Micron-scale geometrical features of microtubules as regulators of microtubule organization
Figures
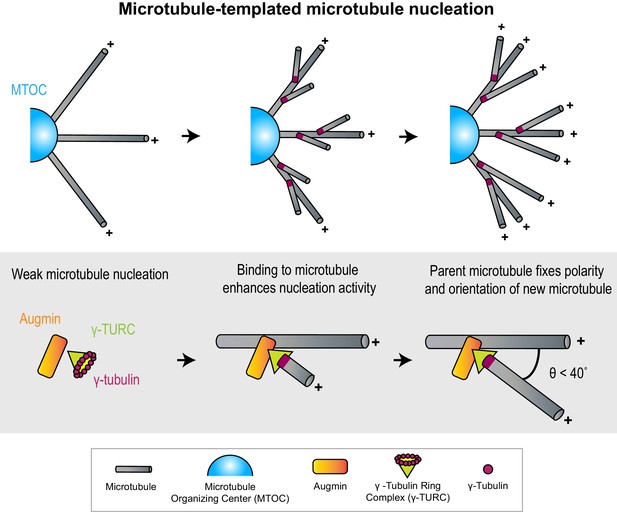
Microtubules act as micron-scale platforms for the generation of new microtubules in an array.
Top: Branching microtubule nucleation increases the density of microtubules growing out from a microtubule organizing center (blue hemisphere), while preserving array polarity. Bottom: The nucleation activity of γ-tubulin ring complex (green cone) and augmin (orange rectangle) is enhanced by their recruitment to a microtubule lattice. The conformation of the ternary complex specifies the orientation (θ) and polarity (+) of the new microtubule with respect to the parent.
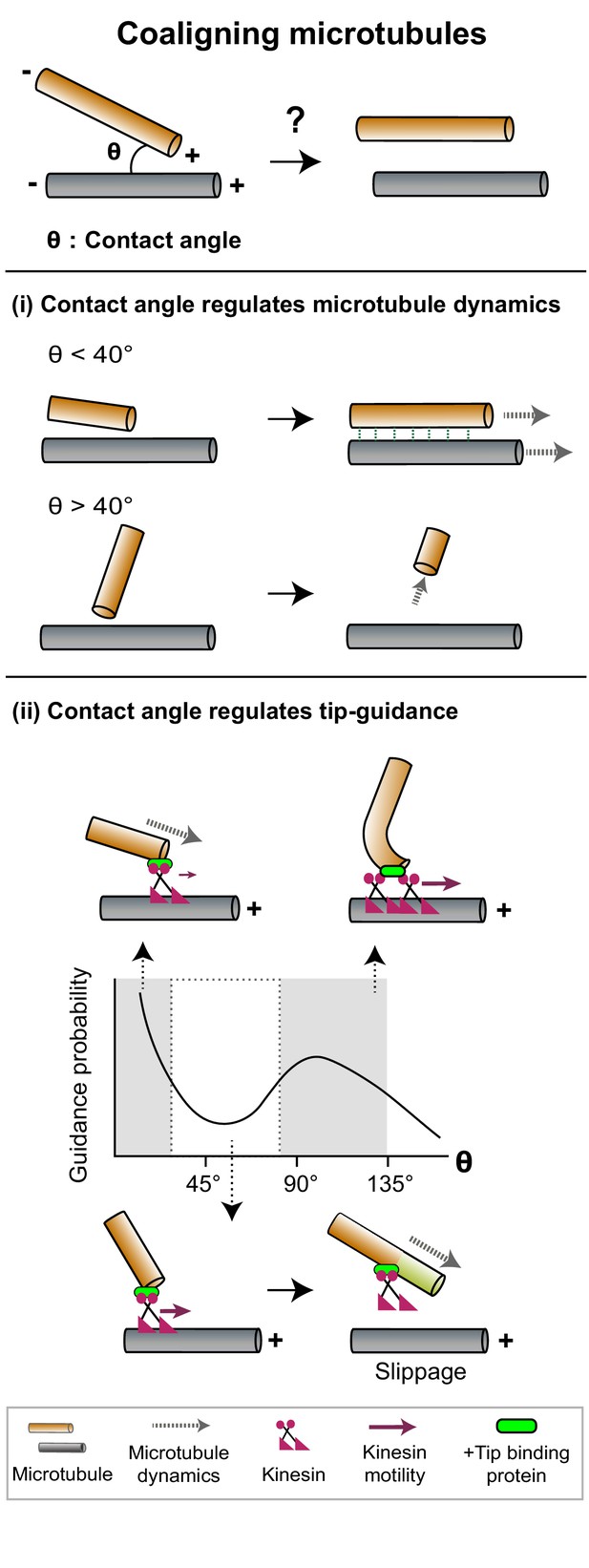
The contact angle between two microtubules regulates mechanisms that promote filament coalignment.
Top: The contact angle (θ), defined as the angle between lines joining the point of contact between two microtubules and their respective minus ends, is an important geometrical feature that regulates microtubule coalignment. (i) A microtubule tip that encounters a lattice at a shallow angle (θ < 40°) gets cross-linked with the lattice and continues growing (top), while a microtubule that makes a steep angle (θ > 40°) undergoes collision-induced catastrophe (bottom). (ii) The probability of occurrence of microtubule tip-guiding events is determined by the contact angle (θ). Top: Tip guiding is mediated by motile kinesins (magenta) and +tip binding proteins (green). Guiding occurs through polymer bending at high contact angles (θ > 85°). Bottom: At intermediate angles (40° < θ < 85°), the need for continuous MAP occupancy of the newly polymerized tip (light green lattice) causes slippage events which decrease guidance probability.
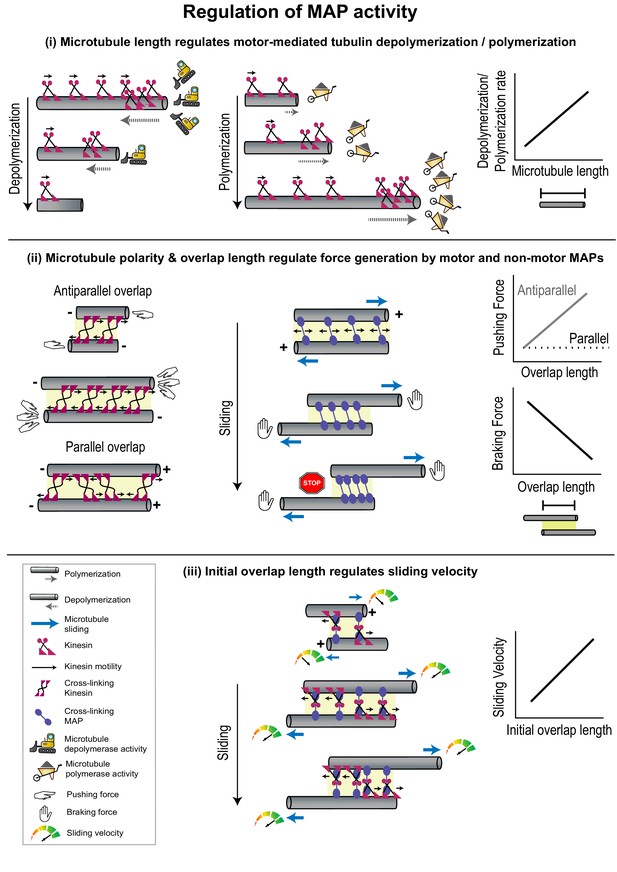
Geometrical features of individual microtubules or polymer networks regulate MAP activity.
(i) The rate of microtubule depolymerization (left) and polymerization (middle) mediated by kinesin motors (magenta) is regulated by microtubule length (right) which determines the number of motors that accumulate at the plus-end. The change in length during depolymerization and polymerization provides feedback for further motor activity. (ii) Pushing forces (left) produced by motors (magenta) and braking forces (middle) produced by non-motor MAPs (violet) are regulated by the relative polarity (+/-) and overlap length (yellow shaded box) of cross-linked microtubules (right). Forces generated by individual cross-linking motors in an anti-parallel overlap are integrated to produce the net pushing force (left). Entropic expansion forces generated by non-motor MAPs increase when the overlap shrinks and MAP density in the overlap increases, to resist motor-mediated sliding (middle). (iii) The initial sliding velocity (red to green speedometer) in a system consisting of microtubules loosely coupled to each other by a motor protein (magenta) bound to a microtubule cross-linker (violet) is determined by initial overlap length and does not change as the overlap shrinks and cross-linker density increases.
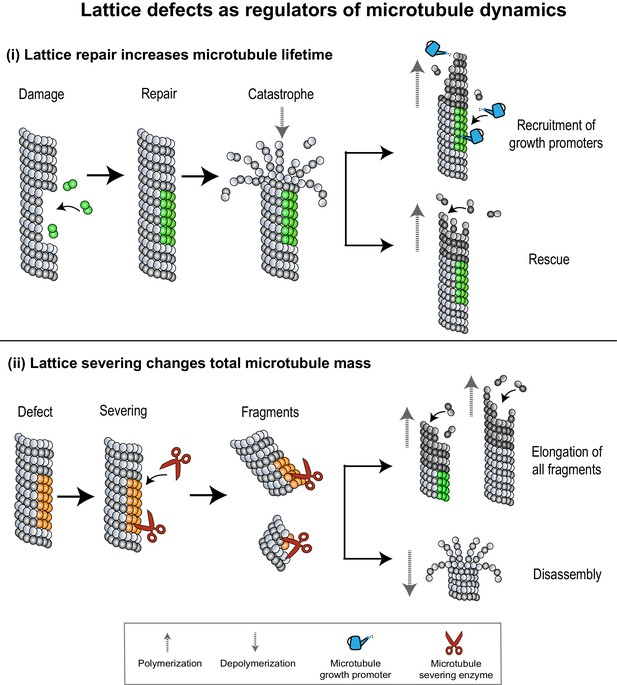
Lattice defects can help recruit MAPs that regulate microtubule dynamics.
(i) Sites of lattice damage can be repaired through the incorporation of GTP-tubulin (green spheres) from solution. These GTP-islands on the lattice help increase microtubule lengths and lifetimes by recruiting microtubule growth promoters (blue watering can) or by serving as points of rescue during subsequent catastrophe events. (ii) Lattice defects (orange spheres) can recruit microtubule severing enzymes (red scissors) to change microtubule density in an array. Severed fragments can either increase total microtubule mass by serving as templates for further polymerization, or be disassembled.
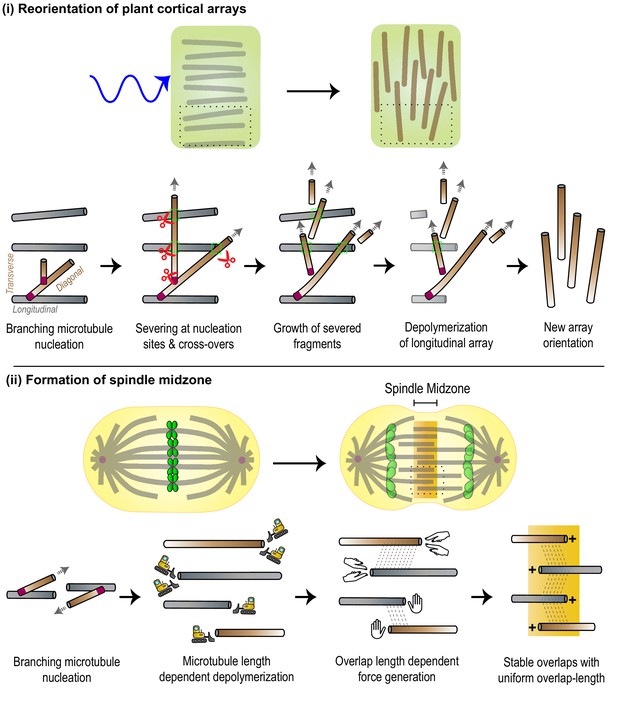
The integration of various microtubule-regulated mechanisms underlies the self-organization and remodeling of cellular arrays.
(i) Top: Plant cortical arrays are reoriented by ~90° in response to blue light. Bottom: Zoomed-in view of region enclosed by black dashed box in top panel. Pre-existing microtubules (gray) of the longitudinal array generate new diagonal and transverse microtubules (brown) through successive branching microtubule nucleation events (magenta). New microtubules are amplified through severing (red scissors) at sites of nucleation and cross-overs (green dashed box), and polymerization of severed fragments. The disassembly of the original array completes the re-orientation process. (ii) Top: The spindle midzone, a cross-linked array of inter-digitating, anti-parallel microtubules is formed during anaphase to specify the site of cell cleavage and ensure error-free genome (green) propagation. Bottom: Zoomed-in view of region enclosed by black dashed box in top panel. Pre-existing microtubules (gray) serve as platforms to generate new parallel microtubules (brown) through branching microtubule nucleation (magenta). The location of the array at the center of the cell and its dimensions are specified by length-dependent microtubule depolymerization and generation of overlap length-dependent sliding and braking forces to produce stable overlaps.
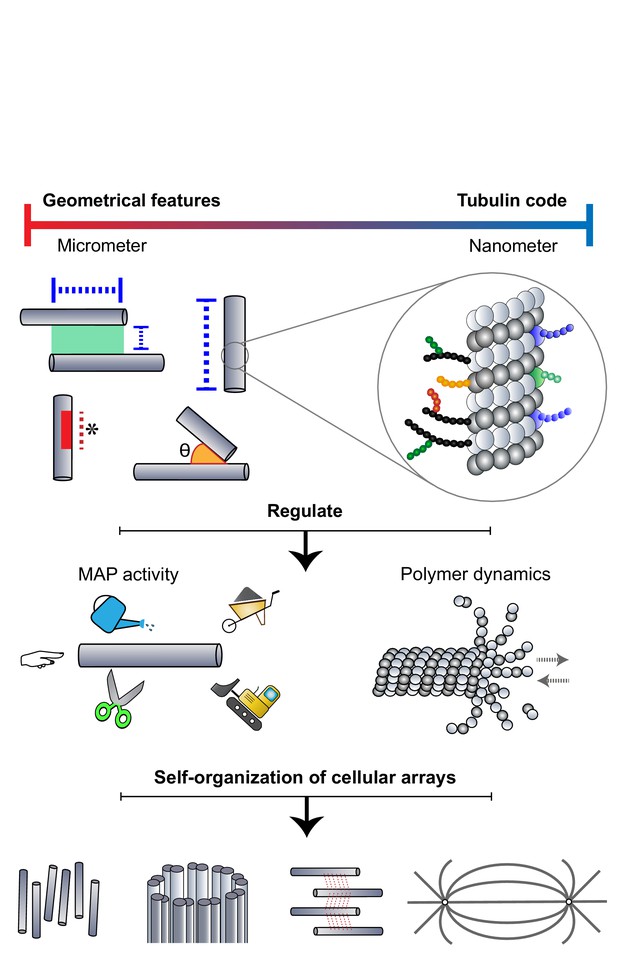
Microtubules adopt a DIY (Do It Yourself) approach toward array building.
The microtubule lattice contains features at two length scales: micron-sized geometrical parameters encompassing the arrangement of polymers and structural defects of the lattice, and nanometer-scale "tubulin codes" comprising post-translational modifications and isotypes of tubulin. Both sets of features regulate MAP activity and polymer dynamics in order to direct the self-organization of microtubules into diverse cellular arrays.





