Coronary plaque composition influences biomechanical stress and predicts plaque rupture in a morpho-mechanic OCT analysis
Figures
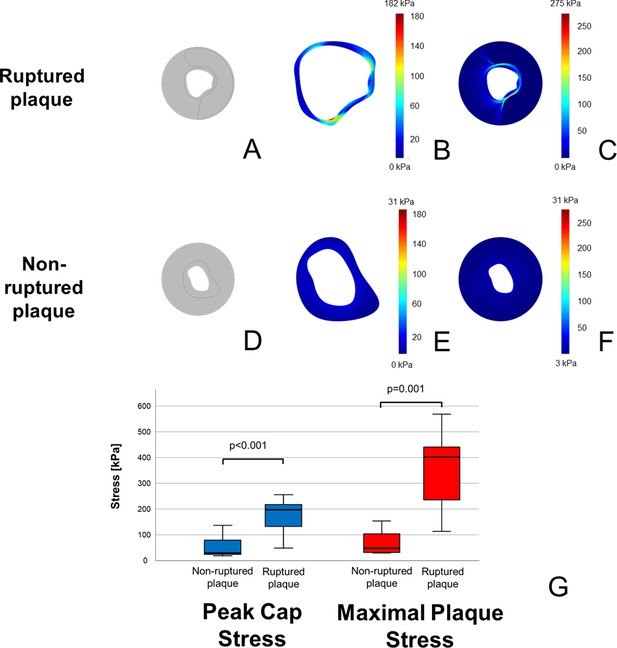
Higher stress concentrations in ruptured plaques than in non-ruptured coronary plaques.
In (A, D), model reconstructions for ruptured and non-ruptured plaques are shown. In both fibrous cap (B) vs. (E) and vessel wall (C) vs. (F), ruptured plaques present higher stress concentrations than non-ruptured ones. Color-code scale is the same for both reconstructions, and maximal stress is shown at the upper edge of the scale. In (G) is shown a box-plot demonstrating the different stress concentrations both on the fibrous cap (in blue) and in the whole plaque (in red).
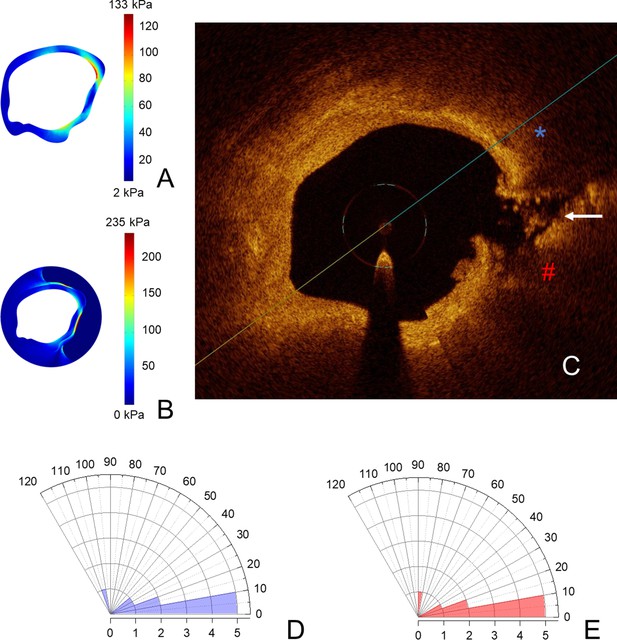
Coronary plaques rupture in the proximity of stress concentrations.
In (A, B), stress concentrations on the fibrous cap and on the vessel wall in the same coronary segment; in (C), the points of maximal stress concentration on the fibrous cap (blue asterisk) and on the vessel wall (red hash) are reported, in the OCT-frame immediately following the one used for reconstruction shown. Here, the rupture is marked with a white arrow. In (D, E), polar histograms showing distribution of angles between rupture and maximal stress on fibrous cap (D) and on the vessel wall (E). Angles higher than 120° have not been detected and for this reason are not shown in the polar graphs.
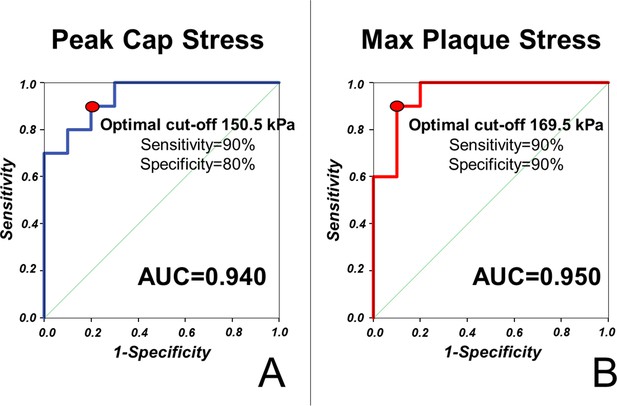
Stress concentration predicts plaque rupture with excellent diagnostic efficiency.
ROC curves for the prediction of plaque rupture are shown for maximal stress in the fibrous cap (A) and in the vessel wall (B) of simulated vessels.
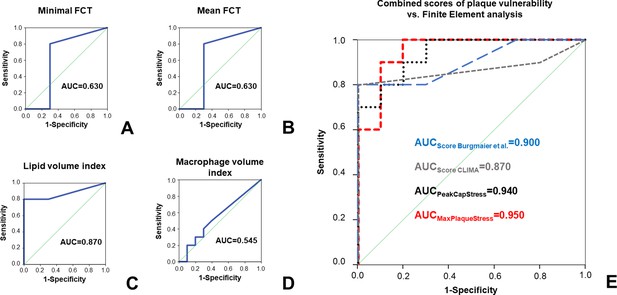
Comparison of stress concentration to ‘classical’ features of plaque vulnerability in prediction of plaque rupture.
ROC curves for the prediction of plaque rupture for minimal FCT (A), mean FCT (B), lipid volume index (C), and macrophage volume index (D). In (E), ROC curves for two established OCT scores combining different features of plaque vulnerability are depicted (respectively: scores calculated according to Burgmaier et al., 2014 in blue and according to the CLIMA study (Prati et al., 2020 in grey) and compared to the ROC curves for peak cap stress (PCS, black dotted line) or maximal plaque stress (MPS, red dashed line).
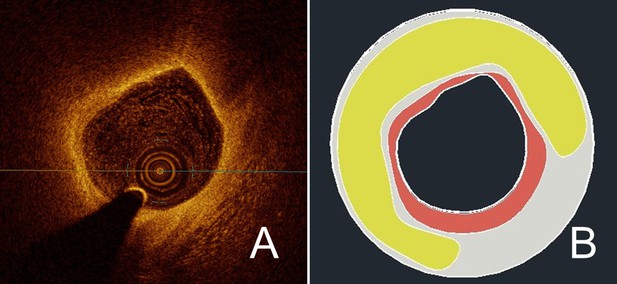
Exemplary reconstruction of a coronary plaque.
Using manual segmentation, a reconstruction of a vessel segment was performed based on OCT images. In (A), the source image from the OCT pullback shows a vulnerable plaque with extensive lipid core and thin fibrous cap. In (B), the result after manual segmentation of the plaque components is shown; here, fibrous cap is in red, lipid core in yellow, and the rest of the vessel wall in light grey.
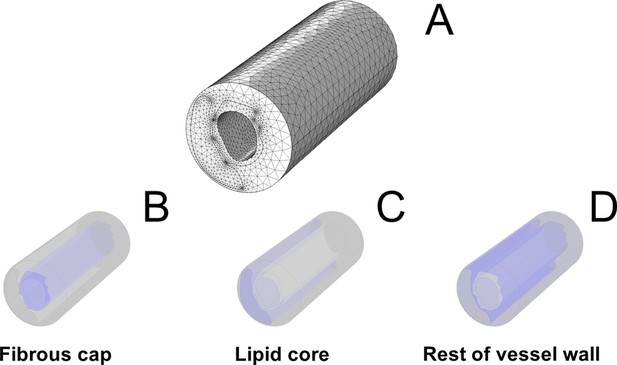
Graphical representation of the finite-element model.
In (A), representation of the generated mesh. In (B-D), single components of the plaque structure are highlighted in blue.
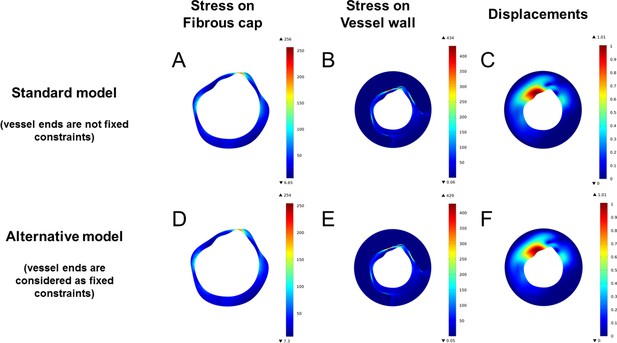
Effects of constraints on the terminal surfaces on stress distribution and displacement.
In (A–C), analysis of stress on the fibrous cap, of stress on the vessel wall, and of displacement in the developed model. In (D–F), the same analyses are repeated after applying constraints on both ends of the simulated vessel segments. As results are almost identical, the model without constraint has been employed throughout the study.
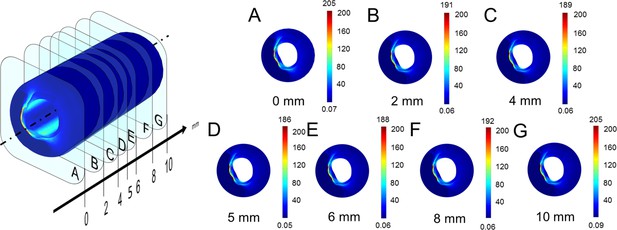
Stress analysis throughout the simulated vessel segment.
Stress distribution on the vessel wall is shown in 2 mm interval throughout the simulated vessel segment (which has a length of 10 mm), as shown in (A–G); legend is shown on the three-dimensional vessel reconstruction on the left side. As results show minimal differences, the 5 mm cross-section (D) has been used for further analysis.
Tables
Patients and lesions characteristics.
Abbreviations: BMI = body mass index, FCT = fibrous cap thickness, TCFA = thin-capped fibroatheroma.
| Non-ruptured | Ruptured | p | |
|---|---|---|---|
| n = 10 | n = 10 | ||
| Clinical characteristics | |||
| Male sex (n, %) | 8 (80%) | 8 (80%) | 1.000 |
| Age (years) | 68 ± 7 | 70 ± 10 | 0.689 |
| Hypertension (n, %) | 10 (100%) | 9 (90%) | 0.305 |
| Hyperlipidemia (n, %) | 7 (70%) | 6 (60%) | 0.639 |
| Nicotine use (n, %) | 3 (30%) | 4 (40%) | 0.639 |
| BMI (kg/m2) | 33 ± 5 | 30 ± 7 | 0.230 |
| Total cholesterol (mg/dl) | 172 ± 43 | 165 ± 33 | 0.663 |
| LDL-c (mg/dl) | 106 ± 41 | 101 ± 32 | 0.724 |
| HDL-c (mg/dl) | 39 ± 6 | 39 ± 6 | 0.972 |
| Triglycerides (mg/dl) | 161 ± 62 | 180 ± 108 | 0.640 |
| HbA1c (%) | 6.1 ± 0.5 | 7.4 ± 1.4 | 0.026 |
| hsCRP | 5.0 ± 2.3 | 26.7 ± 46.9 | 0.175 |
| Aspirine therapy (n, %) | 10 (100%) | 9 (90%) | 0.305 |
| Statine therapy (n, %) | 6 (60%) | 6 (60%) | 1.000 |
| Lesion characteristics | |||
| Minimal FCT (µm) | 97 ± 15 | 49 ± 10 | <0.001 |
| Mean FCT (µm) | 133 ± 12 | 94 ± 17 | 0.006 |
| Maximal lipid arc (°) | 110 ± 8 | 178 ± 39 | 0.001 |
| Lipid volume arc (mm*°) | 3853 ± 1294 | 9876 ± 3088 | 0.011 |
| Presence of TCFA (n, %) | 0 (0%) | 7 (70%) | 0.003 |
| Presence of macrophages (n, %) | 4 (40%) | 6 (60%) | 0.371 |
| Presence of spotty calcifications (n, %) | 7 (70%) | 8 (80%) | 0.527 |
Mechanical properties of the different plaque components.
| Poisson’s ratio (ν) | Young’s modulus | |
|---|---|---|
| Fibrous cap | 0.27 | 244 kPa |
| Necrotic lipid core | 0.48 | 1 kPa |
| Calcification | 0.30 | 10 GPa |
| Vessel wall | 0.27 | 800 kPa |
Additional files
-
Supplementary file 1
Characteristics of the mesh.
Maximum and minimum element size, respectively, limits how big and how small each mesh element can be. Maximum element growth rate limits the size difference of two adjacent mesh elements. Curvature factor limits how big a mesh element can be along a curved boundary. Resolution of narrow regions controls the number of layers of mesh elements in narrow regions.
- https://cdn.elifesciences.org/articles/64020/elife-64020-supp1-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/64020/elife-64020-transrepform-v1.pdf





