Increased theta/alpha synchrony in the habenula-prefrontal network with negative emotional stimuli in human patients
Figures
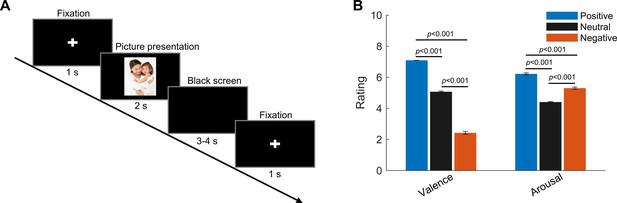
Experimental paradigm and ratings (valence and arousal) of the presented pictures.
(A) Timeline of one individual trial: each trial started with a white cross (‘+’) presented with black background for 1 s, indicating the participants to get ready and pay attention; then a picture was presented in the center of the screen for 2 s. This was followed by a blank black screen presented for 3–4 s (randomized). (B) Valence and arousal ratings for figures of the three emotional categories presented to the participants. Valence: 1 = very negative; 9 = very positive; arousal: 1 = very clam; 9 = very exciting. Error bars indicate the standard deviation of the corresponding mean across participants (N = 9).
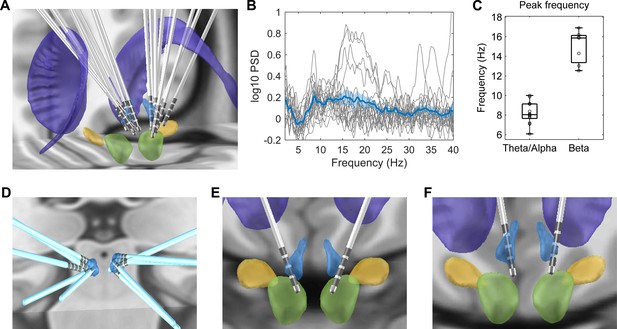
Electrode location and spectral characteristics of local field potentials from recorded habenula at rest.
(A) Electrode locations reconstructed using Lead-DBS, with the structures colored in light blue for the habenula, purple for the caudate nucleus, light green for the red nucleus, and yellow for subthalamic nucleus. (B) The log-transformed oscillatory power spectra fitted using fooof method (after removing the non-oscillatory 1/f components). The bold blue line and shadowed region indicates the mean ± SEM across all recorded hemispheres, and the thin gray lines show measurements from individual hemispheres. (C) Boxplot showing the peak frequencies at theta/alpha and beta frequency bands from all recorded habenula. (D) Positions of the electrodes with theta peaks only during rest. (E) Electrode positions for Case 3, in whom only beta band peaks were detected in the resting activities from both sides. (F) Electrode positions for Case 6, in whom both theta and beta band peaks were present in resting activities from both sides.
-
Figure 2—source data 1
Source data for generating Figure 2B, C.
- https://cdn.elifesciences.org/articles/65444/elife-65444-fig2-data1-v1.mat
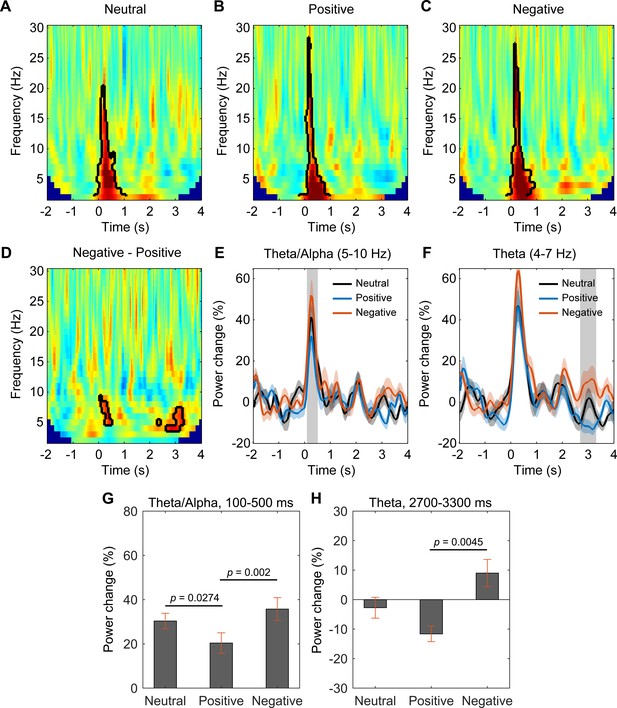
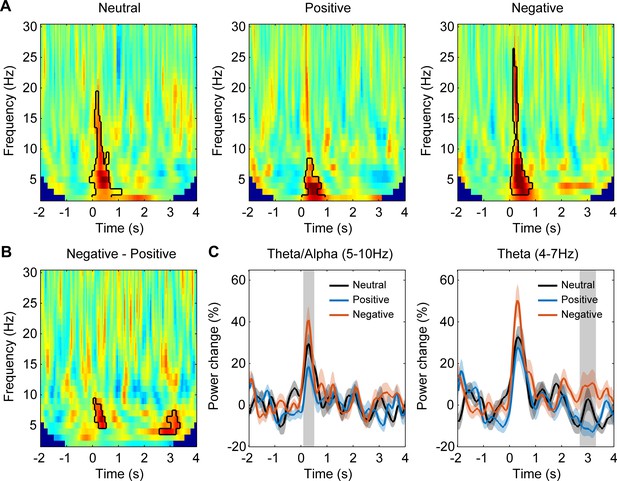
Habenular theta/alpha activity is differentially modulated by stimuli with positive and negative emotional valence (N = 18 habenula local field potential samples from nine subjects).
(A–C) Time-frequency representations of the power response relative to pre-stimulus baseline (−2000 to −200 ms) for neutral (A), positive (B), and negative (C) valence stimuli, respectively. Significant clusters (p<0.05, non-parametric permutation test) are encircled with a solid black line. (D) Time-frequency representation of the power response difference between negative and positive valence stimuli, showing significant increased activity of the theta/alpha band (5–10 Hz) at short latency (100–500 ms) and another increased theta activity (4–7 Hz) at long latencies (2700–3300 ms) with negative stimuli (p<0.05, non-parametric permutation test). (E, F) Normalized power of the activities at theta/alpha (5–10 Hz) and theta (4–7 Hz) band over time. Significant difference between the negative and positive valence stimuli is marked by a shadowed bar (p<0.05, t-test corrected for multiple comparison). (G, H) The average spectral power relative to baseline activity in the identified time period and frequency band for different emotional valence conditions (5–10 Hz, 100–500 ms; 4–7 Hz, 2700–3300 ms). Significant difference was observed in theta/alpha power at 100–500 ms between neutral and positive condition (t-value = 2.4312, p=0.0274, 95% CI of the difference: [1.3203 18.6235]), between negative and positive condition (t-value = 4.5010, p=0.002, 95% CI of the difference: [8.0741 22.6561]), in theta power at 2700–3300 ms between negative and positive condition (t-value = 3.6944, p=0.0045, 95% CI of the difference: [8.8765 32.3104]).
-
Figure 3—source data 1
Source data for generating Figure 3.
- https://cdn.elifesciences.org/articles/65444/elife-65444-fig3-data1-v1.mat
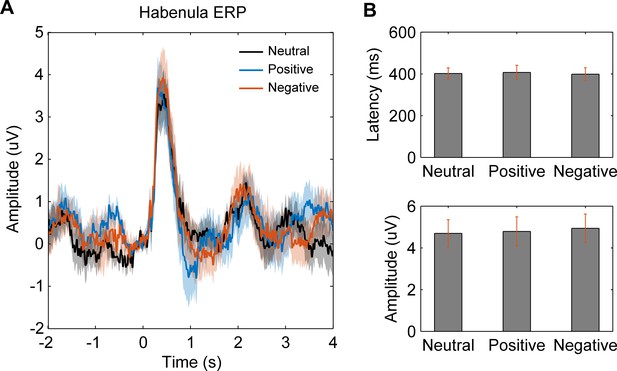
Event-related potential (ERP) in habenula local field potential signals in different emotional valence (neutral, positive, and negative) conditions.
(A) Averaged ERP waveforms across patients for different conditions. (B) Peak latency and amplitude (mean ± SEM) of the ERP components for different conditions.
Non-phase-locked (induced only) activity in different emotional valence (neutral, positive, and negative) conditions (N = 18).
(A) Time-frequency representation of the power changes relative to pre-stimulus baseline for three conditions. Significant clusters (p<0.05, non-parametric permutation test) are encircled with a solid black line. (B) Time-frequency representation of the power response difference between negative and positive valence stimuli, showing significant increased activity the theta/alpha band (5–10 Hz) at short latency (100–500 ms) and another increased theta activity (4–7 Hz) at long latencies (2700–3300 ms) with negative stimuli (p<0.05, non-parametric permutation test). (C) Normalized power of the activities at theta/alpha (5–10 Hz) and theta (4–7 Hz) band over time. Significant difference between the negative and positive valence stimuli is marked by a shadowed bar (p<0.05, corrected for multiple comparison).
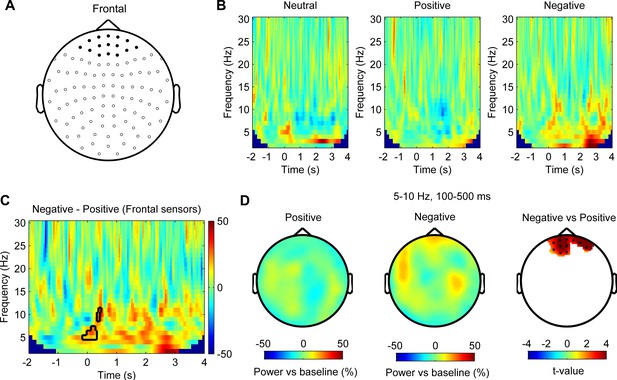
Theta/alpha oscillations in the prefrontal cortex are differentially modulated by stimuli with positive and negative emotional valence (N = 8 magnetoencephalography [MEG] samples from eight subjects).
(A) Layout of the MEG sensor positions and selected frontal sensors (dark spot). (B) Time-frequency representation of the power changes relative to pre-stimulus baseline for neutral, positive, and negative stimuli averaged across frontal sensors (time 0 for stimuli onset). (C) Non-parametric permutation test showed clusters in the theta/alpha band at short latency after stimuli onset with significant difference (p<0.05) comparing negative and positive stimuli across frontal sensors. (D) Scalp plot showing the power in the 5–10 Hz theta/alpha band activity at 100–500 ms after the onset of positive (left), negative (middle) stimuli, and statistical t-values and sensors with significant difference (right) at a 0.05 significance level (corrected for whole-brain sensors).
-
Figure 4—source data 1
Source data for generating Figure 4.
- https://cdn.elifesciences.org/articles/65444/elife-65444-fig4-data1-v1.mat
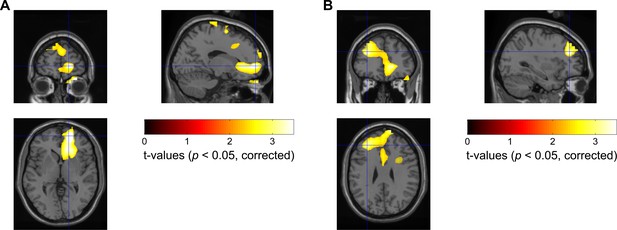
Statistical source maps of t-values (p<0.05; corrected for whole brain) for the comparison of magnetoencephalography (MEG) theta/alpha band (5–10 Hz) power reactivity to negative vs. positive emotional valence stimuli across subjects (N = 8 MEG samples from eight subjects).
Dynamic imaging of coherent source beamformer was applied to the average theta/alpha band power changes from 100 to 500 ms after stimulus onset. The image was transformed to MNI template space and overlaid on the template structural image. The peak emotional valence-induced differences in the theta/alpha power were localized in the right Brodmann area 10, MNI coordinate [16, 56, 0] (shown in Plot A) and left Brodmann area 9, MNI coordinate [−32, 38, 28] (shown in Plot B).
-
Figure 5—source data 1
Source data for generating Figure 5.
- https://cdn.elifesciences.org/articles/65444/elife-65444-fig5-data1-v1.zip
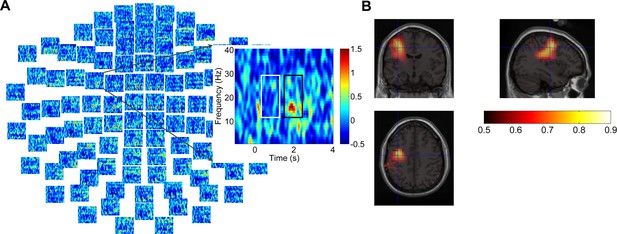
An example of dynamic imaging of coherent source (DICS) beamforming for the movement-related beta power source localization in one participant (Case 8).
(A) Time-frequency maps of magnetoencephalography activity for right-hand button press at sensor level from one participant. (B) DICS beamforming source reconstruction of the areas with movement-related oscillation changes in the range of 12–30 Hz. The peak power was located in the left M1 area, MNI coordinate [−37,–12, 43].
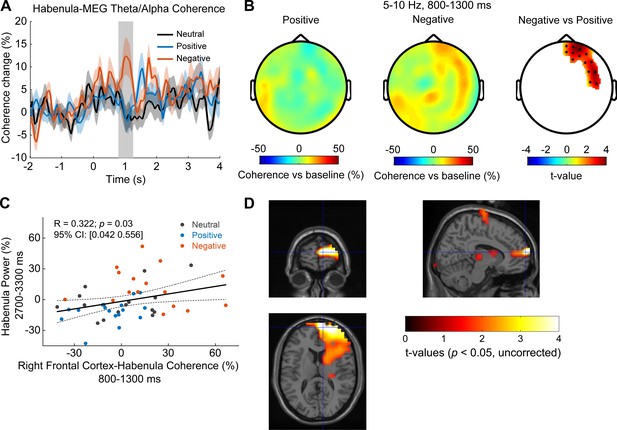
Cortical-habenular coherence in the theta/alpha band is also differentially modulated by stimuli with positive and negative emotional valence (N = 16 local field potential-magnetoencephalography [LFP-MEG] combination samples from eight subjects).
(A) Time-varying theta (5–10 Hz) habenula-cortical coherence changes relative to pre-cue baseline averaged across all MEG channel combinations for each recorded habenula. The thick colored lines and shaded area show the mean and standard error across all recorded habenula. The coherence was significantly higher at 800–1300 ms after the onset of negative emotional stimuli compared to positive stimuli (rectangular shadow showing the time window with p<0.05). (B) Scalp plot showing the cortical-habenula coherence in the theta band during the identified time window (800–1300 ms) for positive stimuli (left), negative stimuli (middle), and statistical t-values and sensors with significant difference (right) masked at p<0.05 (corrected for whole-brain sensors). (C) The increase in the theta band coherence between right frontal cortex and habenula at 800–1300 ms correlated with the theta increase in habenula at 2700–3300 ms after stimuli onset. (D) Statistical source maps of t-values (p<0.05; uncorrected) for the comparison of theta/alpha coherence response in the time window of 800–1300 ms between negative stimuli with positive stimuli. The peak coherence differences were mainly localized in the right Brodmann area 10, MNI coordinate [10, 64, 12].
-
Figure 6—source data 1
Source data for generating Figure 6.
- https://cdn.elifesciences.org/articles/65444/elife-65444-fig6-data1-v1.zip
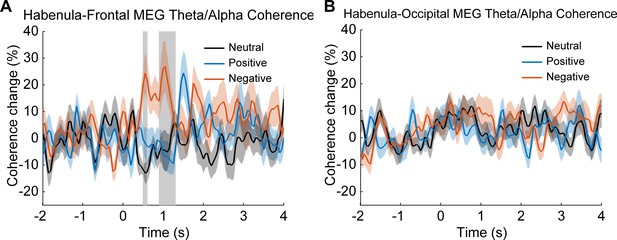
Time-varying coherence changes for frontal cortex-habenula and occipital cortex-habenula separately.
(A) Time-varying theta (5–10 Hz) habenula-cortical coherence changes relative to pre-cue baseline averaged across habenula-frontal magnetoencephalography (MEG) channel combinations for each emotional valence condition. (B) Time-varying theta (5–10 Hz) habenula-cortical coherence changes relative to pre-cue baseline averaged across habenula-occipital MEG channel combinations for each emotional valence condition.
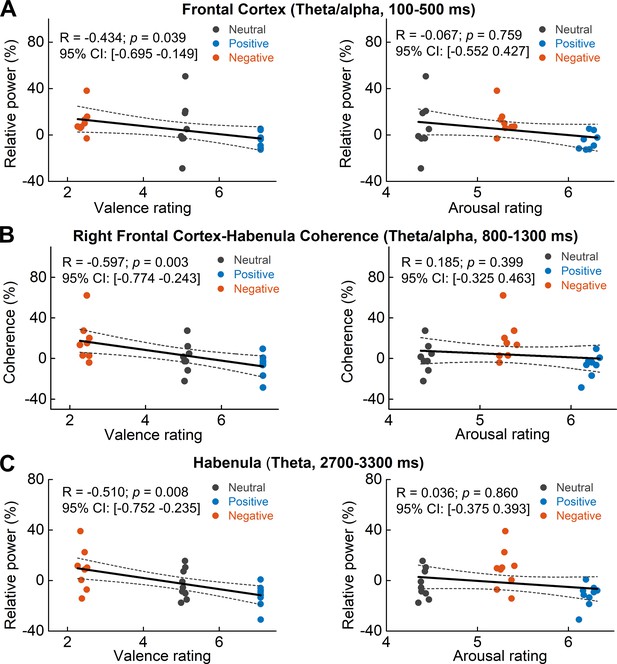
Scatter plots showing how early theta/alpha band power increase in the frontal cortex (A) theta/alpha band frontal cortex-habenula coherence (B) and theta band power increase at a later time window in habenula (C) changed with emotional valence (left column) and arousal (right column).
Each dot shows the average of one participant in each categorical valence condition, which are also the source data of the multilevel modeling results presented in Table 2. The estimated correlation coefficient R and 95% confidence interval (CI), as well as the p value in the figure, are the results of partial correlation considering all data points together.
-
Figure 7—source data 1
- https://cdn.elifesciences.org/articles/65444/elife-65444-fig7-data1-v1.mat
Tables
Characteristics of enrolled subjects.
| Patient | Sex | Age (years) | Duration (years) | Disease | HAMD score | BDI score | Resting oscillation peaks | |
|---|---|---|---|---|---|---|---|---|
| L | R | |||||||
| 1 | M | 21 | 5 | Schiz | NA | 32 | 9.1 Hz | 9.8 Hz |
| 2 | M | 21 | 5 | Dep | 12 | 10 | 7.9 Hz | 8.4 Hz |
| 3 | M | 44 | 10 | Bipolar | 23 | 22 | 14.3 Hz | 15.9 Hz |
| 4 | F | 19 | 4 | Schiz | NA | NA | 10 Hz | 8.1 Hz |
| 5 | M | 21 | 3 | Dep | 24 | 38 | 7.1 Hz | 16.9 Hz |
| 6 | M | 16 | 2 | Schiz | NA | 34 | 9.2 Hz; 13.0 Hz | 7.2 Hz; 12.5 Hz |
| 7 | F | 30 | 8 | Bipolar | 21 | 33 | 6.1 Hz | 7.8 Hz |
| 8 | F | 28 | 13 | Dep | 28 | 37 | No peak | 8.0 Hz |
| 9 | M | 35 | 20 | Dep | 25 | 34 | 16.2 Hz | 7.9 Hz; 16.0 Hz |
-
Hab: habenula; F: female; M: male; Dep: depressive disorder; Bipolar: bipolar disorder; Schiz: schizophrenia; HAMD: Hamilton Depression Rating Scale (17 items); BDI: Beck Depression Inventory; Both HAMD and BDI were acquired before the surgery. NA: not available.
Linear mixed effect modeling details.
| ID | Model | Fixed effect of valence | Fixed effect of arousal | R2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k-Value | 95% CI | p-Value | k-Value | 95% CI | p-Value | |||||||
| 1 | HabTheta1∼ Valence+Arousal+1|SubID | −2.8044 ± 0.9840 | [−4.7800,–0.8289] | 0.0063 | −2.5221 ± 2.5363 | [−7.6139, 2.5697] | 0.3247 | 0.6191 | ||||
| 2 | HabTheta2∼ Valence+Arousal+1|SubID | −4.4526 ± 1.1753 | [−6.8121,–2.0932] | 0.0004 | 0.1975 ± 3.0295 | [−5.8844, 6.2794] | 0.9483 | 0.2557 | ||||
| 3 | PFC_Theta∼ Valence+Arousal+1|SubID | −2.8921 ± 1.0221 | [−4.9507,–0.8334] | 0.0069 | −3.6237 ± 2.6252 | [−8.9112, 1.6637] | 0.1743 | 0.4368 | ||||
| 4 | rPFC_Hab_Coh∼ Valence+Arousal+1|SubID | −6.1031 ± 1.6785 | [−9.4837,–2.7225] | 0.0007 | 3.5242 ± 4.3112 | [−5.1589, 12.2074] | 0.4180 | 0.2766 | ||||
-
HabTheta1: theta/alpha band (5–10 Hz) in habenula LFPs at 100–500 ms ; HabTheta2: theta band (4–7 Hz) in habenula LFPs at 2700–3300 ms; PFC_Theta: theta/alpha band (5–10 Hz) averaged across frontal sensors at 100–500 ms; rPFC_Hab_Coh: theta/alpha band (5–10 Hz) coherence between right PFC and habenula at 800–1300 ms; Valence: valence value for the displayed pictures (1 = unpleasant -> 5 = neutral -> 9 = pleasant); Arousal: arousal value of the displayed pictures (1 = calm -> 9 = exciting); LFP: local field potential; PFC: prefrontal cortex.
Additional files
-
Source code 1
The source code file is a compressed folder containing the MATLAB scripts to generate the figures and separate files for the source data to generate different figures with the file names indicating the figure or table with which the data was associated.
- https://cdn.elifesciences.org/articles/65444/elife-65444-code1-v1.zip
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/65444/elife-65444-transrepform-v1.docx





