Pruriception and neuronal coding in nociceptor subtypes in human and nonhuman primates
Figures
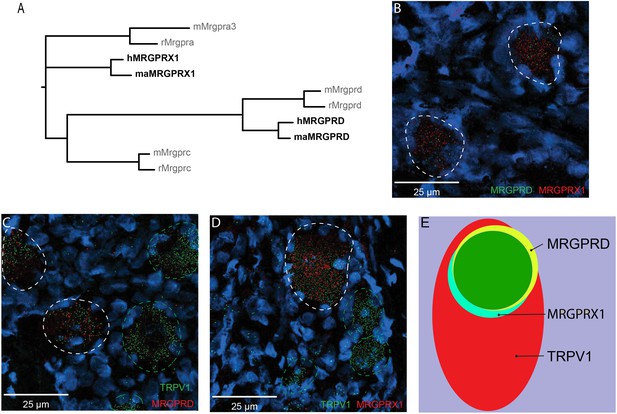
MRGPRX1 and MRGPRD are co-expressed in TRPV1-expressing human dorsal root ganglion (DRG) neurons.
(A) Phylogenetic tree of MRGPRs from mouse (m), rat (r), macaque (ma), and human (h). Note that the MRGPRD gene is conserved among rodents and primates, while the Mrgpra and Mrgprc subfamilies are rodent-specific and the MRGPRX subfamily is primate-specific. For clarity, only one murine Mrgpra gene and only one macaque or human MRGPRX gene is shown. (B–D) Representative double in situ hybridization (ISH) images of a field of human DRG with neurons stained for MRGPRD (B, green; C, red), MRGPRX1 (B and D, red), and TRPV1 (C and D, green). Double-positive and single-positive neurons are outlined with white and green dashed lines, respectively. DAPI counterstain is displayed in blue. (E) Venn diagram summarizing the relative expression overlap of MRGPRD (yellow), MRGPRX1 (blue), and TRPV1 (red) in human DRG. Note that MRGPRD and MRGPRX1 are expressed in a largely overlapping population (green, i.e., MRGPRD + MRGPRX1/MRGPRD = 89.6 ± 1.5%; MRGPRX1 + MRGPRD/MRGPRX1 = 93.9 ± 2.0%) in about 1/3 of all TRPV1-positive neurons (MRGPRD + TRPV1/TRPV1 = 36.5 ± 4.2%; MRGPRX1 + TRPV1/TRPV1 = 32.5 ± 3.7%). Expression analysis for all three markers was performed in DRG tissue from four individuals, and data are stated as mean ± standard error of the mean (SEM). Green filled area indicates the overlap in expression of MRGPRD and MRGPRX1.
-
Figure 1—source data 1
Expression of MRGPRX1 and MRGPRD in nonhuman primate DRG.
- https://cdn.elifesciences.org/articles/64506/elife-64506-fig1-data1-v1.xlsx
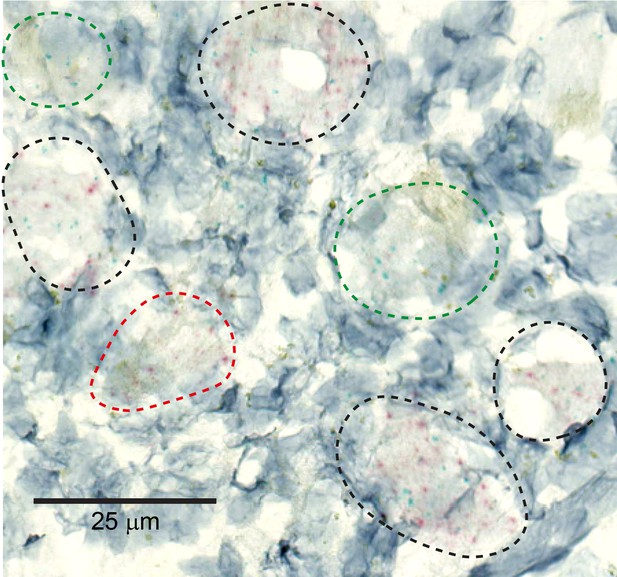
MRGPRX1 and MRGPRD are co-expressed in macaque dorsal root ganglion (DRG) neurons.
Representative double in situ hybridization image of a field of macaque DRG with neurons stained for MRGPRD (red) and MRGPRX1 (green). Double-positive and single-positive neurons are outlined with black and red/green dashed lines, respectively. Hematoxylin counterstain was used to identify nuclei.
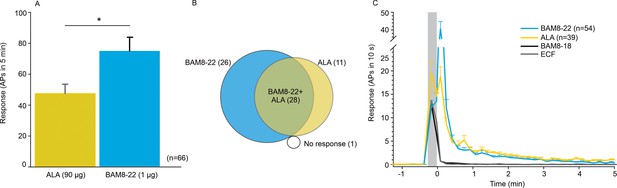
CMHs respond to bovine adrenal medulla peptide 8–22 (BAM8-22) more vigorously than to β-alanine (ALA) but with a similar time course.
(A) The average evoked response of all CMHs to BAM8-22 (blue) was significantly larger than that to ALA (yellow) injection (paired t-test: t(65) = −2.142, p=0.0359). (B) Venn diagram of the number of CMHs responsive to BAM8-22 or ALA or both. (C) The time course of action potential activity (plotted as number of action potentials in 10 s bins) was similar after BAM8-22 and ALA injection, except for the greater response to BAM8-22 within the first 10 s following injection. The average responses of the same populations to vehicle (extracellular fluid [ECF] and BAM8-18) are graphed with gray and black lines, respectively. Error bars represent standard error of the mean (SEM). Gray box marks time of needle insertion and injection.
-
Figure 2—source data 1
Activation of CMHs by ALA and BAM8-22.
- https://cdn.elifesciences.org/articles/64506/elife-64506-fig2-data1-v1.xlsx
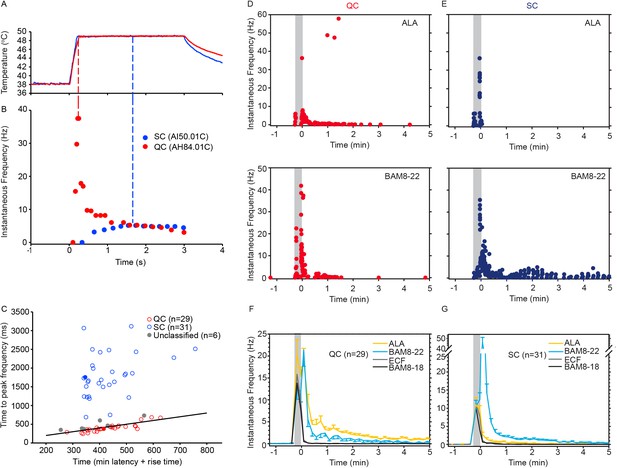
CMH subpopulations, QCs and SCs, exhibit differences in their response to heat stimulation and in the magnitude and time course of their response to β-alanine (ALA) and bovine adrenal medulla peptide 8–22 (BAM8-22).
(A) Temperature waveform of the CO2 laser-evoked heat stimulus. The skin was first pre-heated to 38 °C, 3 s baseline temperature and then rapidly raised (rise time ~200 ms) to 49 °C for 3 s. (B) Specimen recording showing the response of an individual QC fiber (red circles) and an SC fiber (blue circles) to the laser-heat stimulus described in (A). The instantaneous discharge frequency is plotted versus time. Each dot represents the occurrence of an action potential (AP). The response in the QC fiber starts during the temperature rise and reaches peak frequency at the end of the ramp (red dashed line). The SC fiber reached the peak instantaneous discharge frequency during the plateau phase of the heat stimulus (blue dashed line). (C) The time of peak discharge for each fiber is plotted against the sum of stimulus rise time + the minimal AP conduction time as measured in response to transcutaneous electrical stimulation from the proximal edge of the receptive field (RF). Data points above the line of equality correspond to fibers in which the peak discharge occurred in the plateau phase of the heat stimulus (SCs, blue circles). Data points falling below the line of equality are from those fibers whose peak discharge occurred during the rising phase of the heat stimulus (QCs, red circles). The filled circles represent the data from the specimen recordings shown in (B) and also of the specimen responses to ALA and BAM8-22 shown in (D) and (E). Gray circles indicate data from fibers that were unclassified. Examples of responses in (D) of a QC fiber and (E) an SC fiber to ALA (top panels) and each to BAM8-22 (bottom panels). Responses to ALA and BAM8-22 are from the same fiber. The instantaneous frequency of each AP is plotted versus the time of its occurrence. The time course of neuronal activity induced by ALA, BAM8-22, and vehicle controls in the population of (F) QC fibers and (G) SC fibers. The average number of APs recorded over 10 s intervals during the 5 min observation period following injection is plotted. In QC fibers, ALA and BAM8-22 produced marked excitation, whereas in SC fibers, only BAM8-22 produced long-lasting activity. Error bars represent standard error of the mean (± SEM). Gray boxes mark time of needle insertion and injection.
-
Figure 3—source data 1
Activation of QCs and SCs by ALA and BAM8-22.
- https://cdn.elifesciences.org/articles/64506/elife-64506-fig3-data1-v1.xlsx
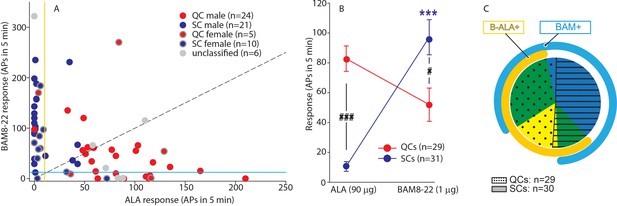
QCs and SCs from both male and female monkeys differ in sensitivity and incidence of activation by β-alanine (ALA) and bovine adrenal medulla peptide 8–22 (BAM8-22).
(A) For each C-fiber, the response to BAM8-22 was plotted against its response to ALA. Solid red and blue circles indicate QC and SC fibers, respectively, recorded from male animals. Red and blue circles with a gray border indicate data obtained from female animals. Solid gray circles indicate data from C fibers with an unclassified heat response. The diagonal line indicates equal response to both compounds. Vertical and horizontal lines indicate the ‘threshold’ (≥10 action potentials [APs]) for an afferent to be counted as being responsive to an agonist (yellow and blue, respectively, for responses to ALA and BAM8-22). (B) Population responses of QCs and SCs to ALA and BAM8-22. In SCs, intradermal injection of BAM8-22 produces a significantly greater response than ALA (*** p<0.0001). The response to ALA was significantly larger in QCs than SCs (###p<0.0001), whereas the response to BAM8-22 was significantly larger in SCs than QCs (#p=0.002). Data were analyzed with repeated measures ANOVA (RMANOVA) with ‘fiber type’ as between-subjects factor and ‘pruritogen’ as a within-subjects factor (‘fiber type’ × ‘pruritogen’: F(1,58)=29,55; p<0.0001), followed by Scheffe test for post hoc analysis. (C) ALA activated 27/29 QCs and 7/31 SCs. BAM8-22 activated 21/29 QCs and 29/31 SCs. The occurrence of QCs and SCs responding to ALA only (eight and one afferents, respectively) was fairly rare. The number of QCs responding only to BAM8-22 (two units) was also small, whereas the majority of SCs (23/31) only responded to BAM8-22. Of the 31 SCs, one did not respond to either agonist. 19 QCs and 6 SCs responded to both, ALA and BAM8-22.
-
Figure 4—source data 1
Preferrential activation of QCs and SCs by ALA and BAM8-22.
- https://cdn.elifesciences.org/articles/64506/elife-64506-fig4-data1-v1.xlsx
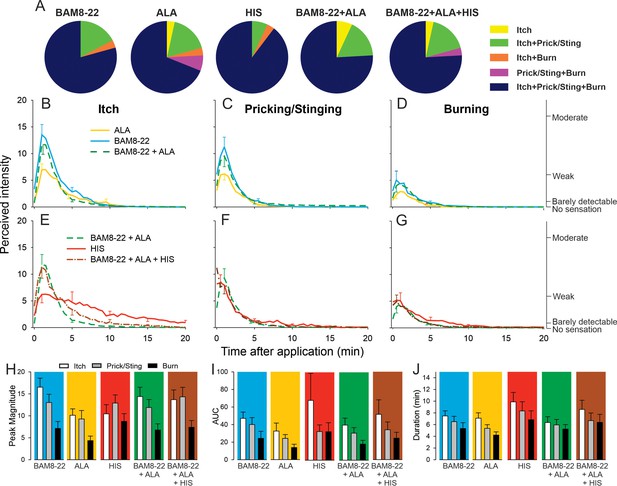
In humans, co-injection of β-alanine (ALA) and bovine adrenal medulla peptide 8–22 (BAM8-22) with or without histamine (HIS) does not change the itch or nociceptive sensations compared to the effects of one component pruritogen given alone.
(A) The majority of subjects (n = 29) reported itch, pricking/stinging, and burning sensations after administration of each of the pruritogens or their combinations. (B) Magnitude of itch, (C) stinging/pricking, and (D) burning sensations evoked by BAM8-22, ALA, and a combination of BAM8-22 and ALA are plotted for successive 30 s intervals after injection averaged across all 29 subjects. (E) Magnitude of itch, (F) stinging/pricking, and (G) burning sensations evoked by HIS, a combination of BAM8-22 and ALA, and a combination of BAM8-22, ALA, and HIS. For clarity, the standard error of the mean (SEM) is plotted only every 5 min starting with the peak rating for each quality. On the right vertical axis, the locations of three verbal descriptors are shown in correspondence with the ratings of perceived intensity indicated on the left vertical axis (see Materials and methods). (H) Peak magnitude of perceived sensation intensity (n = 29), (I) computed area under the curve (n = 17), or (J) duration of itch, stinging/pricking, or burn ratings (n = 17) were each not significantly different for the five different stimuli, that is, three single pruritogens and two combinations (repeated measures ANOVA [RMANOVA], see text for further details). For (I) and (J), subjects (n = 12) without a measurable area under the curve (AUC) or duration of any sensation during the time course were not included in the analysis, and therefore only 17 subjects were used for within-subjects analysis and post hoc comparisons. Data represent mean ± SEM.
-
Figure 5—source data 1
Magnitude of sensation, AUC and Duration of sensation in response to single pruritogen or their combinations for itch, prcking/stinging and burning.
- https://cdn.elifesciences.org/articles/64506/elife-64506-fig5-data1-v1.xlsx
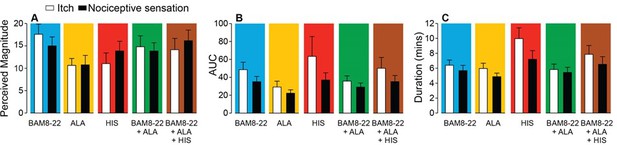
In humans, co-injection of β-alanine (ALA) and bovine adrenal medulla peptide 8–22 (BAM8-22) with or without histamine (HIS) does not change the itch or nociceptive sensation compared to the effects of one of the component pruritogens given alone.
(A) Magnitude of itch, (B) AUC and (C) Duration of sensation. Data are plotted in the same format as Figure 5H–J for the 25 subjects that, in response to each single pruritogen, reported a greater than zero peak magnitude of both itch and either type of nociceptive sensation (whichever elicited the greater area under the curve [AUC]). This increased the sample size from n = 17 in Figure 5I, J to n = 25 by including the data for eight additional subjects that always felt at least one type of nociceptive sensation but not always both. A 5 ‘stimulus’ × 2 ‘sensory quality’ repeated measures ANOVA (RMANOVA) was performed for peak magnitude, AUC, and duration. For peak magnitude, only ‘stimulus’ had a significant effect (F(4,96)=2.85, p<0.03) with BAM8-22 producing a higher peak magnitude of sensation than ALA (p<0.034, Bonferroni test). The interaction between ‘stimulus’ and ‘sensory quality’ was not significant (F(4,96)=1.89, p=0.12). For AUC, only ‘sensory quality’ was significant (F(1,24.0) = 5.25, p<0.032), with the mean AUC for itch being significantly larger than that for nociceptive sensation (p<0.032, Bonferroni test). The interaction between ‘stimulus’ and ‘sensory quality’ was not significant (F(1.7,42.2) = 0.91, p=0.40). For duration, ‘stimulus’ and ‘sensory quality’ were each significant (F(2.75, 66.0)=4.25, p=0.010 and F(1,24.0) = 13.03, p=0.001, respectively), but their interaction was not (F(2.78, 66,8)=2.1, p=0.11). HIS-induced sensations lasted significantly longer than those induced by ALA or by BAM8-22 + ALA (p<0.01 and p<0.05, respectively). Furthermore, the duration of itch was significantly greater than for the nociceptive sensation (p=0.001, Bonferroni test).
-
Figure 5—figure supplement 1—source data 1
Magnitude of of sensation, AUC and Duration of sensation in response to single puritogen or their combinations for itch and nociceptive sensation.
- https://cdn.elifesciences.org/articles/64506/elife-64506-fig5-figsupp1-data1-v1.xlsx
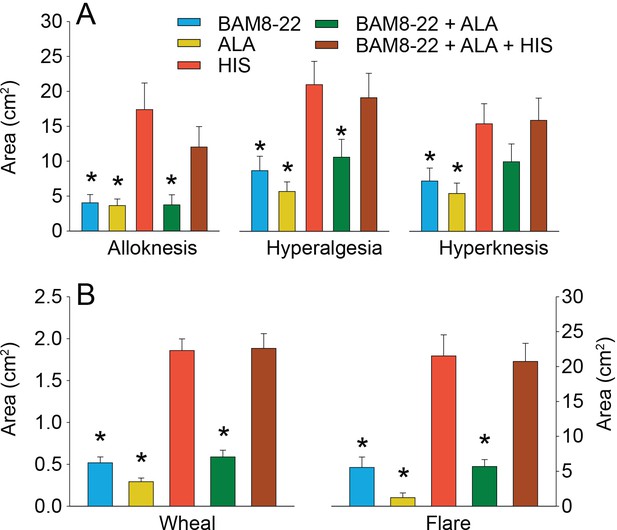
In humans, co-injection of β-alanine (ALA) and bovine adrenal medulla peptide 8–22 (BAM8-22) with or without histamine (HIS) does not change the areas of dysesthesia or skin reactions compared to the effects of one of the component pruritogens given alone.
(A) Areas of alloknesis, hyperalgesia, and hyperknesis and (B) areas of wheal and flare produced after injection of each pruritogen (BAM8-22, ALA), HIS, and combination of pruritogens. Data were analyzed with repeated measures ANOVA (RMANOVAs). Greenhouse–Geisser corrections were made for all analyses to correct for non-sphericity followed by Bonferroni post hoc tests (see main text and Supplementary file 2 for p-values). * indicates significant differences compared to HIS or BAM8-22 + ALA + HIS. Data from all subjects (n = 29) were included in the analysis. Data represent mean ± standard error of the mean (SEM).
-
Figure 6—source data 1
Areas of dysethesias, wheal and flare in response to a single pruritogen or their combination.
- https://cdn.elifesciences.org/articles/64506/elife-64506-fig6-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Macaca nemestrina) | Macaca nemestrina | Johns Hopkins, NIH-supported pigtailed macaque breeding colony | ||
| Biological sample (Homo sapiens) | DRG tissue | Tissue For Research (http://www.biobankonline.com) and the NIH NeuroBioBank | See Supplementary file 4 | |
| Biological sample (pigtailed monkey) | DRG Tissue (Macaca nemestrina) | Johns Hopkins, NIH-supported pigtailed macaque breeding colony | ||
| Peptide, recombinant protein | Bovine adrenal medulla peptide 8–22 (BAM8-22) | Tocris | Cat no. 1763 | |
| Peptide, recombinant protein | Bovine adrenal medulla peptide 8–18 (BAM8-18) | Keck Lab, Yale University, New Haven Sikand et al., 2011b | ||
| Commercial assay or kit | hTRPV1 in situ hybridization probe | Advanced Cell Diagnostics | Cat no. 415381 | |
| Commercial assay or kit | hMRGPRX1 in situ hybridization probe | Advanced Cell Diagnostics | Cat no. 517011 | |
| Commercial assay or kit | hMRGPRD in situ hybridization probe | Advanced Cell Diagnostics | Cat no. 524871 | |
| Commercial assay or kit | RNAscope multiplex fluorescent development kit | Advanced Cell Diagnostics | Cat no. 320850 | |
| Commercial assay or kit | BaseScope Probe - BA-Hs-MRGPRX1-1zz-st | Advanced Cell Diagnostics | Cat no. 717091 | |
| Commercial assay or kit | BaseScope Probe BA-Hs-MRGPRD-1zz-st | Advanced Cell Diagnostics | Cat no. 878101-C2 | |
| Commercial assay or kit | BaseScope Duplex Reagent Kit | Advanced Cell Diagnostics | Cat no. 323800 | |
| Chemical compound, drug | β-Alanine (ALA) | Sigma-Aldrich | 146064-25G, A9920-100G | |
| Chemical compound, drug | Histamine dihydrochloride (HIS) | Sigma-Aldrich | H7250-5G | |
| Chemical compound, drug | Ketamine hydrochloride | Phoenix Pharmaceutical, St. Louis, MO | ||
| Chemical compound, drug | Sodium pentobarbital | Akorn Pharmaceutical, Lake Forest, IL | ||
| Chemical compound, drug | Cefazolin | West-Ward Pharmaceutical Corp, Eatontown, NJ | ||
| Chemical compound, drug | Buprenorphine SR | ZooPharm, Laramie, WY | ||
| Software, algorithm | Data acquisition processing system (DAPSYS), version 8 | Brian Turnquist (Bethel University) Sikand et al., 2011a; Wooten et al., 2014 | http://www.dapsys.net/ | |
| Software, algorithm | SigmaPlot 10 | Systat Software Inc, San Jose, CA | ||
| Software, algorithm | Statistica, version 13 | Tibco | ||
| Software, algorithm | Corel Draw (version 9.439 or 2020) | Corel Corporation, Ottawa, Canada | ||
| Software, algorithm | Adobe Illustrator | Adobe, San Jose, CA | ||
| Software, algorithm | ImageJ | NIH, Bethesda, MD | ||
| Software, algorithm | T-Coffee | EMBL-EBI, Hinxton, UK | https://www.ebi.ac.uk/Tools/msa/tcoffee/ | |
| Software, algorithm | FastTree 2.1 | Price et al., 2010 | https://usegalaxy.eu/ | |
| Software, algorithm | Newick Display | Dress et al., 2008 | https://usegalaxy.eu/ |
Additional files
-
Supplementary file 1
Expression of MRGPRD and MRGPRX1 was assessed in three macaque dorsal root ganglions (DRGs) using double-labeling in situ hybridization (ISH).
The number of single- and double-positive neurons is given as aggregated number for each DRG.
- https://cdn.elifesciences.org/articles/64506/elife-64506-supp1-v1.docx
-
Supplementary file 2
Statistical analysis of areas (cm2) of alloknesis, hyperalgesia, hyperknesis, wheal and flare, or local erythema evoked by injection of a bovine adrenal medulla peptide 8–22 (BAM8-22), β-alanine (ALA), histamine (HIS), a combination of BAM8-22 and ALA, and a combination of BAM8-22 and ALA and HIS.
*Bonferroni post hoc p-values compared to HIS. **Bonferroni post hoc p-values compared to BAM8-22 + ALA + HIS. Units are in cm2 and calculated as mean ± standard error of the mean (SEM). Data from all 29 subjects were included in the analysis.
- https://cdn.elifesciences.org/articles/64506/elife-64506-supp2-v1.docx
-
Supplementary file 3
Protein sequences used for phylogenetic tree construction were fetched from Ensembl.
EnsemblGeneIDs, gene names, and the species of the gene are shown.
- https://cdn.elifesciences.org/articles/64506/elife-64506-supp3-v1.docx
-
Supplementary file 4
Clinical data for human dorsal root ganglion (DRG) donors.
DRG tissue was obtained from Tissue For Research (donors 1 and 2) and the NIH NeuroBioBank (donors 3 and 4). Basic clinical data for each donor are summarized.
- https://cdn.elifesciences.org/articles/64506/elife-64506-supp4-v1.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/64506/elife-64506-transrepform-v1.docx





