Urocortin-3 neurons in the mouse perifornical area promote infant-directed neglect and aggression
Figures
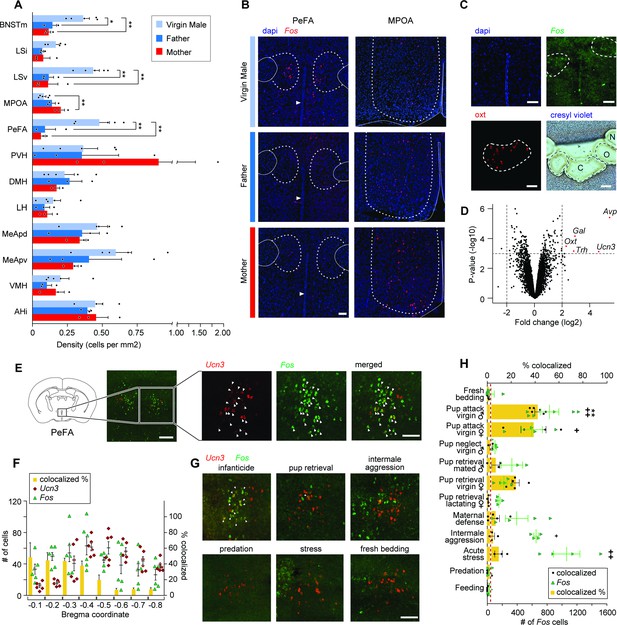
The neuropeptide gene urocortin-3 (Ucn3) in the perifornical area (PeFA) marks neurons activated during infanticide.
(A) Quantification of Fos+ cells per mm2 in brain areas of interest (oriented from top to bottom, rostral to caudal) in virgin males (attacking), mated males (parental), and mated females (parental) after pup exposure. One-way ANOVA followed by Tukey’s multiple comparison test reveals significant differences in the number of Fos+ cells identified between parental and infanticidal animals in medial bed nucleus of stria terminalis (BNSTm) (F2,8 = 12.47, p<0.0035), ventral lateral septum (LSv) (F2,8 = 14.31, p<0.0023), medial preoptic area (MPOA) (F2,8 = 8.536, p<0.0104), and PeFA (F2,8 = 12.02, p<0.0039) (virgin male n = 5; fathers n = 3; mothers n = 3). (B) Representative in situ hybridization images of Fos (red channel) expression in PeFA and MPOA after animal exposure to pups with counterstain DAPI (blue channel) (scale bar: 100 µm). Dotted lines in right and left denote the boundary of brain regions of interest (PeFA and MPOA, respectively), solid lines denote the fornix, arrowheads indicate location of third ventricle in the left panels, and solid lines denote the edge of the brain and third ventricle in the right panel. (C) Laser-capture microscopy strategy to identify markers of cells activated during infanticide (scale bar 100 µm). Tissue material corresponding to areas labeled by Fos (C) after pup-mediated attack, as well as oxytocin+ (Oxt) (O) and adjacent negative (N) area was laser-dissected and the corresponding transcripts characterized by microarray analysis. (D) Volcano plot showing the fold change (log base 2) against the p-value (log base 10) for all genes. Transcripts with greater than a twofold change in expression in the Fos+ compared to negative region and a p-value less than 0.001 are shown in red: vasopressin (Avp), thyrotropin-releasing hormone (Trh), galanin (Gal), Oxt, and Ucn3 (n = 3 biological replicates, each containing pooled tissue from six males). (E) Representative in situ hybridization showing colocalization of Ucn3 (red) and Fos (green) expression after infanticide (scale bar 100 µm), arrowheads indicate colocalization. (F) Plot depicting number (#) of neurons expressing Fos (green triangles), Ucn3 (red circles) and percent of cells expressing both Fos and Ucn3 (yellow bars) per brain section across Bregma coordinates (n = 6) indicate high colocalization in rostral PeFA. Overall representation of Ucn3+ neurons in the PeFA (2.74%) is indicated by the red line. (G) Representative in situ hybridization showing expression of Ucn3 (red) and Fos (green) after infanticide and other behaviors in the rostral PeFA, with colocalization (white arrowheads) only observed after infanticide (scale bar 100 µm). (H) Percentage of Ucn3+ cells colocalized with Fos after various behaviors (yellow bars; *significance) and number of Fos cells induced by various behaviors across sections of the rostral PeFA (green triangles; significance #) (n = 3–6/group). Kruskal–Wallis test followed by Dunn’s multiple comparisons test reveals significant differences in number of Fos+ cells in rostral PeFA between fresh bedding control exposure and pup attack in virgin males and virgin females, intermale aggression, and acute stress (#p<0.0001); yet, despite the observed widespread activation of PeFA cells during various behaviors, only pup-directed attacks by virgin males induced significant Fos expression in PeFAUcn3 neurons (*p<0.0002).
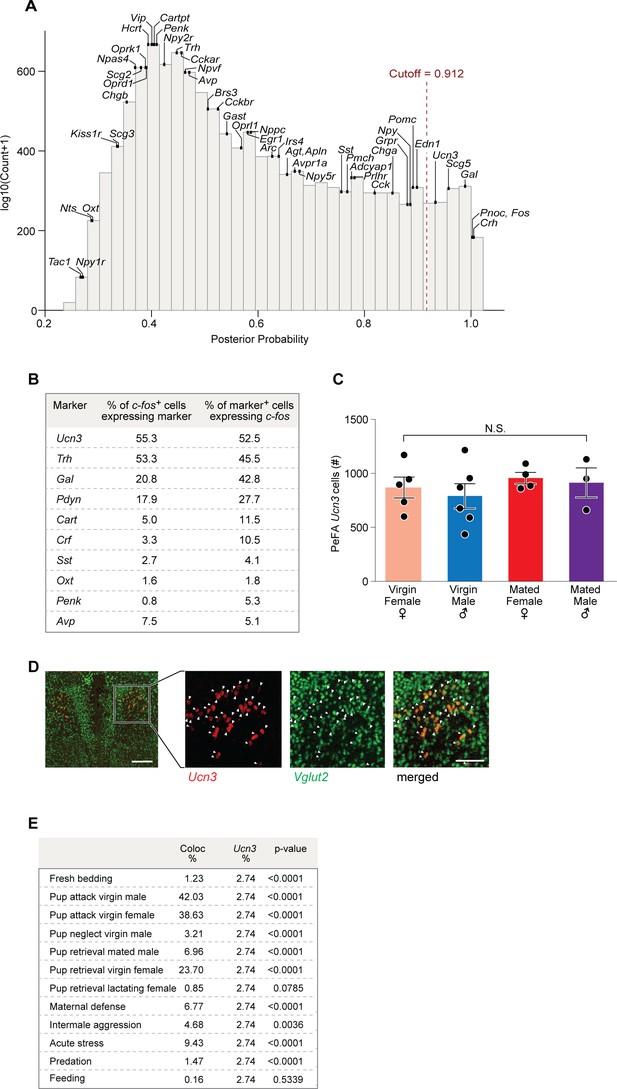
Expression of halorhodopsin in PeFA urocortin-3 neurons.
(A) Distribution of the posterior probabilities of expression levels expressed as log10(Counts) in immunoprecipitated (IP) minus input in infanticidal males exposed to pups compared to males exposed to fresh bedding. Vertical, red-dashed line marks the empirically defined significant posterior-probability cutoff. Expression of neuropeptides and their receptors is indicated by their posterior probabilities. (B) Relative enrichment in infanticide-induced Fos and candidate marker gene expression in perifornical area (PeFA) neurons. (C) Analysis of the number of urocortin-3 (Ucn3+) neurons in the PeFA among virgin and mated males and females reveals no significant difference. (D) Representative in situ hybridization images showing Ucn3 (red) co-expression with Vglut2 (green) in the PeFA (scale bar 100 µm), arrowheads indicate colocalization. (E) Colocalization of Fos and Ucn3 expression (Coloc %) relative to the representation of Ucn3+ neurons in the total pool of PeFA neurons (Ucn3).
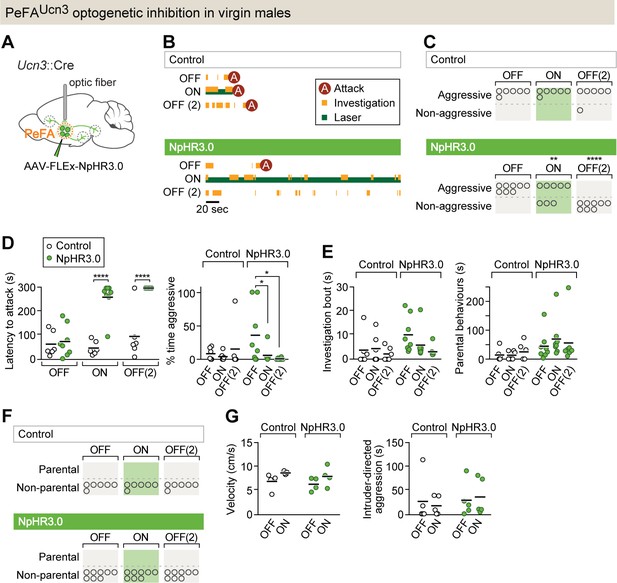
PeFAUcn3 neurons are required for infant-directed aggression in virgin males.
(A) Setup for optogenetic silencing of PeFAUcn3 neurons in infanticidal virgin males. (B) Representative behavior trace of a control and a NpHR3.0-expressing male across three consecutive sessions (scale bar 20 s of 5 min test). (C) Numbers of control and NpHR3.0-expressing males showing aggressive pup-directed behavior (accelerated failure regression model with first session as time-point baseline and controls as treatment baseline, significant difference between control (n = 6) and NpHR3.0 (n = 8) during laser inhibition p<0.01 and during second laser OFF session p<0.0005). (D, left) Pup-directed attack latency across sessions comparing control (open dots) and NpHR3.0 (green dots)-infected males. Two-way repeated measures ANOVA control vs. NpHR3.0 F(1,12) = 35.05, p<0.0001, sessions F(2,24) = 22.71, p<0.0001, and interaction F(2,24) = 17.30, p<0.0001 followed by Bonferroni’s multiple comparisons test. (Right) Percentage of time displaying aggression toward pups across three sessions in control (open dots) and NpHR3.0 (green dots) males. Two-way repeated measures ANOVA showed no main effect while post-hoc Tukey’s multiple comparisons test revealed significant differences between sessions in the NpHR3.0 group. (E, left) Pup investigation bout length across three sessions in control and NpHR3.0 males. (Right) Duration of parental care behaviors across three sessions in control and NpHR3.0 males. (F) Number of males in control and NpHR3.0-expressing groups displaying parental behaviors. (G, left) Locomotion velocity in control and NpHR3.0-expressing males with and without laser stimulation. (Right) Duration of aggressive displays during adult male interactions in control and NpHR3.0-expressing males with and without laser stimulation.
Representative image of AAV-mediated NpHR3.0 expression in the perifornical area (PeFA) of Ucn3::Cre males (scale bar 100 µm).
Fornix indicated by outline, arrowhead indicates ventricle, and f denotes fiber tract.
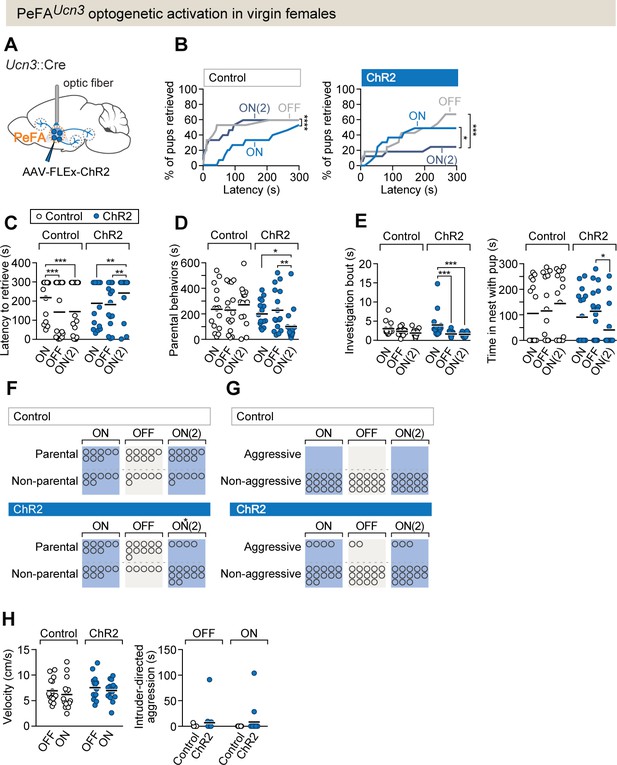
PeFAUcn3 optogenetic activation in virgin females suppresses parental behavior.
(A) Setup for optogenetic activation of PeFAUcn3 neurons in virgin female mice. (B, left) Cumulative retrieval of pups in virgin females injected with a control virus (n = 15). Friedman statistic(3, 27) = 41.84 followed by Dunn’s multiple comparisons tests. (Right) Cumulative retrieval of pups in virgin females injected with conditional virus expressing ChR2 (n = 16). Friedman statistic (3, 24) = 17.19, followed by Dunn’s multiple comparisons test. (C) Latency to retrieve in control (open dots) and ChR2-expressing (blue dots) females across three sessions. Two-way repeated measures ANOVA reveals no main effect of virus treatment F1-29 = 0.7892, but significant effects of session F2,58 = 6.181 (p=0.0037) and interaction F2,58 = 13.42 (p<0.0001). Post-hoc analysis by Bonferroni’s multiple comparisons. (D) Duration of parental behaviors displayed by control (open dots) and ChR2-expressing (blue dots) females. Two-way repeated measures ANOVA reveals no main effect of virus F1−29 = 2.721 or session F2,58 = 1.178, but a significant main interaction effect F2,58 = 4.747 (p=0.0123) followed by Tukey’s post-hoc analysis. (E, left) Duration of time spent investigating pups in a single bout across three sessions in control (open dots) versus ChR2 (blue dots) females. Two-way repeated measures ANOVA reveals no significant main effect of virus treatment F1-29 = 1.637 or session F2,58 = 0.6324, but a significant interaction effect F2,58 = 3.746 (p=0.0295). Post-hoc analysis by Tukey’s multiple comparisons test. (Right) Time spent in the nest with pups. Two-way repeated measures ANOVA shows a significant main interaction effect F2,58 = 3.746 (p<0.03). Post-hoc analysis by Tukey’s multiple comparisons test. (F) Number of females displaying parental behaviors across three sessions in control and ChR2 females (accelerated failure regression model with first session as time-point baseline and control as treatment baseline, significant difference between control and ChR2 females in second laser ON session p<0.04). (G) Number of females in control or ChR2 groups displaying aggressive behaviors toward pups. (H, left) Locomotion velocity in control or ChR2 females with or without laser stimulation is not significantly affected. (Right) Aggression toward an adult male intruder is unaffected.
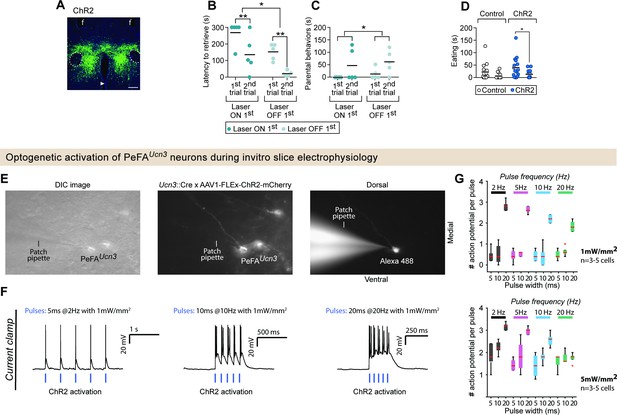
Optogenetic stimulation of PeFA urocortin-3 expressing neurons leads to behavioral and physiological effects.
(A) Representative image of viral-mediated ChR2 expression in the perifornical area (PeFA) of Ucn3::Cre virgin females (scale bar 100 µm). Fornix indicated by outline, arrowhead indicates ventricle, and f denotes fiber tract. (B) Latency to retrieve pups in virgin females injected with ChR2 in either laser ON first conditions (dark green dots; n = 5) or laser OFF first conditions (light green dots; n = 5). Two-way repeated measures ANOVA reveals a significant main effect of laser order F1,8 = 8.235, p<0.02 and session F(1,8) = 39.16, p<0.0002, Sidak’s multiple comparisons test for ON first condition and OFF first condition significant. (C) Parental behavior duration also showed a significant main effect of session F1,8 = 6.519, p<0.034 with no significant post-hoc effects. (D) Eating duration of an appetitive food was significantly altered with laser treatment (two-way repeated measures ANOVA F1,29 = 8.504, p<0.0068, Bonferroni’s multiple comparisons ON vs. OFF for ChR2 group). (E) Raw images depicting (left) DIC, (middle) PeFAUcn3 neurons labeled with mCherry (Ucn3::Cre injected with AAV1-eF1a-DoubleFlox hChR2(H134R)-mCherry-WPRE-HgHpA, 8–12-week-old male mice, n = 2) and (right) a recorded PeFAUcn3 neuron filled with Alexa 488 hydrazide. (F) Single traces showing activation of ChR2-positive PeFAUcn3 neurons for (left) 5 ms light pulses at 2 Hz and 1 mW/mm2, (middle) 10 ms light pulses at 10 Hz and 1 mW/mm2, (left) 20 ms light pulses at 20 Hz and 1 mW/mm2 from a custom-built 465 nm (1–5 mW/mm2, CBT-90-B-L11, Luminus) light source. (G) Summary data showing the activation of ChR2-positive PeFAUcn3 neurons (n = 5 neurons) in response to different pulse widths (5, 10, 20 ms) for four different stimulation frequencies; 2 Hz (black), 5 Hz (pink), 10 Hz (blue), and 20 Hz (green) for two different stimulation intensities of 1 mW/mm2 (top) and 5 mW/mm2 (bottom).
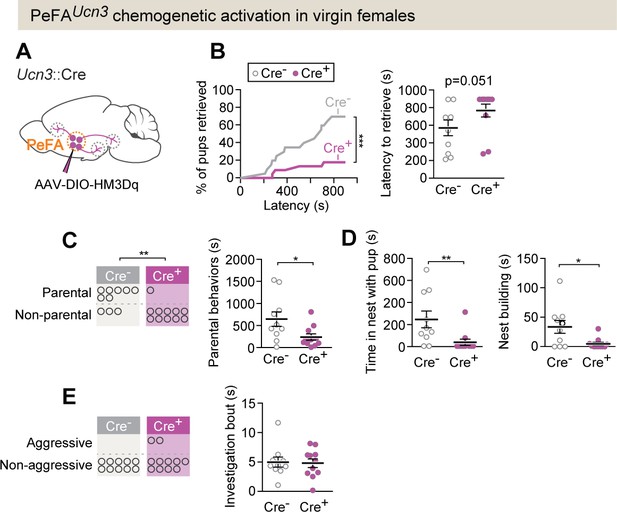
PeFAUcn3 chemogenetic activation in virgin females suppresses parental behavior.
(A) Setup for chemogenetic activation of PeFAUcn3 neurons in virgin females. (B, left) Cumulative retrieval in Cre+ (n = 11) or Cre- control (n = 10) females. Kolmogorov–Smirnov test for significance p<0.0001. (Right) Latency to retrieve first pup, Mann–Whitney test p=0.051. (C, left) Number of females in Cre + or Cre- (control) displaying parental behaviors. Significance determined by Fisher’s exact test (p=0.0075). (Right) Duration of parental behaviors, t-test p<0.0291. (D, left) Duration in nest, Mann–Whitney test, p=0.0039. (Right) Nest building, Mann–Whitney test p=0.0422. (E, left) Number of females in Cre + or Cre - (control) displaying infant-directed aggression. (Right) Investigation bout length is unaffected.
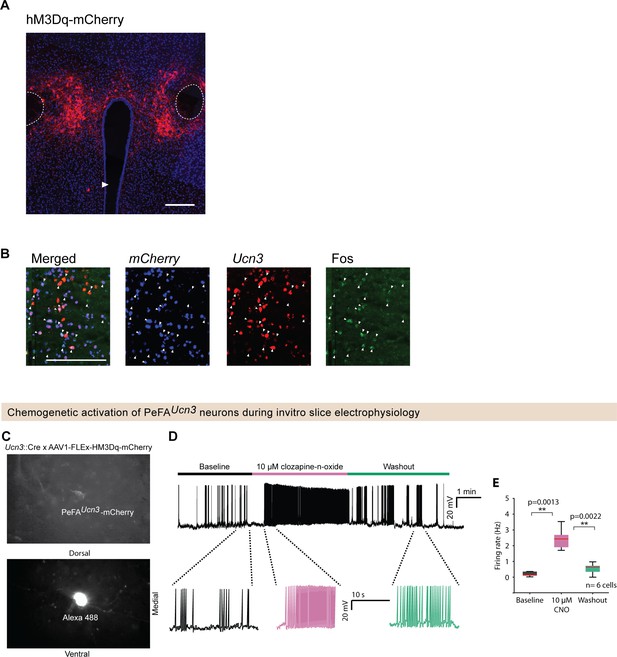
Chemogenetic stimulation of PeFA urocortin-3 expressing neurons significantly increases cell firing rate.
(A) Representative fluorescence image of excitatory DREADD (hM3Dq) viral-mediated expression in the perifornical area (PeFA) of a Ucn3::Cre virgin female (scale bar 100 µm) Fornix indicated by outline, arrowhead indicates ventricle. (B) Representative in situ hybridization images of mCherry, Ucn3, and Fos in a Ucn3::Cre virgin female 30 min after treatment with 0.3 mg/kg clozapine-n-oxide (CNO) (scale bar 100 µm), arrowheads indicate colocalization. (C) Fluorescence images showing (top) PeFAUcn3 neurons labeled with mCherry (Ucn3::Cre male mice injected with pAAV-hSyn-DIO-hM3D(Gq)-mCherry) and (bottom) a recorded PeFAUcn3 neuron filled with Alexa 488 hydrazide. (D) Example trace of a PeFAUcn3 neuron with bath application of CNO (3–5 min of baseline followed by 3–5 min of bath application of 10 μM CNO and 3–5 min of washout of CNO). (E). Summary plot showing significant increase in the firing rate of the neurons (p=0.0013 and 0.0022 compared to baseline and washout, Wilcoxon rank-sum method) in response to application of CNO (six cells for 8–12-week-old male mice, n = 2 animals).
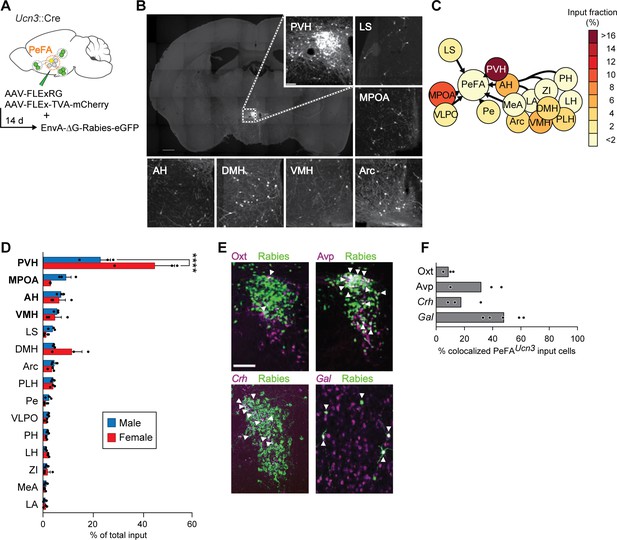
Inputs of PeFAUcn3 neurons.
(A) Schematic of monosynaptic retrograde tracing from PeFAUcn3 neurons. (B) Representative images of labeled monosynaptic inputs (scale bar 200 µm). (C) Heatmap of input fractions by brain area in virgin males. (D) Quantification of input fraction in virgin males (n = 3) and virgin females (n = 3). Regions are ranked by effect size largest (top) to smallest (bottom) relative to values observed in males using a regression analysis. Paraventricular hypothalamus (PVH) and medial preoptic area (MPOA), and to a lesser extent amygdalohippocampal area (AH) and ventromedial hypothalamus (VMH), showed significantly enriched input to the PeFAUcn3 neurons compared to chance (p<0.0001, indicated in bold), post-hoc test significant for PVH male vs. female. (E) Representative images of immunofluorescence (Oxt, Avp) or in situ hybridization (corticotropin-releasing hormone Crh, Gal) co-staining with Rabies-eGFP (scale bar, 200 µm), arrowheads indicate colocalization. (F) Percentages of presynaptic eGFP-positive neurons expressing Oxt, Avp, Crh, or Gal (n = 3–5/group). MPOA input neurons largely colocalize with galanin expression (48.02% ± 5.63%).
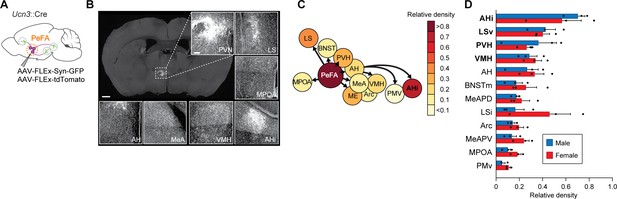
Projections of PeFAUcn3 neurons.
(A) Anterograde tracing strategy. (B) Representative images of synaptophysin densities in projection areas (scale bar 200 µm). (C) Heatmap of relative densities of PeFAUcn3 projections. (D) Quantification of relative densities of PeFAUcn3 projections in males (n = 3) and females (n = 3), regression analysis reveals significant enrichment of amygdalohippocampal area (AHi), ventral lateral septum (LSv), paraventricular hypothalamus (PVH), and ventromedial hypothalamus (VMH) projections compared to chance (indicated in bold).
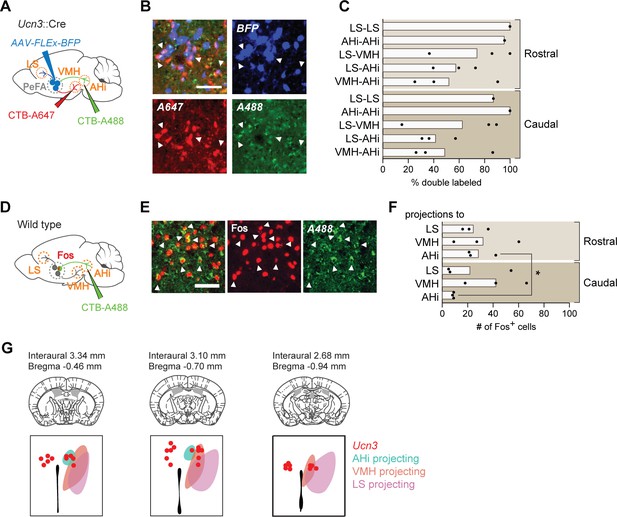
Projection tracing reveals bifurcating but behaviorally distinguishable sub-populations of PeFAUcn3 neurons.
(A) Schematic of retrograde cholera toxin B (CTB) tracing combined with PeFAUcn3 reporter. (B) Representative image of CTB experiment (scale bar 100 µm). (C) Quantification of Ucn3+ neurons labeled with CTB. (D) Schematic of CTB tracing combined with Fos staining in infanticidal males. (E) Representative image of CTB and Fos labeling in perifornical area (PeFA) after CTB injection in amygdalohippocampal area (AHi). (F) Quantification of number of Fos-positive cells labeled with CTB in the rostral and caudal PeFA after CTB injection in a given projection (n = 3/group). Rostral versus caudal percentages are significantly different for AHi-projecting neurons (paired t-test p<0.03). (G) Schematic of topographical arrangement of PeFAUcn3 neurons in PeFA according to their projection sites.
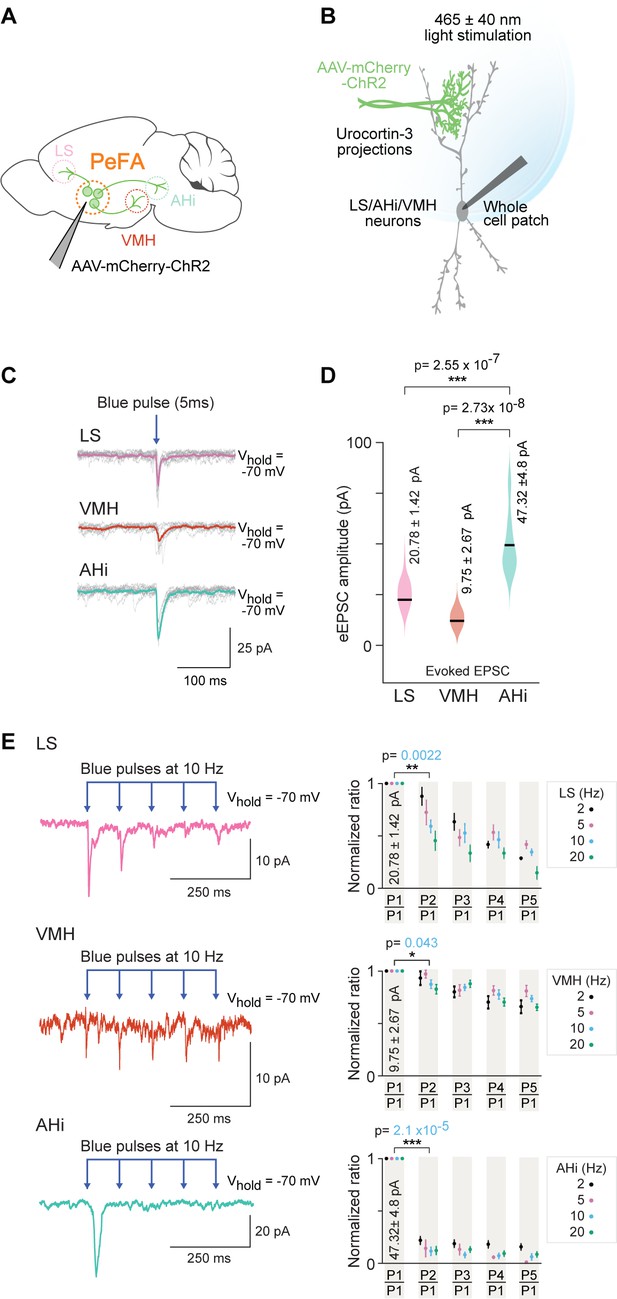
Optogenetic investigation of functional connectivity between PeFAUcn3 neurons and specific targets.
(A) Experimental design depicting selective targeting of PeFAUcn3 with ChR2 (Ucn3::Cre injected with AAV1-eF1a-DoubleFlox hChR2(H134R)-mCherry-WPRE-HgHpA, 8–12-week-old male mice, n = 8, five males and three females). (B) Schematic depicting in vitro slice electrophysiological recordings of neurons in lateral septum (LS), ventromedial hypothalamus (VMH), and amygdalohippocampal area (AHi) slices in response to optogenetic activation of PeFAUcn3 projections labeled with ChR2. (C) Electrophysiological responses to activation of PeFAUcn3 projections (single light pulse: 5 ms duration, 465 nm at 5 mW/mm2) for 10 trials with average traces shown in pink (LS), orange (VMH), and green (AHi). (D) Summary plot showing significantly higher amplitude of evoked excitatory post-synaptic currents (eEPSCs) in neuronal populations of AHi (green) compared to LS (pink) (Wilcoxon rank-sum test, p<0.0001) and VMH (orange) (Wilcoxon rank-sum test, p<0.0001). (E) Electrophysiological trace of an LS neuron (top left), a VMH neuron (middle left), and an AHi neuron (bottom left) (mean response of five consecutive trials) depicting paired pulse depression of EPSCs in response to activation of PeFAUcn3 projections (light pulses: 5 ms duration, 465 nm at 10 Hz). Summary plots showing paired pulse depression of optogenetic activation of PeFAUcn3 projections on LS neurons (top right, Wilcoxon rank-sum test, p=0.0022), VMH neurons (middle right, Wilcoxon rank-sum test, p=0.043), and AHi neurons (bottom right, Wilcoxon rank-sum test, p<0.001) as a function of stimulation frequency.
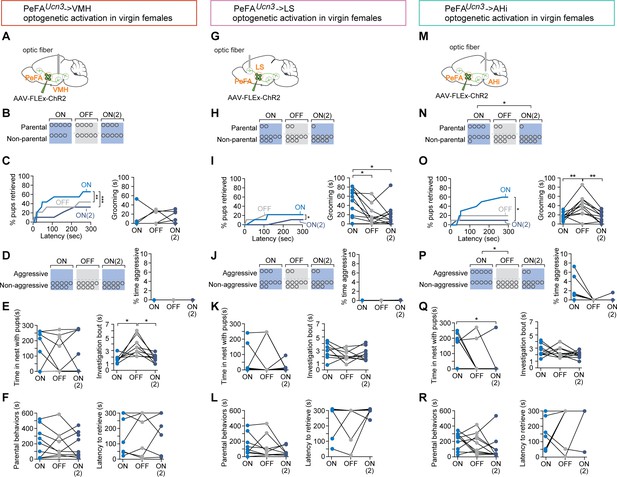
Specific projections from PeFAUcn3 neurons mediate infant-directed neglect and aggression.
(A) Optogenetic stimulation of PeFAUcn3 to ventromedial hypothalamus (VMH) fiber terminals. (B) Number of females showing parental behavior. (C, left) Cumulative retrieval of pups is significantly different among groups (Friedman statistic (3-15) = 19.57, p<0.0001; n = 9). Dunn’s multiple comparisons test reveals difference between ON vs. ON2 (***) and OFF vs. ON2 (*). (Right) One-way repeated measures ANOVA revealed a significant main effect in investigation bout length F(1, 11) = 10.12 (p<0.005) among groups. Tukey’s multiple comparisons revealed differences between ON vs. OFF (*) and OFF vs. ON2 (*). (D, left) Number of females showing pup-directed aggression and (right) percent time displaying aggression show no differences across sessions. (E, left) Time in the nest with pups and (right) grooming were not altered by PeFAUcn3 to VMH fiber stimulation. (F, left) Duration of parental behaviors and (right) latency to retrieve pups were not changed. (G) Optogenetic stimulation of PeFAUcn3 to lateral septum (LS) terminals. (H) Number of females showing parental behavior is not affected (n = 10). (I, left) Cumulative retrieval of pups is suppressed and significantly affected over the course of the experiment analyzed by Friedman test, Friedman statistic (3-7) = 10.21 (p<0.0041). Dunn’s multiple comparisons test reveals significant differences in OFF vs. ON2 comparison (*). (Right) One-way repeated measures ANOVA reveals that grooming is significantly reduced over the course of the experiment F2-16 = 6.368 (p<0.0102). Tukey’s multiple comparisons test reveals significant differences between ON vs. OFF (*) and ON vs. ON2 (*). (J, left) Number of females showing aggression and (left) time spent aggressive toward pups is not changed. (K, left) Time in the nest and (right) investigation bout length are not affected. (L, left) Parental behaviors and (right) latency to retrieve are unaffected. (M) Schematic of optogenetic stimulation of PeFAUcn3 to amygdalohippocampal area (AHi) terminal stimulation. (N) Number of females showing parental behaviors was significant reduced between ON vs. ON2 conditions (chi-square p<0.0191). (Right) One-way repeated measures ANOVA revealed reversibly reduced grooming by stimulating of PeFAUcn3 to AHi axonal terminals F2,18 = 11.40 (p<0.0006). Tukey’s multiple comparisons test showed differences between ON vs. OFF (**) and OFF vs. ON2 (**). (O, left) Friedman test reveals that cumulative retrieval of pups is significantly affected (p<0.0095). Dunn’s multiple comparisons shows significant differences between ON vs. ON2 (*). (Right) Time spent in the nest with pups was significantly reduced between ON vs. ON2 conditions (repeated measures ANOVA F2,14 = 4.297, p<0.0351, followed by Tukey’s post-hoc test). (P, left) Number of females showing aggressive behaviors toward pups is significantly different between laser ON and laser OFF groups (chi-square test p<0.02; n = 10). (Right) Friedman test reveals a significant difference in the time spent displaying aggressive behavior over the course of the experiment (p<0.0247). (Q, left) Time spent in the nest with pups was significantly reduced between ON vs. ON2 conditions (repeated measures ANOVA F(2,14) = 4.297, p<0.0351, followed by Tukey’s post-hoc test) (right) and investigation bout length were unaffected. (R, left) Duration of parental behaviors and (right) latency to retrieve were unchanged across sessions.
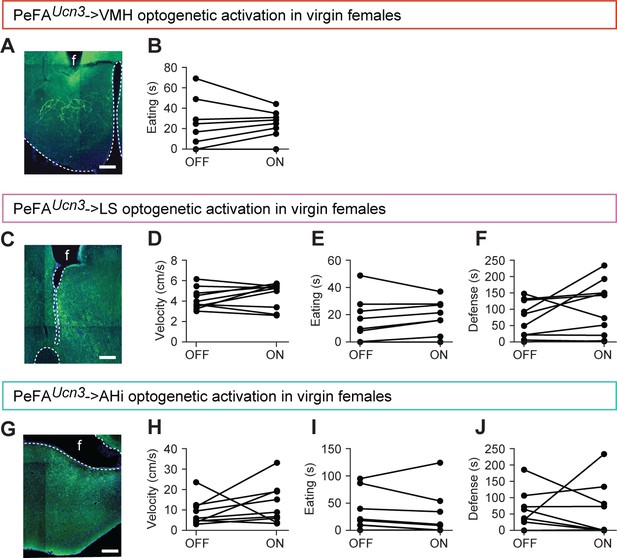
Optogenetic stimulation of PeFA urocortin-3 expressing neuronal projections does not impact locomotion, feeding, or inter-adult defensive behaviors.
(A) Representative image of viral-mediated ChR2 expression in Ucn3::Cre virgin females with fiber implant (f) directed toward the ventromedial hypothalamus (VMH), scale bar 100 µm, ventricle outlined. (B) Time spent eating a cookie was not affected by laser condition (n = 8). (C) Representative image of viral-mediated ChR2 expression in Ucn3::Cre virgin females with fiber implant (f) directed toward the lateral septum (LS), scale bar 100 µm, ventricle outlined. (D) Velocity in locomotion test (n = 10). (E) Time spent eating a cookie (n = 9). (F) Time spent showing defensive behavior toward a castrated male intruder swabbed with intact male urine (n = 10). (G) Representative image of viral-mediated ChR2 expression in Ucn3::Cre virgin females with fiber implant (f) directed toward the amygdalohippocampal area (AHi), scale bar 100 µm, ventricle outlined. (H) Velocity in locomotion test (n = 9). (I) Time spent eating a cookie (n = 9). (J) Time spent showing defensive behavior toward a castrated male intruder swabbed with intact male urine (n = 9).
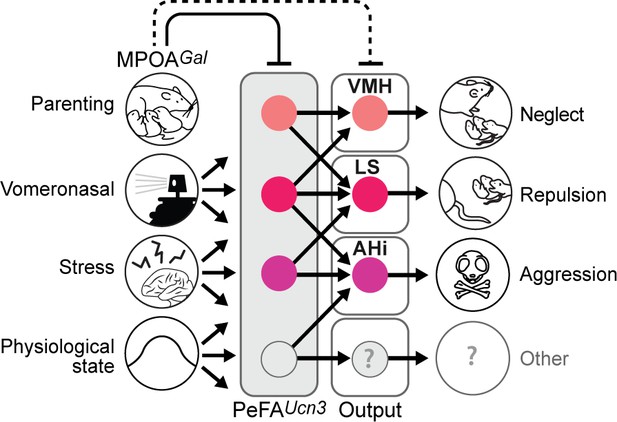
Model of the organization and function of PeFAUcn3 neurons in infant-mediated neglect and aggression.
PeFAUcn3 neurons receive input from brain areas involved in processing social cues and physiological state, stress, as well as parenting control. In turn, PeFAUcn3 neurons send bifurcating projections to major targets such as the amygdalohippocampal area (AHi), lateral septum (LS), and ventromedial hypothalamus (VMH). Stimulation of these projections specifically impacts various aspects and degrees of pup-directed agonistic behavior from avoidance (VMH), repulsion (LS), to aggression (AHi).
Videos
Optogenetic stimulation of PeFAUcn3 neurons in a virgin female mouse during pup-directed behavior.
A newborn pup is presented to a female in the home cage. Optogenetic stimulation is indicated by the white dot in the upper center of the frame. In a subset of females, we observe tail rattling behavior toward the pup during light illumination as seen in this example.
Optogenetic stimulation of PeFAUcn3 neuronal projections to the ventromedial hypothalamus in a virgin female mouse during pup-directed behavior.
Most females showed reduced interaction with the pup during light illumination as seen here.
Optogenetic stimulation of PeFAUcn3 neuronal projections to the lateral septum in a virgin female mouse during pup-directed behavior.
Most females showed avoidance of the pup during light illumination, and in a subset of females we observed escape-like behavior upon light stimulation as in this example.
Optogenetic stimulation of PeFAUcn3 neuronal projections to the amygdalohippocampal area in a virgin female mouse during pup-directed behavior.
Most females showed reduced parental behavior toward pups, and a subset of females displayed pup-directed aggression such as aggressively carrying the pups around the cage without retrieving upon light illumination as in this example.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (mouse, male and female, crossed to C57) | Ucn3::Cre | MMRRC | Mmcd 032078-UCD | Resuscitated in lab from frozen sperm |
| Recombinant DNA reagent (AAV2/1) | AAV1-EF1a-DIO-eNpHR3.0-EYFP | Addgene | RRID:Addgene_26966-AAV1 | |
| Recombinant DNA reagent (AAV2/1) | AAV1-EF1a-DIO-EYFP | Addgene | RRID:Addgene_27056-AAV1 | |
| Recombinant DNA reagent (AAV2/1) | AAV1-EF1a-DIO-hChR2(H134R) | Wu et al., 2014 (gift of Karl Deisseroth) | ||
| Recombinant DNA reagent (AAV2/1) | AAV1-dio-hM3Dq-mCherry | Kohl et al., 2018 | ||
| Recombinant DNA reagent (AAV2/1) | AAV1-FLEx-RG | Kohl et al., 2018 | ||
| Recombinant DNA reagent (AAV2/1) | AAV1-FLEx-TVA-mCherry | Kohl et al., 2018 | ||
| Recombinant DNA reagent (AAV2/1) | AAV1-FLEx-synaptophysin-eGFP | Kohl et al., 2018 | ||
| Recombinant DNA reagent (AAV2/1) | AAV1-FLEx-tdTomato | Kohl et al., 2018 | ||
| Recombinant DNA reagent (EnvA g-deleted rabies) | EnvA deltaG Rabies-eGFP | Salk Vector Core | ||
| Antibody | Anti-C-Fos (rabbit polyclonal) | Synaptic Systems | (1:2000) | |
| Antibody | Anti-oxytocin (rabbit polyclonal) | Immunostar | (1:1000) | |
| Antibody | Anti-vasopressin (rabbit polyclonal) | Immunostar | (1:1000) | |
| Antibody | Anti-GFP (rabbit polyclonal) | Novus Biologicals | (1:2000) | |
| Antibody | Anti-phospho-S6 (rabbit polyclonal) | Life Technologies | Cat# 44923G | (4 µg/IP) |
| Other | CTB-A488 | Molecular Probes | Cat# C34775 | (0.5% wt/vol) |
| Other | CTB-A647 | Molecular Probes | Cat# C34778 | (0.5% wt/vol) |
| Software, algorithm | Ethovision XT 8.0 | Noldus Technology | ||
| Software, algorithm | Observer XT 11 | Noldus Technology | ||
| Software, algorithm | BRAIM | https://gitlab.com/dulaclab/ucn3_neuron_microarray/-/tree/master/braimSourceCode/braim.R | ||
| Software, algorithm | Microarray analysis | https://gitlab.com/dulaclab/ucn3_neuron_microarray |





