of the sections. (c, d) 300-μm-thick sections from a transcardially perfused mouse (c, n = 4 sections) and a mouse from which acute ECS-preserved sections were collected (d, n = 4 sections). Sections were labeled with Alexa Fluor-488 conjugated anti-NeuN without permeabilization. 2P images as in panels (a, b). Scale bars: 100 μm.
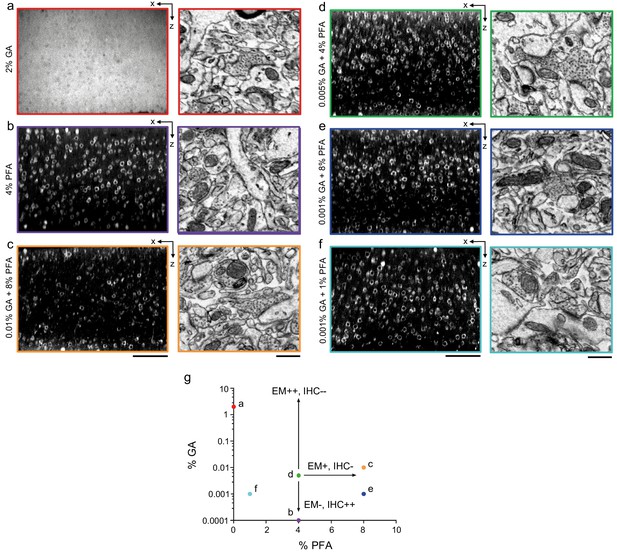
Optimization of fixation parameters.
(a–f) 300-μm-thick acute extracellular space (ECS)-preserved sections from mouse cerebral cortex. Slices were fixed with different concentrations of glutaraldehyde (GA) and paraformaldehyde (PFA) and then incubated with Alexa Fluor-488 conjugated anti-NeuN. Two-photon image volumes were acquired. X–Z reslices (left panels) are 10 µm average intensity projections from the center of the sections. Sections were then fixed with 2% GA, stained for electron microscopy, and a region from the center of the section was examined for ultrastructural integrity (right panels). (g) The points in parameter space that were tested are color-coded to match the panels in (a–f). A combination of 0.005% GA + 4% PFA (d) was selected as an optimal tradeoff between ultrastructural quality and antibody penetration. Scale bars: (a) 100 µm; (b–d) 100 µm (upper panels), 0.5 µm (lower panels).
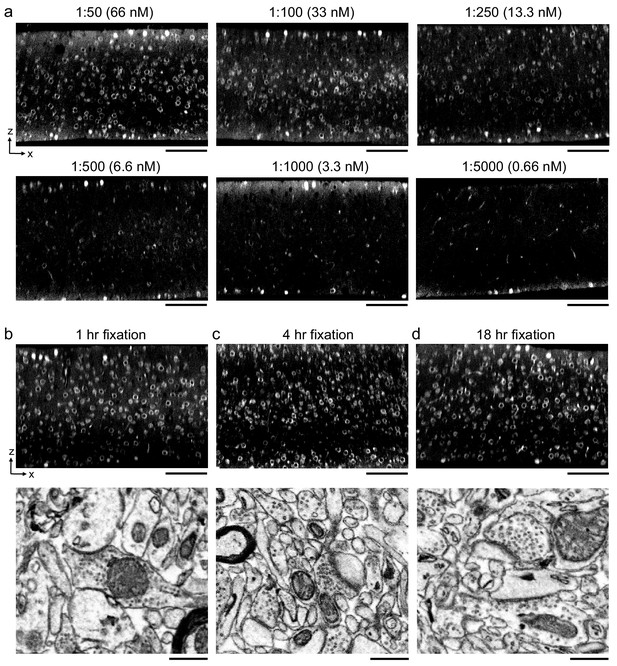
Optimal antibody concentration and duration of primary fixation.
(a) 300-μm-thick acute extracellular space (ECS)-preserved sections from mouse cerebral cortex. Sections were incubated in different concentrations of Alexa Fluor-488 conjugated anti-NeuN as indicated for 72 hr. Two-photon (2P) image volumes were acquired. X–Z reslices are 10 µm average intensity projections from the center of the sections. (b–d) 300-μm-thick acute ECS-preserved sections from mouse cerebral cortex. Sections were fixed for varying durations (1, 4, or 18 hr) with 0.005% glutaraldehyde + 4% paraformaldehyde prior to incubation with Alexa Fluor-488 conjugated anti-NeuN. 2P image volumes were acquired. X–Z reslices (upper panels) are 10 µm average intensity projections from the center of the sections. Sections were then stained for electron microscopy, and a region from the center of the section was examined for ultrastructural integrity (lower panels). Scale bars, upper panels: 100 μm; lower panels: 0.5 μm.
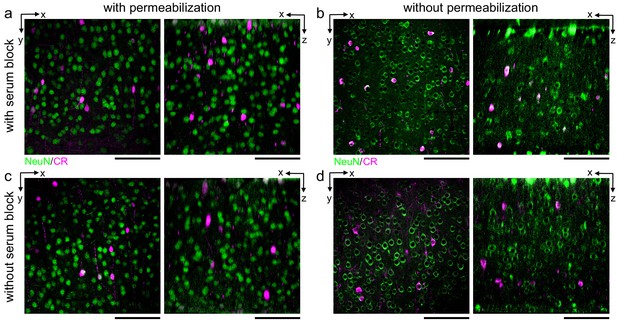
Comparison of the effect of serum blocking on labeling thick sections.
300-μm-thick acute extracellular space-preserved sections from mouse cerebral cortex. Sections were incubated with Alexa Fluor-488 conjugated anti-NeuN and anti-calretinin (CR) either with (a, b) or without (c, d) 3% donkey serum and with (a, c) or without (b, d) permeabilization with 0.3% Triton. Two-photon image volumes were acquired. X–Y (left panels) and X–Z reslices (right panels) are 10 µm average intensity projections from the center of the sections. Scale bars: 100 μm.
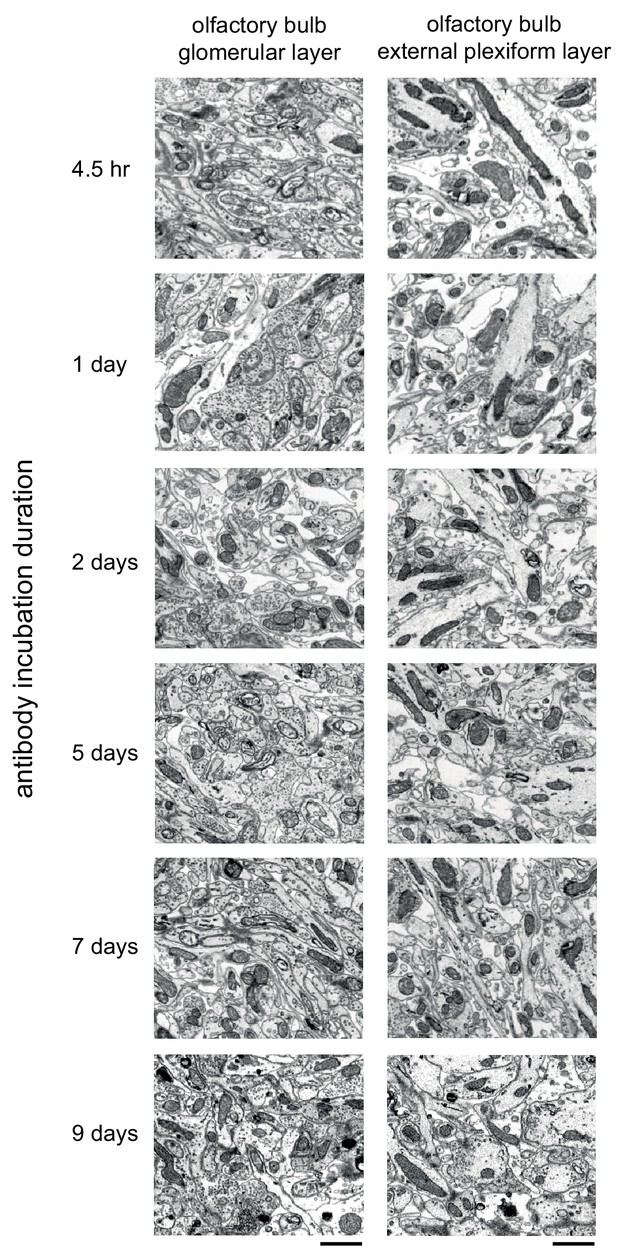
Comparison of the effect of prolonged antibody incubation on ultrastructure quality.
300-μm-thick acute extracellular space-preserved sections from mouse olfactory bulb. Sections were incubated with Alexa Fluor-488 conjugated anti-NeuN for varying durations (4.5 hr to 9 days). Sections were then stained for electron microscopy, and a region from the center of the section was examined for ultrastructural integrity in the glomerular layer (left panels) and external plexiform layer (right panels). Scale bars: 1 μm.
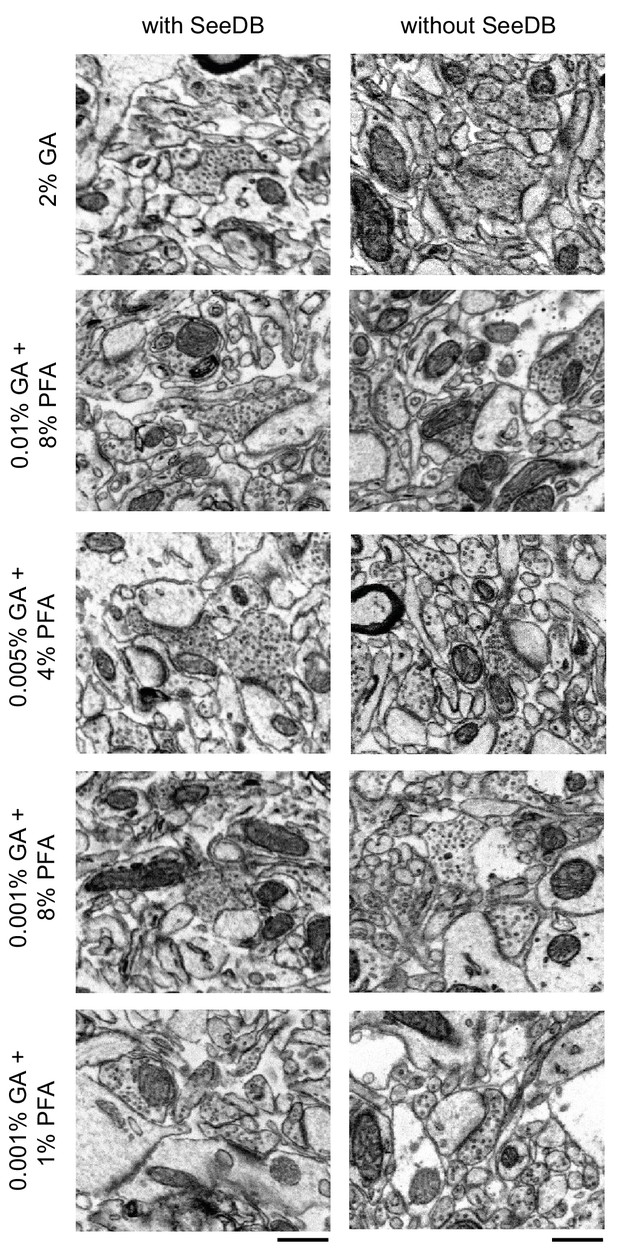
Comparison of the effect of SeeDB clearing on ultrastructure quality.
300-μm-thick acute extracellular space-preserved slices from mouse cerebral cortex. Slices were fixed with different concentrations of glutaraldehyde (GA) and paraformaldehyde (PFA) as indicated and were then incubated with Alexa Fluor-488 conjugated anti-NeuN. Half of the slices then underwent the SeeDB procedure (see Materials and methods), and the others remained in buffer. The SeeDB slices were then rehydrated to buffer, and then all sections were refixed in 2% GA and stained for electron microscopy. Scale bars: 0.5 μm.
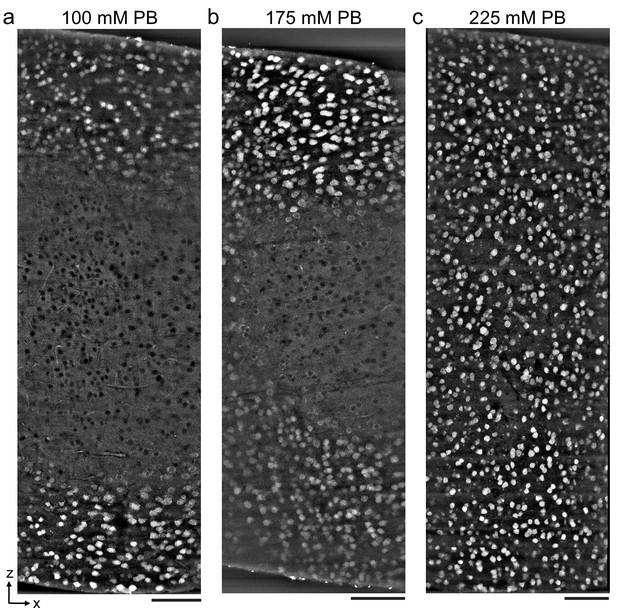
Antibody depth penetration increases with fixation osmolarity.
Approximately 2 × 1 × 1-mm-thick acute extracellular space (ECS)-preserved sections from the mouse cerebral cortex, fixed with increasing buffer concentrations to yield (a) low (215 mOsm), (b) medium (360 mOsm), or (c) high (480 mOsm) osmolarities corresponding to increasing ECS volume fractions. Incubation with Alexa Fluor-488 conjugated anti-NeuN was performed without Triton to label neuronal somata. Tissue sections were hemisected and the cut surface was two-photon imaged to investigate the penetration depth. Images are 10 µm maximum intensity projections from the cut surface. Scale bars: 100 μm.
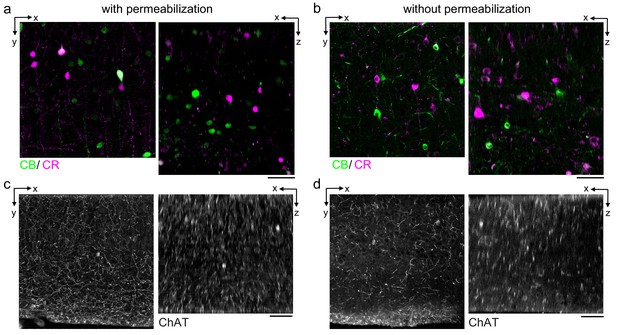
Permeabilization-free labeling of cell types and axons.
(a, b) 300-µm-thick acute extracellular space (ECS)-preserved sections from the mouse cerebral cortex. Simultaneous incubation with anti-calbindin (CB) and anti-calretinin (CR) was performed with (a) and without (b) 0.3% Triton to label interneuron somata. X–Y slices (left panels) and X–Z reslices (right panels) of two-photon image volumes are 10 µm average intensity projections from the center of the sections. (c, d) Same as in (a, b) but 300-µm-thick acute ECS-preserved sections from the mouse medial prefrontal cortex were labeled with anti-choline acetyltransferase (ChAT) with (c) and without (d) 0.3% Triton to label cholinergic axons. Scale bars: 50 µm.
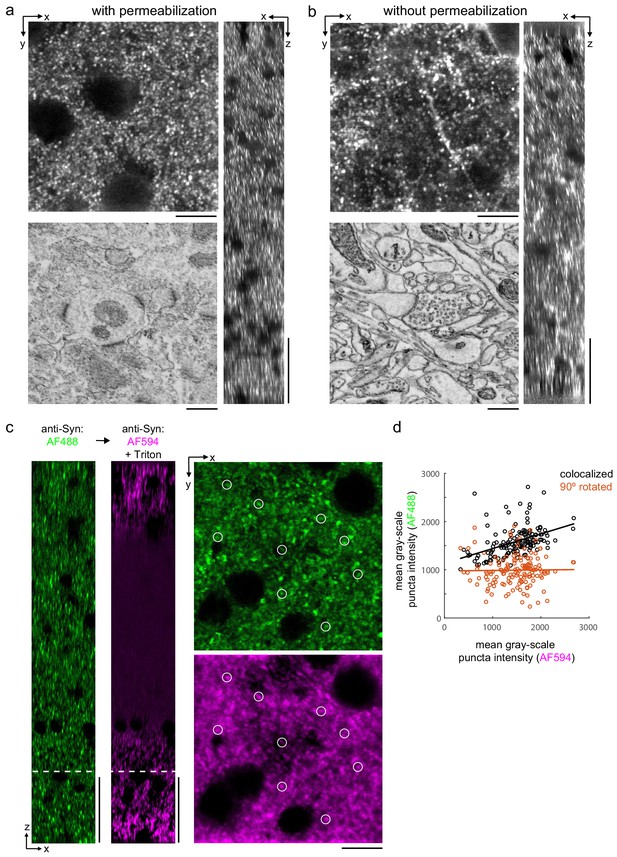
Permeabilization-free labeling of synaptic proteins.
(a, b) 300-µm-thick acute extracellular space (ECS)-preserved sections from the mouse cerebral cortex. Incubation with anti-Homer was performed with (a) and without (b) 0.3% Triton. X–Y slices (upper-left panels) and X–Z reslices (right panels) of two-photon image volumes are taken from the center of the sections. Sections were then stained for electron microscopy, and a region from the center of the section was examined for ultrastructural integrity (lower-left panels). (c) 300-µm-thick acute ECS-preserved slices from the mouse cerebral cortex. Sequential incubation with Alexa Fluor-488 (AF488) conjugated anti-synaptophysin (anti-Syn) without Triton (left, green) followed by Alexa Fluor-594 (AF594) conjugated anti-Syn with 0.3% Triton (left, magenta) shown as X–Z reslices. Puncta were outlined in X–Y images at the depth indicated (left, white dashed line) in the AF594 channel (lower-right panel, representative white circles) for co-localization analysis with the AF488 channel (upper-right panel). (d) Co-localization analysis of 150 puncta from image stacks in (c) comparing co-localized puncta (black) to a 90° rotation of the AF488 channel (orange). Scale bars: (a, b) (upper-left panels: 10 µm; right panels: 50 µm; lower-left panels: 0.5 µm), (c) (left panels: 50 µm; right panels: 10 µm).
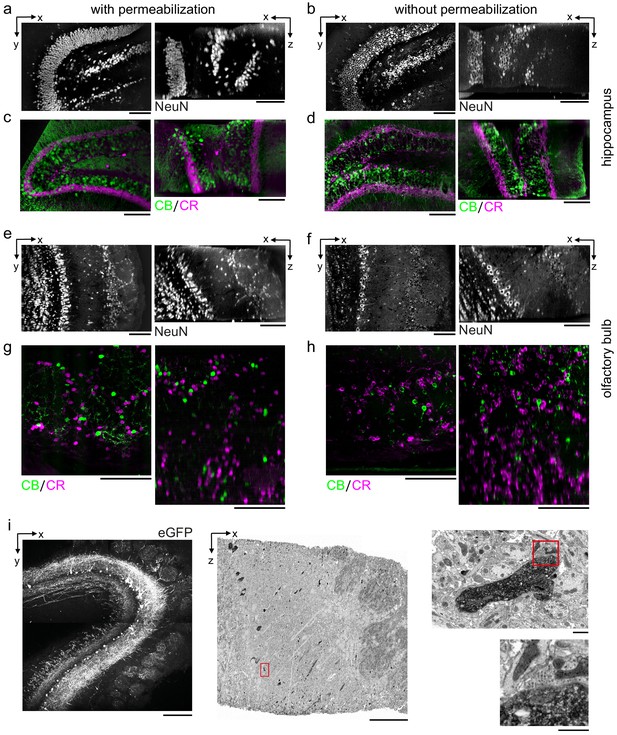
Permeabilization-free labeling of diverse brain regions and genetically expressed proteins.
(a–d) 300-µm-thick acute extracellular space (ECS)-preserved sections from the mouse hippocampus. Incubation with Alexa Fluor-488 conjugated anti-NeuN (a, b) or simultaneous incubation with anti-calbindin (CB) and anti-calretinin (CR) (c, d) was performed with (a, c) and without (b, d) 0.3% Triton to label interneuron somata. X–Y slices (left panels) and X–Z reslices (right panels) of two-photon (2P) image volumes are 10 µm average intensity projections from the center of the sections. (e–h) 300-µm-thick acute ECS-preserved sections from the mouse olfactory bulb. Incubation with Alexa Fluor-488 conjugated anti-NeuN (e, f) or simultaneous incubation with anti-CB and anti-CR (g, h) was performed with (e, g) and without (f, h) 0.3% Triton to label neuronal somata. 2P image slices as in (a–d). (i) Horseradish peroxidase (HRP) labeling of eGFP expressing mitral cells (MCs) in the mouse olfactory bulb. 300-µm-thick acute ECS-preserved sections containing MCs expressing eGFP following adeno-associated virus transfection (left panel). Incubation with anti-GFP and HRP-conjugated secondary antibody was performed without Triton, followed by polymerization of diaminobenzidine (DAB) and electron microscopy staining (middle panel). Enlarged regions (red rectangles) demonstrate confinement of the DAB product to MC dendrites (right, upper panel) and a presynaptic terminal formed on a labeled dendrite (right, lower panel). Scale bars: (a–h) 100 µm; (i) 200 µm (left), 50 µm (middle), 1 µm (right).
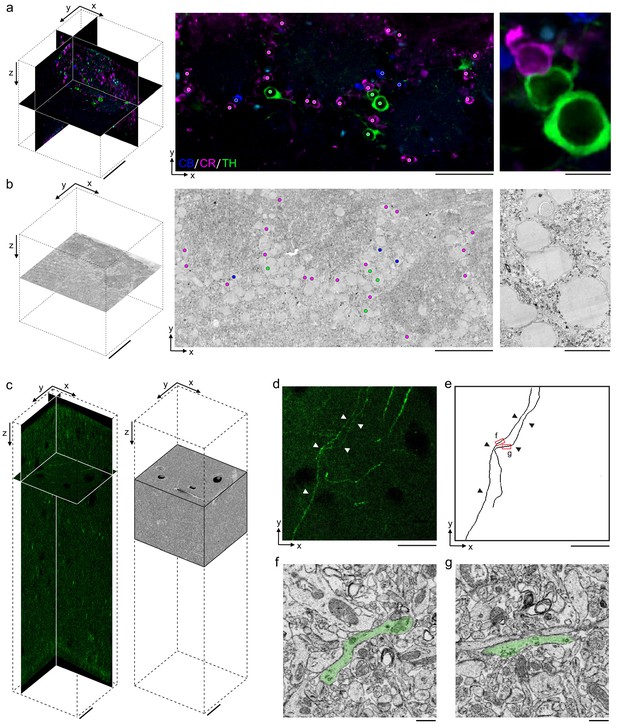
Permeabilization-free labeling for correlative microscopy.
(a, b) Correlative microscopy of calbindin-positive (CB+), calretinin-positive (CR+), and tyrosine hydroxylase-positive (TH+) interneurons in the mouse olfactory bulb. (a) 300-µm-thick acute extracellular space (ECS)-preserved section (left panel) incubated with anti-CB (blue), anti-CR (magenta), and anti-TH (green) without Triton. An X–Y slice (middle panel) from the two-photon (2P) image volume located ~150 µm deep in the section. Colored dots indicate the positive labeling by the respective antibodies. Enlarged region illustrates three somata (right panel). (b) 35 nm electron microscopy (EM) slice cut from ~150 µm deep in the section from panel (a) (left panel). Colored dots indicate correspondence between soma locations in the EM section and the 2P section (middle panel). Enlarged region illustrates three somata (right panel). (c–g) Correlative microscopy of TH+ axons in the mouse medial prefrontal cortex. (c) 2P image stack from a 300-µm-thick ECS-preserved acute section that was incubated with anti-TH without Triton to label dopaminergic axons (left panel). A serial block-face scanning electron microscopy (SBEM) volume centered on branded fiducial marks spans a depth of 50–125 µm in the 2P image stack (right panel). (d) A 10-µm-thick X–Y maximum intensity projection from panel (c) with TH+ axons indicated (white arrowheads). (e) Anatomical reconstructions of corresponding TH+ axons from the SBEM volume with matching axons indicated (black arrowheads). (f, g) Example EM images of the reconstructed axons from the regions indicated in panel (e). Axons are false-colored (green). Scale bars: (a, b) 100 µm (left), 50 µm (middle), 10 µm (right); (c–e) 20 µm; (f, g) 1 µm.
Additional files
-
Supplementary file 1
Optimized permeabilization-free immunohistochemical (IHC) protocol.
Incubation solutions, durations, and temperatures are reported along with reference to relevant supplementary figures. The duration of antibody incubation was varied dependent on section thickness. Asterisk (*) indicates sodium phosphate buffer (PB) concentration should be varied to achieve the desired extracellular space (ECS) volume fraction in a tissue-dependent manner. ACSF: artificial cerebral spinal fluid; PFA: paraformaldehyde; GA: glutaraldehyde; Ab: antibody; CB: sodium cacodylate buffer; NIRB: near-infrared branding; RT: room temperature; o/n: overnight.
- https://cdn.elifesciences.org/articles/63392/elife-63392-supp1-v1.pdf
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/63392/elife-63392-transrepform-v1.docx





