A gustatory receptor tuned to the steroid plant hormone brassinolide in Plutella xylostella (Lepidoptera: Plutellidae)
Figures
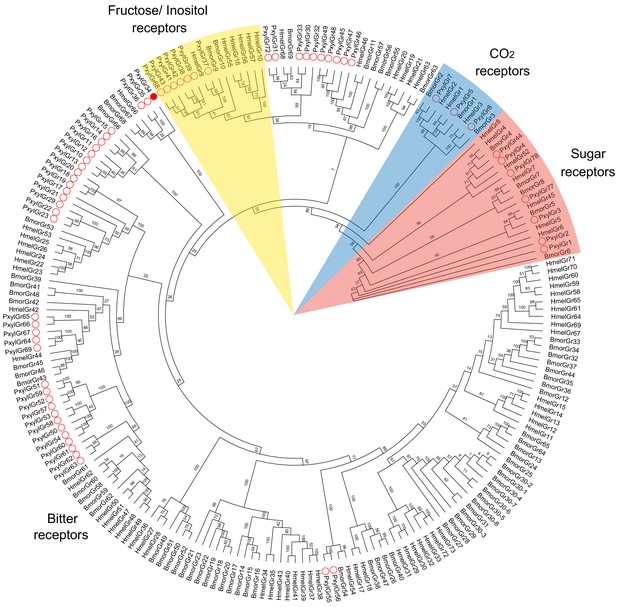
Phylogenetic tree of gustatory receptors (GRs).
Amino acid sequences are based on previously reported GRs. Bootstrap values are based on 1000 replicates. Abbreviations: Hmel, Heliconius melpomene; Bmor, Bombyx mori; Pxyl, Plutella xylostella. ○, GRs of P. xylostella; ●, PxylGR34.
-
Figure 1—source data 1
Amino acid sequences of GRs in Plutella xylostella.
- https://cdn.elifesciences.org/articles/64114/elife-64114-fig1-data1-v2.xlsx
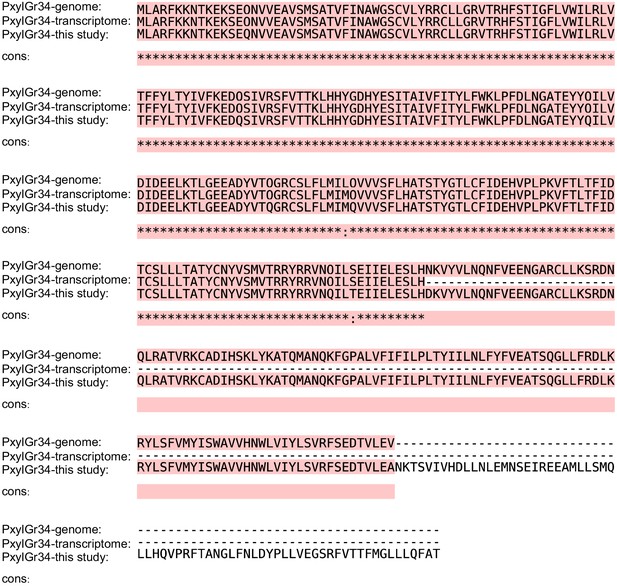
Alignment of amino acid sequences of PxylGr34 from genomic data (Engsontia et al., 2014), transcriptomic data (Yang et al., 2017), and this study.
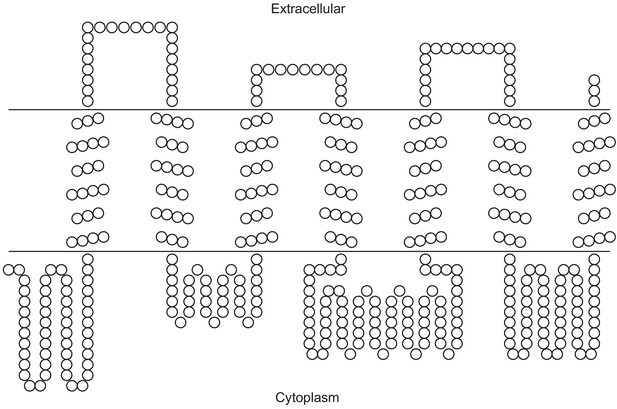
Secondary structure prediction of PxylGr34.
Image was constructed using TOPO2 software (http://www.sacs.ucsf.edu/TOPO2/) based on secondary structure predicted by TOPCONS (topcons.net) models (Tsirigos et al., 2015). Only the model with a reliable seven-transmembrane structure was adopted.
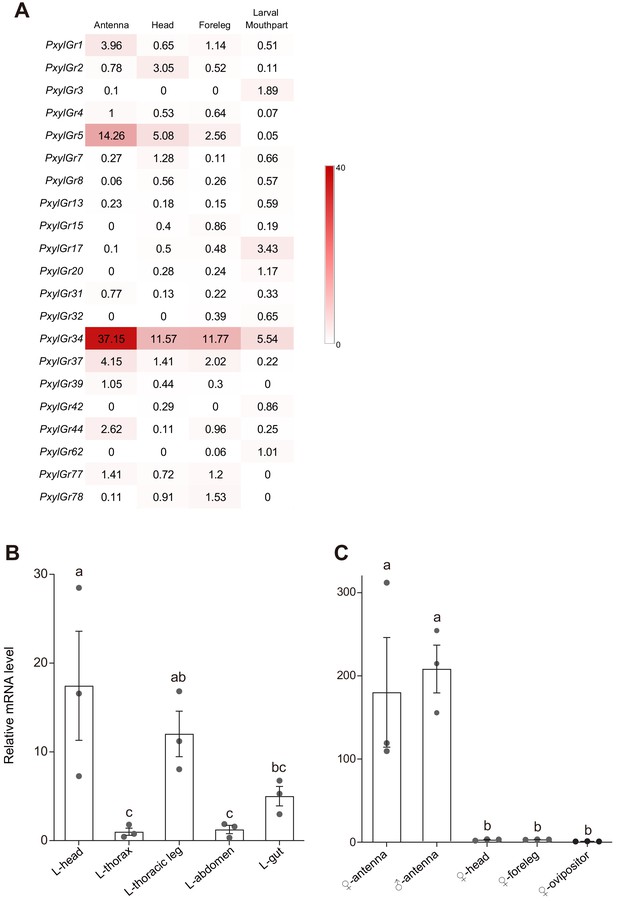
Tissue expression pattern of gustatory receptors (GRs) in Plutella xylostella as determined by Illumina read-mapping and qPCR analysis.
(A) Transcripts per million (TPM) value of each GR is indicated in box. Color scales were generated using Microsoft Excel. Antenna, head, and foreleg were from the moth; larval mouthpart was from 4th instar larvae. The GRs that undetectable in the TPM analysis were not listed. Relative PxylGr34 transcript levels in (B) 4th instar larval tissues and (C) mated moth tissues of Plutella xylostella as determined by qPCR. Data are mean ± SEM. n = 3 replicates of 40–200 tissues each. For 4th instar larvae, p=0.0004; for the moth, p<0.0001 (one-way ANOVA, Tukey’s HSD test).
-
Figure 2—source data 1
Source data for Figure 2B and C.
- https://cdn.elifesciences.org/articles/64114/elife-64114-fig2-data1-v2.xlsx
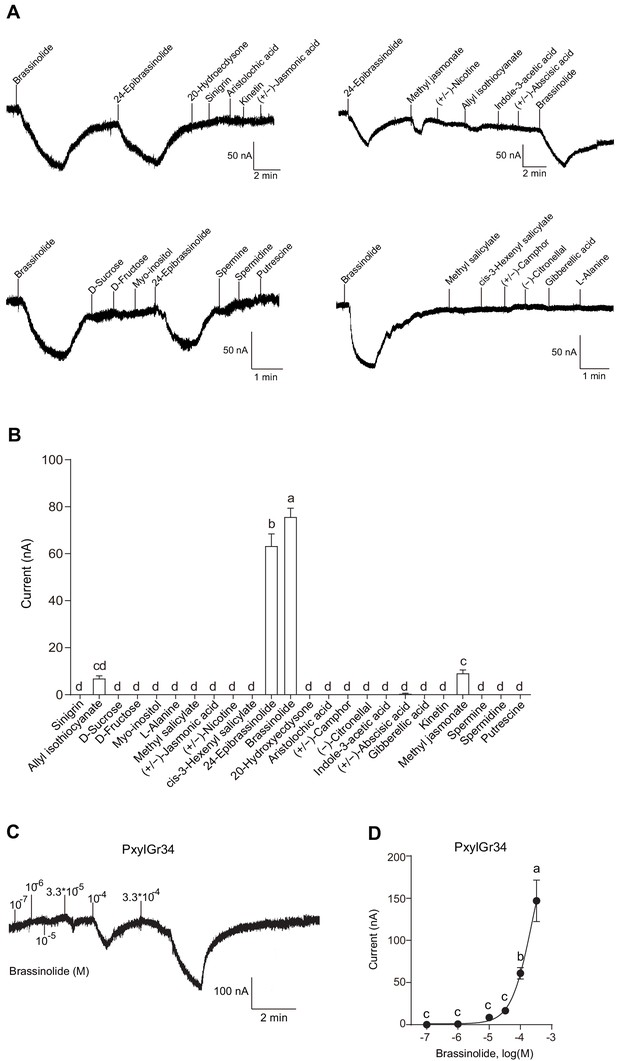
Brassinolide and 24-epibrassinolide induced a strong response in the oocytes expressing PxylGr34.
(A) Representative inward current responses of Xenopus oocytes expressing PxylGr34 in response to ligands at 10−4 M. (B) Response profiles of Xenopus oocytes expressing PxylGr34 in response to ligands at 10−4 M. Data are mean ± SEM. n = 7 replicates of cells. p<0.0001 (one-way ANOVA, Tukey's HSD test). (C) Representative inward current responses of Xenopus oocytes expressing PxylGr34 in response to BL at a range of concentrations. (D) Response profiles of Xenopus oocytes expressing PxylGr34 in response to BL at a range of concentrations. Data are mean ± SEM; n = 6–8 replicates of cells. p<0.0001 (one-way ANOVA, Tukey’s HSD test).
-
Figure 3—source data 1
Source data for Figure 3B and D.
- https://cdn.elifesciences.org/articles/64114/elife-64114-fig3-data1-v2.xlsx
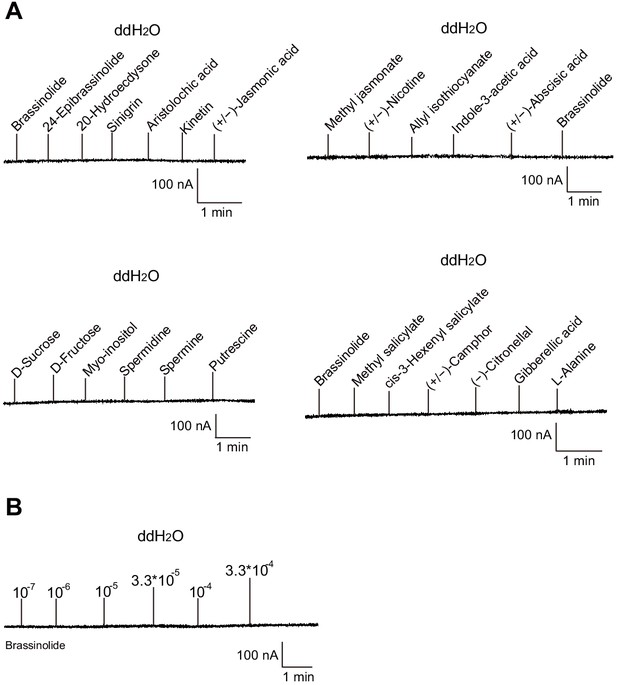
Two-electrode voltage-clamp recordings of Xenopus oocytes injected with distilled water and stimulated with test compounds.
No inward current responses of distilled-water-injected Xenopus oocytes to tested ligands (A) or BL at a range of concentrations (B).
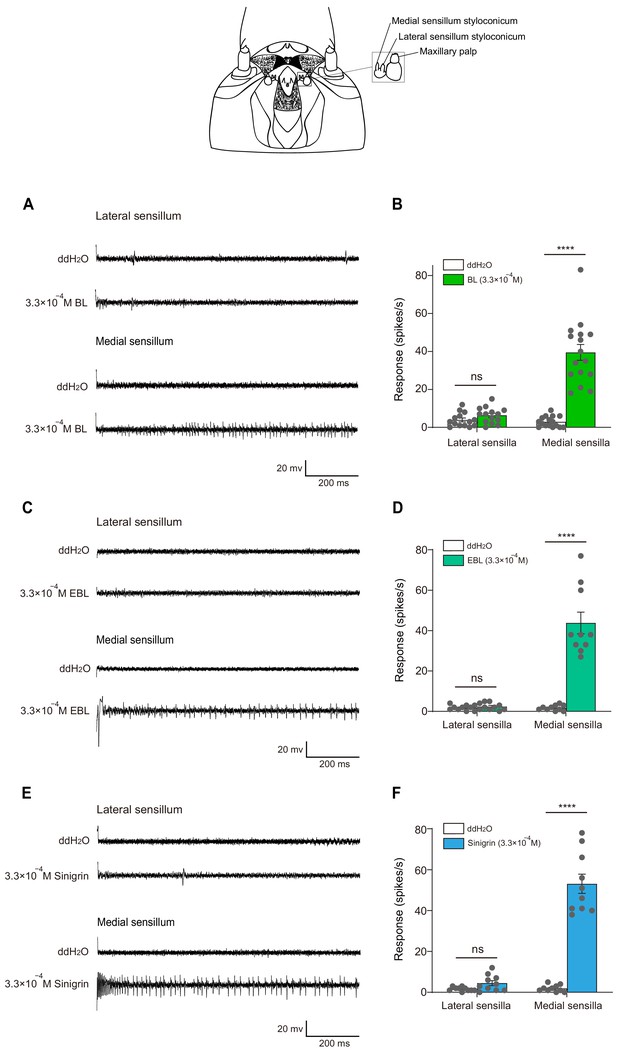
The larval medial sensilla styloconica exhibited vigorous responses in P.
xylostella to brassinolide (BL) and 24-epibrassinolide (EBL). Typical electrophysiological recordings in response to water and BL (A), EBL (C), and sinigrin (E) at 3.3 × 10−4 M for 1 s obtained by tip-recording from a neuron innervating the lateral and medial sensillum styloconica on maxillary galea of P. xylostella 4th instar larvae. Response profiles of the two lateral and medial sensilla styloconica on the maxilla of P. xylostella 4th instar larvae to water and BL (B), EBL (D), and sinigrin (F) at 3.3 × 10−4 M. Data are mean ± SEM. For lateral sensilla styloconica, n = 14, p=0.0905 to BL; n = 10, p=0.3869 to EBL; n = 10, p=0.0672 to sinigrin; for medial sensilla styloconica, n = 16, p<0.0001 to BL; n = 10, p<0.0001 to EBL; n = 10, p<0.0001 to sinigrin. Data were analyzed by paired-samples t-test.
-
Figure 4—source data 1
Source data for Figure 4B,D and F.
- https://cdn.elifesciences.org/articles/64114/elife-64114-fig4-data1-v2.xlsx
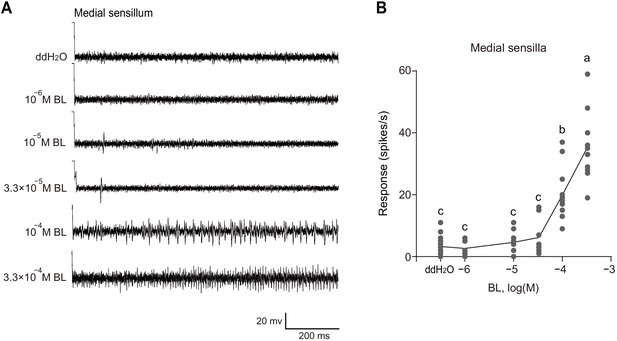
The medial sensilla styloconica showed a dose-dependent response to brassinolide (BL).
(A) Typical electrophysiological recordings in response to water and BL at a series of concentrations for 1 s obtained by tip-recording from a neuron innervating the medial sensillum styloconicum on the maxillary galea of P. xylostella 4th instar larvae. (B) Response profiles of medial sensilla styloconica on maxilla of P. xylostella 4th instar larvae to water and BL at a series of concentrations. Data are mean ± SEM. n = 8–14 replicates of larvae, p<0.0001 (one-way ANOVA, Tukey’s HSD test).
-
Figure 5—source data 1
Source data for Figure 5B.
- https://cdn.elifesciences.org/articles/64114/elife-64114-fig5-data1-v2.xlsx
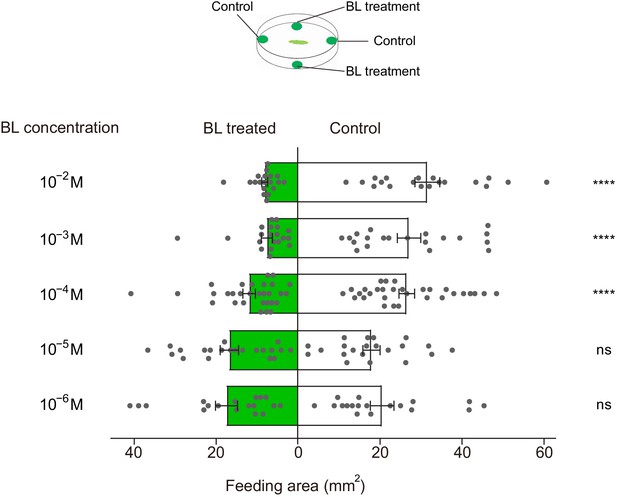
Brassinolide (BL)-induced feeding deterrence effect on P.xylostella larvae.
Dual-choice feeding tests were conducted using pea leaf discs. Total feeding area of control (white bars) and BL-treated discs (green bars). BL was diluted in 50% ethanol to 10−6, 10−5, 10−4, 10−3, and 10−2 M. Data are mean ± SEM. For 10−6 M, n = 18, p=0.3947; for 10−5 M, n = 21, p=0.5967; for 10−4 M, n = 30, p<0.0001; for 10−3 M, n = 20, p<0.0001; for 10−2 M, n = 19, p<0.0001. Data were analyzed by paired-samples t-test.
-
Figure 6—source data 1
Source data for Figure 6.
- https://cdn.elifesciences.org/articles/64114/elife-64114-fig6-data1-v2.xlsx
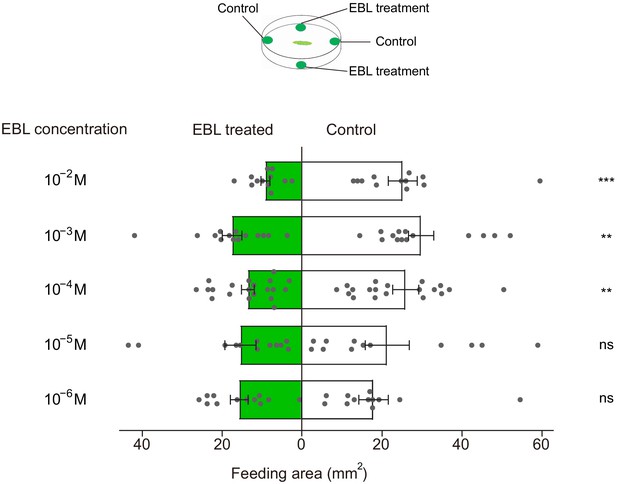
24-Epibrassinolide (EBL)-induced feeding deterrence effect on P. xylostella larvae.
Dual-choice feeding tests were conducted using pea leaf discs. Total feeding area of control (white bars) and EBL-treated discs (green bars). EBL was diluted in 50% ethanol to 10−6, 10−5, 10−4, 10−3, and 10−2 M. Data are mean ± SEM. For 10−6 M, n = 12, p=0.5151; 10−5 M, n = 12, p=0.336; 10−4 M, n = 20, p=0.0024; 10−3 M, n = 14, p=0.0023; 10−2 M, n = 12, p=0.0004. Data were analyzed by paired-samples t-test.
-
Figure 6—figure supplement 1—source data 1
Source data for Figure 6—figure supplement 1.
- https://cdn.elifesciences.org/articles/64114/elife-64114-fig6-figsupp1-data1-v2.xlsx
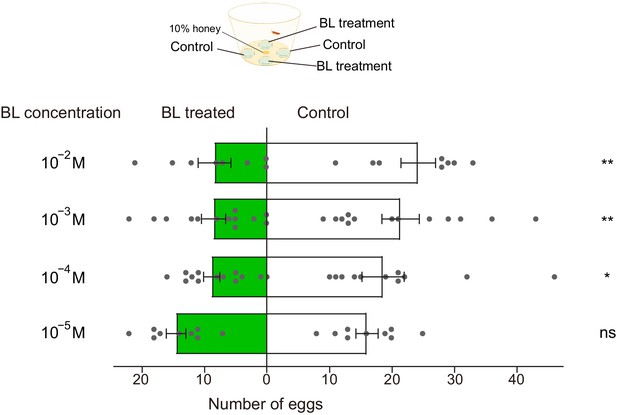
Brassinolide (BL)-induced oviposition deterrence to P. xylostella females.
Dual-choice oviposition tests were conducted using plastic film coated with cabbage leaf juice. Both control (50% ethanol) and BL (diluted in 50% ethanol) were painted evenly onto plastic film. After 24 hr, total number of eggs laid by a single mated female on control films (white bars) and BL-treated films (green bars) were counted. Data are mean ± SEM. For 10−5 M, n = 9, p=0.4364; for 10−4 M, n = 12, p=0.0275; for 10−3 M, n = 13, p=0.0015; 10−2 M, n = 8, p=0.0038. Data were analyzed using paired-samples t-test.
-
Figure 7—source data 1
Source data for Figure 7.
- https://cdn.elifesciences.org/articles/64114/elife-64114-fig7-data1-v2.xlsx
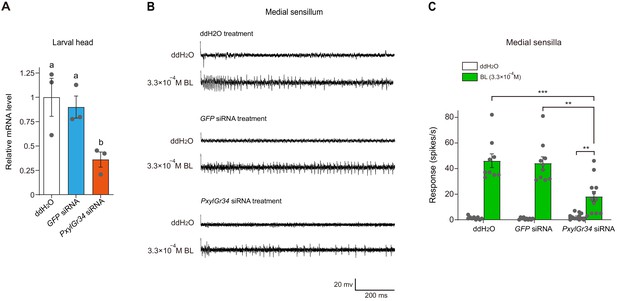
PxylGr34 siRNA-treated P. xylostella larvae inhibit the responses of sensilla to brassinolide (BL).
(A) Effect of PxylGr34 siRNA on transcript levels of PxylGr34 in 4th instar larval head. Heads were collected after feeding larvae with cabbage leaf discs coated with PxylGr34 siRNA, GFP siRNA, or ddH2O. Relative transcript levels of PxylGr34 in each treatment were determined by qPCR. Data are mean ± SEM. n = 3 replicates of 21–24 heads each, p=0.0237 (one-way ANOVA, Tukey’s HSD test). (B) Typical electrophysiological recordings in response to water and BL at 3.3 × 10−4 M for 1 s obtained by tip-recording from a neuron innervating the medial sensillum styloconica, on maxillary galea of P. xylostella 4th instar larvae with cabbage leaf discs coated with PxylGr34 siRNA, GFP siRNA, or ddH2O. (C) Response profiles of the medial sensilla styloconica on the maxilla of P. xylostella 4th instar larvae with cabbage leaf discs coated with PxylGr34 siRNA, GFP siRNA, or ddH2O, to water and BL at 3.3 × 10−4 M. Data are mean ± SEM. The data of each treatment to water and BL at 3.3 × 10−4 M were analyzed by paired-samples t-test. For ddH2O treatment, n = 9, p<0.0001; for GFP siRNA treatment, n = 10, p<0.0001; for PxylGr34 siRNA treatment, n = 12, p=0.0019. The data of different treatments to BL at 3.3 × 10−4 M were analyzed by one-way ANOVA, Tukey’s HSD test, p=0.0002.
-
Figure 8—source data 1
Source data for Figure 8A and C.
- https://cdn.elifesciences.org/articles/64114/elife-64114-fig8-data1-v2.xlsx
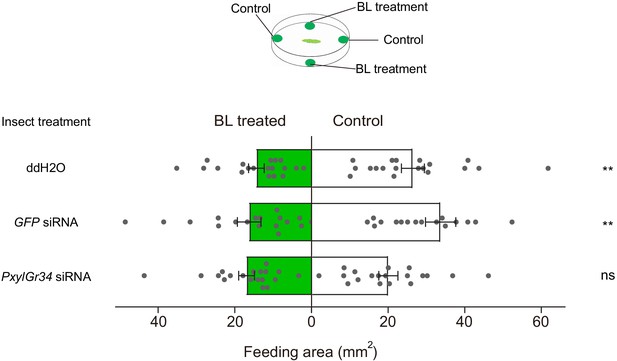
PxylGr34 siRNA-treated P. xylostella larvae alleviated the feeding deterrent effect of brassinolide (BL).
Choice assay using 4th instar larvae fed on cabbage leaf discs treated with PxylGr34 siRNA, GFP siRNA, or ddH2O. In dual-choice assay, larvae chose between control pea leaf discs (treated with 50% ethanol) and those treated with BL at 10−4 M (diluted in 50% ethanol). Figure shows total feeding area of control (white bars) and treated discs (green bars). Data are mean ± SEM. For ddH2O treatment, n = 19, p=0.0093; for GFP siRNA treatment, n = 18, p=0.0044; for PxylGr34 siRNA treatment, n = 19, p=0.1864. Data were analyzed by paired-samples t-test.
-
Figure 9—source data 1
Source data for Figure 9.
- https://cdn.elifesciences.org/articles/64114/elife-64114-fig9-data1-v2.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Plutella xylostella) | Muscle actin gene | NCBI | GenBank: AB282645.1 | |
| Commercial assay or kit | RNeasy Plus Universal Mini Kit | Qiagen | Cat# 73404 | |
| Commercial assay or kit | Q5 High-Fidelity DNA Polymerase | NEB | Cat# M0491 | |
| Commercial assay or kit | M-MLV reverse transcriptase | Promega | Cat# M1701 | |
| Commercial assay or kit | SYBR Premix Ex TaqII | Takara | Cat# RR820 | |
| Commercial assay or kit | mMESSAGE mMACHINE SP6 | Ambion | Cat# AM1340 | |
| Chemical compound, drug | (+/−)-Abscisic acid | Sigma-Aldrich | CAS: 21293-29-8 | |
| Chemical compound, drug | L-Alanine | Sigma-Aldrich | CAS: 56-41-7 | |
| Chemical compound, drug | Allyl isothiocyanate | Sigma-Aldrich | CAS: 57-06-7 | |
| Chemical compound, drug | Aristolochic acid | Shanghai Macklin Biochemical Co.,Ltd, China | CAS: 313-67-7 | |
| Chemical compound, drug | Brassinolide | Yuanyeshengwu Co., Ltd, China | CAS: 72962-43-7 | |
| Chemical compound, drug | (+/−)-Camphor | Beijing Mreda Technology Co., Ltd, China | CAS: 76-22-2 | |
| Chemical compound, drug | (−)-Citronellal | Tokyo Chemical Industry Co., Ltd, Japan | CAS: 5949-05-3 | |
| Chemical compound, drug | 24-Epibrassinolide | Shanghai Macklin Biochemical Co., Ltd, China | CAS: 78821-43-9 | |
| Chemical compound, drug | D-Fructose | Sigma-Aldrich | CAS: 7660-25-5 | |
| Chemical compound, drug | Gibberellic acid | Yuanyeshengwu Co., Ltd, China | CAS: 77-06-5 | |
| Chemical compound, drug | cis-3-Hexenyl salicylate | Shanghai Macklin Biochemical Co., Ltd, China | CAS: 65405-77-8 | |
| Chemical compound, drug | 20-Hydroxyecdysone | Yuanyeshengwu Co., Ltd, China | CAS: 5289-74-7 | |
| Chemical compound, drug | Indole-3-acetic acid | Yuanyeshengwu Co., Ltd, China | CAS: 87-51-4 | |
| Chemical compound, drug | (+/−)-Jasmonic acid | Tokyo Chemical Industry Co., Ltd, Japan | CAS: 6894-38-8 | |
| Chemical compound, drug | Kinetin | Sigma-Aldrich | CAS: 525-79-1 | |
| Chemical compound, drug | Methyl jasmonate | Sigma-Aldrich | CAS: 1211-29-6 | |
| Chemical compound, drug | Methyl salicylate | Sigma-Aldrich | CAS: 119-36-8 | |
| Chemical compound, drug | Myo-inositol | Sigma-Aldrich | CAS: 87-89-8 | |
| Chemical compound, drug | (+/−)-Nicotine | Sigma-Aldrich | CAS: 22083-74-5 | |
| Chemical compound, drug | Putrescine | Alfa Aesar | CAS: 110-60-1 | |
| Chemical compound, drug | Sinigrin | Sigma-Aldrich | CAS: 3952-98-5 | |
| Chemical compound, drug | Spermidine | Sigma-Aldrich | CAS: 124-20-9 | |
| Chemical compound, drug | Spermine | Sigma-Aldrich | CAS: 71-44-3 | |
| Chemical compound, drug | D-Sucrose | Sigma-Aldrich | CAS: 57-50-1 | |
| Software, algorithm | GraphPad Prism | GraphPad Prism | RRID:SCR_002798 | 8.3 |
| Software, algorithm | Adobe Illustrator | Adobe systems | RRID:SCR_014198 | CC2018 |
| Software, algorithm | pCLAMP software | pCLAMP software | RRID:SCR_011323 |
Additional files
-
Supplementary file 1
Sequence information for gustatory receptors of Plutella xylostella.
- https://cdn.elifesciences.org/articles/64114/elife-64114-supp1-v2.docx
-
Supplementary file 2
Primers used for qPCR, Xenopus oocyte expression (Xe), and siRNA synthesis.
- https://cdn.elifesciences.org/articles/64114/elife-64114-supp2-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/64114/elife-64114-transrepform-v2.docx





