Group phenotypic composition in cancer
Figures
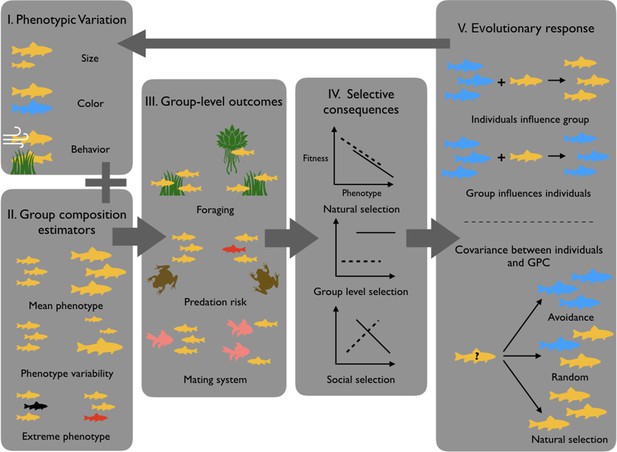
GPC and its ecological/evolutionary implications.
Because individuals show significant phenotypic variation (I), groups may vary in their GPC depending on which individuals constitute the group (II). This may in return influence group-level outcomes (III), which as a consequence differentially impacts individual fitness (IV). (V) GPC then drives different evolutionary responses, like for instance decisions to join or leave particular groups or phenotypic plasticity in response to GPC. These phenomena can then influence the distribution of phenotypes in subsequent generations (figure modified from Farine et al., 2015).
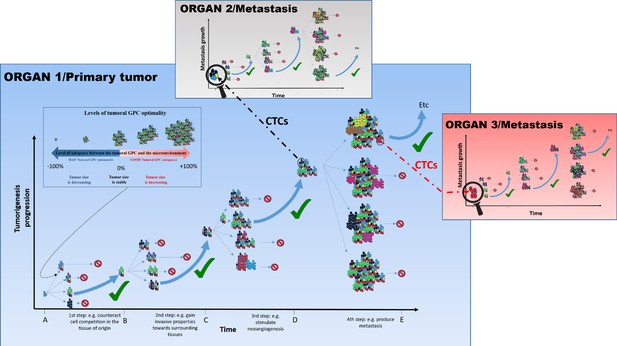
The tumoral GPC framework.
Cancer cell proliferation and mutation in a tumor can produce different possibilities of tumoral GPCs, depending on the relative fitness of cancer cells at a given time (different colored cells represent distinct evolutionary lineages). Depending on the resulting tumoral GPC, the tumor, viewed as the habitat in which malignant cells live and evolve, possesses specific group properties (e.g., quality of the vascular network, level of immunogenicity, etc.). These properties can, in return, affect (positively or negatively) cell fitness, and hence tumor growth. In the absence of selection at the group level, or of an encoded tumorigenesis program, it is potentially frequent that conditions that increase cell-level fitness of one clonal lineage can result in a non-optimal or even detrimental tumoral GPCs, which can slow down or stop tumor growth, and/or even induce its size reduction. Since tumors of different sizes have different requirements and interactions with their changing microenvironment, tumoral GPC varies with the tumor stages and the microenvironment; that is, there is no single optimal tumoral GPC that is maintained throughout cancer progression. Only tumors that achieve a successful/adequate tumoral GPC at each step of tumorigenesis will evolve into metastatic tumors. The tumors that fail to generate an adequate tumoral GPC at a given step do not necessarily disappear, they just do not continue to expand. Those that produce an inadequate tumoral GPC, for instance leading to higher immunogenicity, may become reduced in size and even disappear. This hypothesis can explain why we can develop many neoplasms in the body, but the majority of them never grow until the metastatic stage or even regress (Folkman and Kalluri, 2004). Circulating tumor cells (CTCs), especially clusters that can be either homogeneous (organ 3) or heterogeneous (organ 2), can disseminate and initiate metastasis where a novel process of diversification is required so as to harbor the right GPC in a given organ and develop into advanced metastasis.





