Prolonging the integrated stress response enhances CNS remyelination in an inflammatory environment
Figures
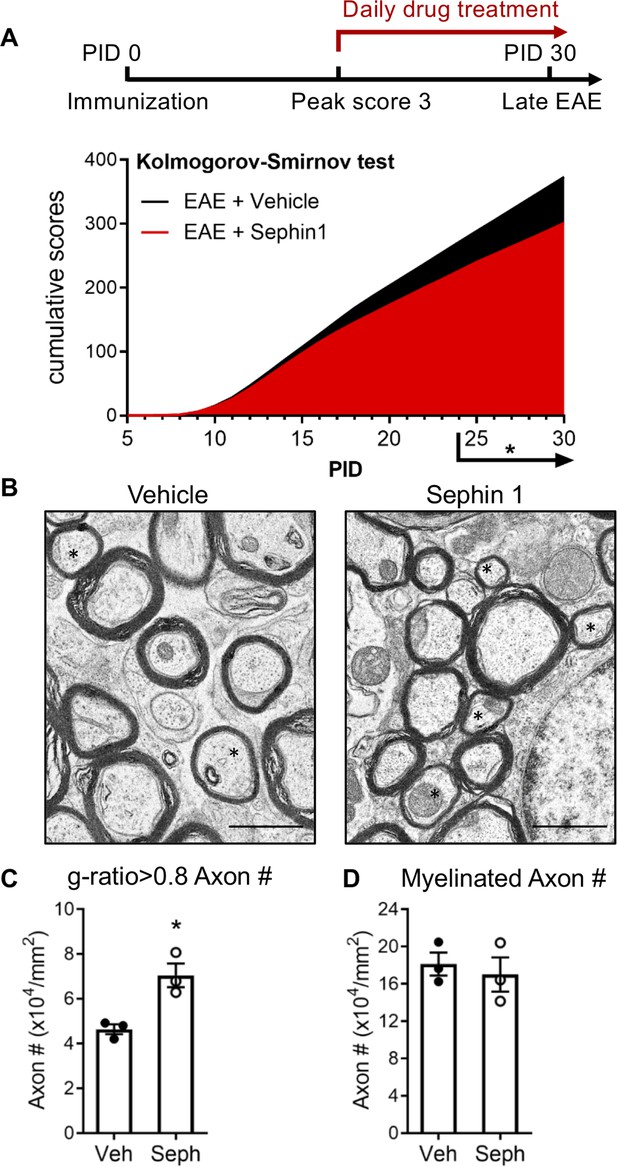
Sephin1 treatment enhances remyelination after inflammatory demyelination.
(A) Cumulative clinical scores of C57BL/6J female mice immunized with MOG35-55/CFA to induce chronic EAE, treated with vehicle (n = 7) and 8 mg/kg Sephin1 (n = 7) from the peak disease. *p<0.05. Significance based on Kolmogorov-Smirnov test. (B) Representative EM images of axons in the spinal cord white matter tracts of EAE mice treated with vehicle or Sephin1 at PID30. Remyelinated axons in the EAE spinal cord were identified by thinner myelin sheaths (*). Scale bar = 1 µm. (C) Density of myelinated axons with g-ratio <0.8 in the EAE spinal cord. (D) Density of total myelinated axons in the EAE spinal cord. Data are presented as the mean ± SEM (n = 3 mice/group). Over 300 axons were analyzed per mouse. *p<0.05. Significance based on unpaired t-test.
-
Figure 1—source data 1
Disease scores and axon measurement of late stage of EAE.
- https://cdn.elifesciences.org/articles/65469/elife-65469-fig1-data1-v1.xlsx
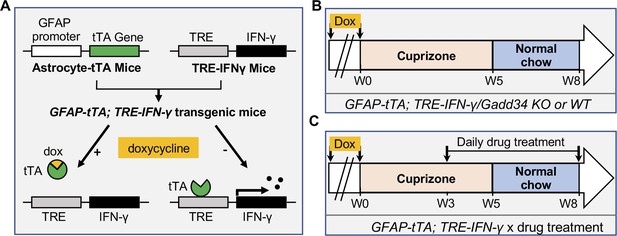
Schematics of GFAP-tTA;TRE-IFN-γ double-transgenic mouse model of cuprizone demyelination/remyelination.
(A) GFAP-tTA mice are mated with TRE-IFN-γ mice to produce double-positive animals. When these mice are maintained on doxycycline (Dox), the expression of the IFN-γ is repressed. When they are released from Dox, IFN-γ is expressed in the CNS. (B) Cuprizone demyelination/remyelination model of GFAP-tTA;TRE-IFN-γ/GADD34 KO or WT. Dox is removed and cuprizone chow is added when mice are at 6-week-old (W0). After 5 weeks of cuprizone exposure (W5), mice were placed back on normal chow for up to 3 weeks to allow remyelination. (C) Cuprizone demyelination/remyelination model of GFAP-tTA;TRE-IFN-γ with designed treatment. Drug treatment is started at 3 weeks of cuprizone exposure (W3) and lasts to the end of remyelination (W8).
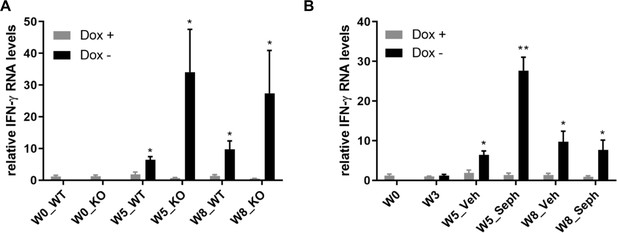
GFAP-tTA;TRE-IFN-γ mice express IFN-γ after release from doxycycline.
Real-time qPCR analyses for detection of mRNA levels of ectopically expressed IFN-γ in the brains of GFAP-tTA;TRE-IFN-γ mice. (A) The expression of IFN-γ in the GFAP-tTA;TRE-IFN-γ/GADD34 KO or WT with the doxycycline (Dox+) and without doxycycline (Dox-). (B) The expression of IFN-γ in the GFAP-tTA;TRE-IFN-γ treated with vehicle and Sephin1 with the doxycycline (Dox+) and without doxycycline (Dox-). Data are presented as the mean ± SEM (n = 4 mice/group). *p<0.05, **p<0.01. Significance based on ANOVA.
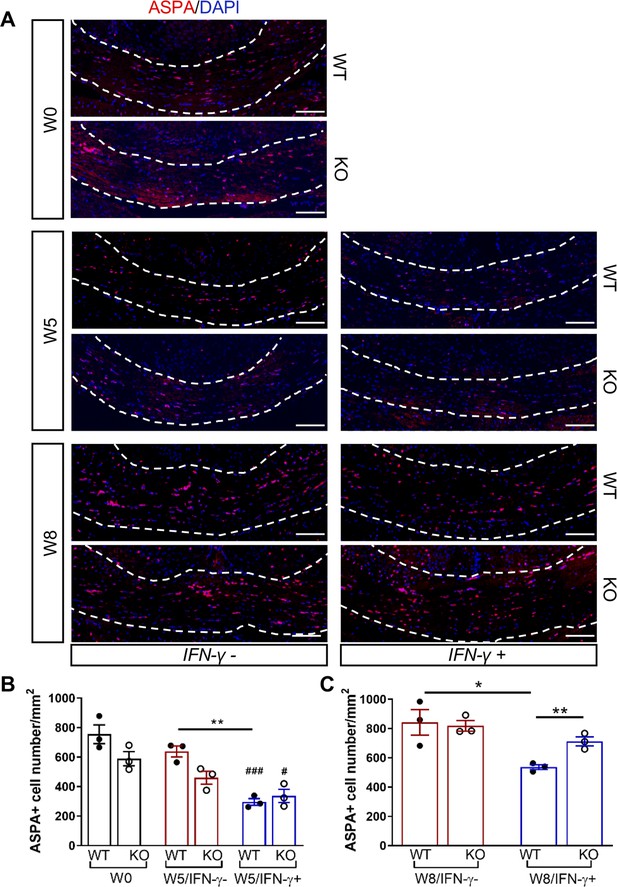
GADD34 deficiency protects remyelinating oligodendrocytes in the presence of IFN-γ.
The corpora callosa of GFAP-tTA;TRE-IFN-γ/GADD34 KO or WT were taken at W0, W5, and W8. (A) Immunofluorescent staining for ASPA (a mature oligodendrocyte marker) and DAPI (nuclei). Scale bar = 100 µm. (B) Quantification of cells positive for ASPA in the corpus callosum areas at W0 and W5 in the absence (IFN-γ-) or presence of IFN-γ (IFN-γ+). Data are presented as the mean ± SEM (n = 3 mice/group). WT: eight males and one female; KO: five males and four females. **p<0.01, #p<0.05 (#vs W0/WT), ###p<0.001 (# vs W0/KO). Significance based on ANOVA. (C) Quantification of cells positive for ASPA in the corpus callosum areas at W8 in the absence or presence of IFN-γ. WT: two males and four females; KO: four males and two females. Data are presented as the mean ± SEM (n = 3 mice/group). *p<0.05, **p<0.01. Significance based on ANOVA.
-
Figure 3—source data 1
The number of ASAP+ cells in WT and KO.
- https://cdn.elifesciences.org/articles/65469/elife-65469-fig3-data1-v1.xlsx
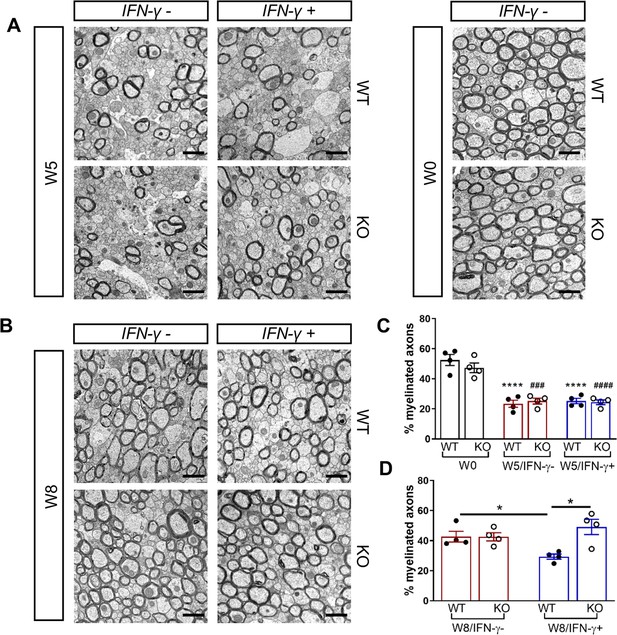
GADD34 deficiency enhances remyelination in the presence of IFN-γ.
The corpora callosa of GFAP-tTA;TRE-IFN-γ/GADD34 KO or WT were harvested for EM processing. (A) Representative EM images of axons in the corpus callosum at W0 and W5. Scale bar = 1 µm. (B) Representative EM images of axons in the corpus callosum at W8. Scale bar = 1 µm. (C) Percentage of myelinated axons at W0 and W5. WT: nine males and three females; KO: six males and six females. ****p<0.0001 (* vs W0/WT); ###p<0.001, ####p<0.0001 ( #vs W0/KO). Significance based on ANOVA. (D) Percentage of myelinated axons at W8. WT: four males and four females; KO: five males and three females. Data are presented as the mean ± SEM (n = 4 mice/group). *p<0.05 Significance based on ANOVA.
-
Figure 4—source data 1
The number of myelinated axons in WT and KO.
- https://cdn.elifesciences.org/articles/65469/elife-65469-fig4-data1-v1.xlsx
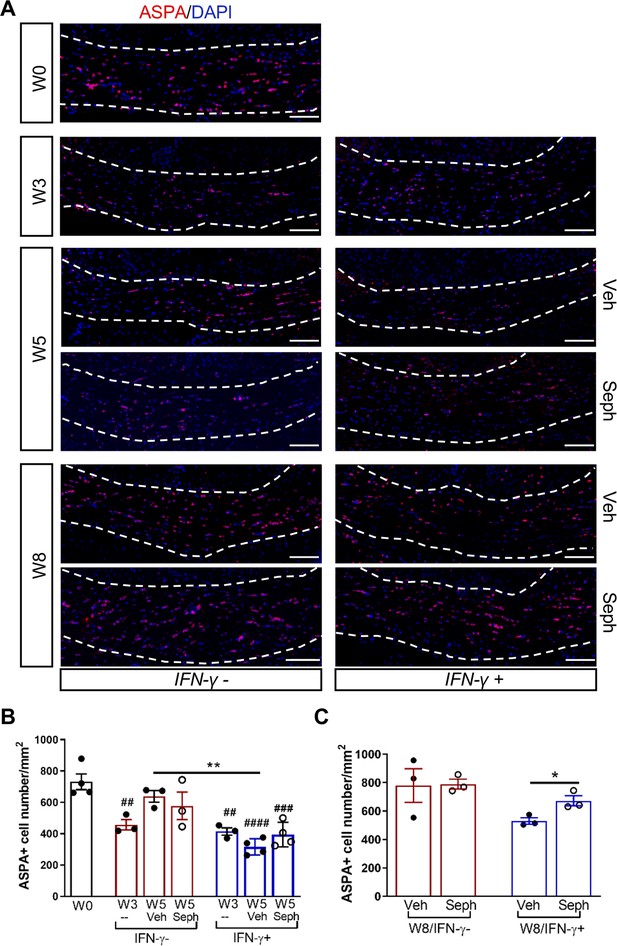
Sephin1 protects remyelinating oligodendrocytes in the presence of IFN-γ.
The corpora callosa of GFAP-tTA;TRE-IFN-γ were taken at W0 and W3 prior to any treatment as well as after either vehicle or Sephin1 treatment at W5 and W8. (A) Immunofluorescent staining for ASPA (a mature oligodendrocyte marker) and DAPI (nuclei). Scale bar = 100 µm. (B) Quantification of cells positive for ASPA in the corpus callosum areas at W0, W3, and W5 in the absence (IFN-γ-) or presence of IFN-γ (IFN-γ+). Data are presented as the mean ± SEM (n = 3–4 mice/group). W0: four males; W3: five males and one female; W5 (Veh): four males and two females; W5 (Seph): four males and two females. **p<0.01, ##p<0.01, ###p<0.001, ####p<0.0001 (#vs W0). Significance based on ANOVA. (C) Quantification of cells positive for ASPA in the corpus callosum areas at W8 in the absence or presence of IFN-γ. Data are presented as the mean ± SEM (n = 3 mice/group). W8 (Veh): five males and one female; W8 (Seph): five males and one female. *p<0.05. Significance based on ANOVA.
-
Figure 5—source data 1
The number of ASAP+ cells in Veh and Seph groups.
- https://cdn.elifesciences.org/articles/65469/elife-65469-fig5-data1-v1.xlsx
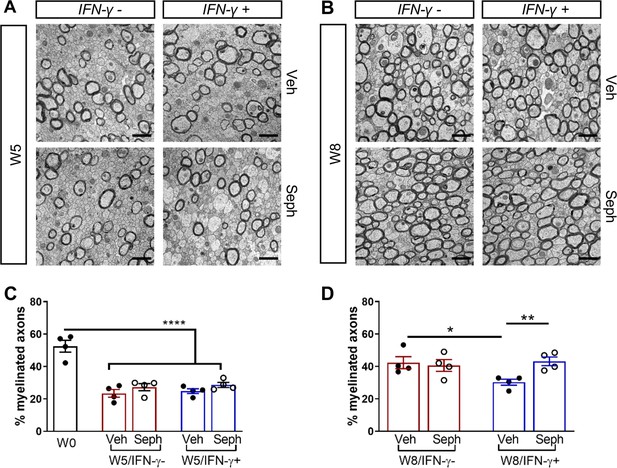
Sephin1 enhances remyelination in the presence of IFN-γ.
The corpora callosa of GFAP-tTA;TRE-IFN-γ were harvested for EM processing. (A) Representative EM images of axons in the corpus callosum at W5. Scale bar = 1 µm. (B) Representative EM images of axons in the corpus callosum at W8. Scale bar = 1 µm. (C) Percentage of remyelinated axons at W0 and W5. W0: four males; W5 (Veh): five males and three females; W5 (Seph): five males and three females. (D) Percentage of remyelinated axons at W8. Data are presented as the mean ± SEM (n = 4 mice/group). W8 (Veh): six males and two females; W8 (Seph): six males and two females. *p<0.05, **p<0.01, ****p<0.0001. Significance based on ANOVA.
-
Figure 6—source data 1
The number of myelinated axons in Veh and Seph groups.
- https://cdn.elifesciences.org/articles/65469/elife-65469-fig6-data1-v1.xlsx
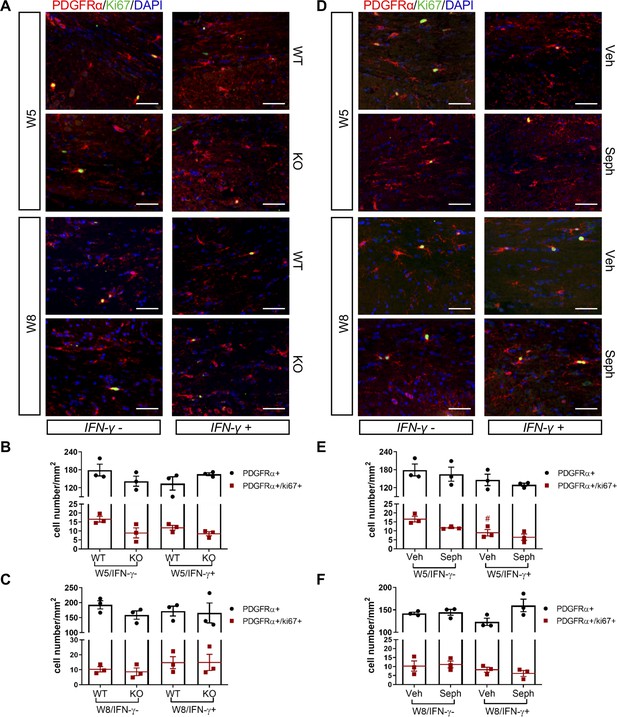
GADD34 deficiency or Sephin1 does not affect OPC proliferation during remyelination.
(A) Immunofluorescent staining for PDGFRα (an OPC marker), Ki67 and DAPI (nuclei) from the corpus callosum of GFAP-tTA;TRE-IFN-γ/GADD34 KO or WT was taken at W5 and W8. Scale bar = 50 µm. Quantification of cells positive for PDGFRα and cells positive for both PDGFRα and Ki67 in the corpus callosum areas of GFAP-tTA;TRE-IFN-γ/GADD34 KO or WT in the absence (IFN-γ-) or presence of IFN-γ (IFN-γ+) at W5 (B) and W8 (C). Data are presented as the mean ± SEM (n = 3 mice/group). W5 (WT): five males and one female; W5 (KO): four males and two females. W8 (WT): two males and four females; W8 (KO): four males and two females. (D) Immunofluorescent staining for PDGFRα, Ki67 and DAPI from the corpus callosum of GFAP-tTA;TRE-IFN-γ was taken after either vehicle or Sephin1 treatment at W5 and W8. Quantification of cells positive for PDGFRα and cells positive for both PDGFRα and Ki67 in the corpus callosum areas of GFAP-tTA;TRE-IFN-γ treated with treatment in the absence (IFN-γ-) or presence of IFN-γ (IFN-γ+) at W5 (E) and W8 (F). Data are presented as the mean ± SEM (n = 3 mice/group). W5 (Veh): four males and two females; W5 (Seph): four males and two females. W8 (Veh): five males and one female; W8 (Seph): five males and one female. #p<0.05 (vs. veh from W5/IFN-γ-). Significance based on ANOVA.
-
Figure 7—source data 1
The number of total OPCs and proliferating OPCs.
- https://cdn.elifesciences.org/articles/65469/elife-65469-fig7-data1-v1.xlsx
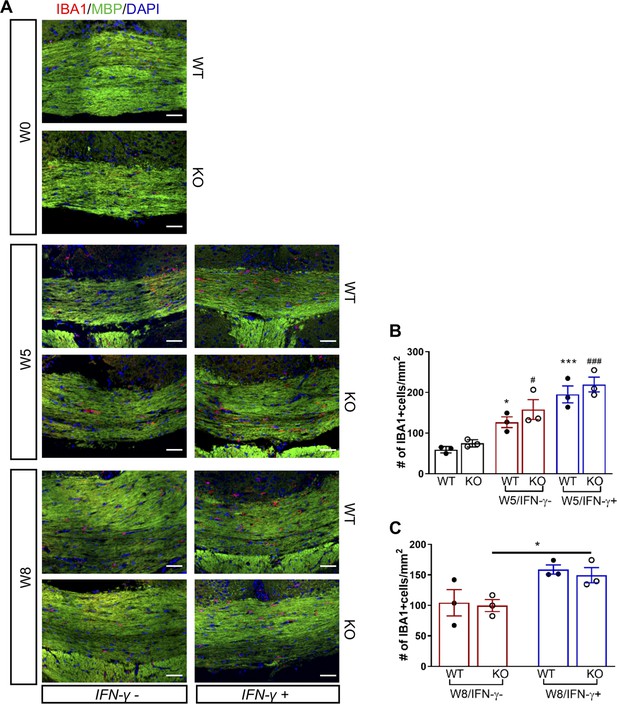
GADD34 deficiency does not affect microglial activation during remyelination in the presence of IFN-γ.
The corpus callosum of GFAP-tTA;TRE-IFN-γ/GADD34 KO or WT was taken at W0, W5 and W8. (A) Immunofluorescent staining for IBA1 (a microglia marker), MBP (a myelin marker) and DAPI. Scale bar = 50 µm. (B) Quantification of cells positive for IBA1 in the corpus callosum areas at W0 and W5 in the absence (IFN-γ-) or presence of IFN-γ (IFN-γ+). Data are presented as the mean ± SEM (n = 3 mice/group). *p<0.05, ***p<0.001 (*vs W0/WT), #p<0.05, ###p<0.001 (# vs W0/KO). Significance based on ANOVA. (C) Quantification of cells positive for IBA1 in the corpus callosum areas at W8 in the absence (IFN-γ-) or presence of IFN-γ (IFN-γ+). Data are presented as the mean ± SEM (n = 3 mice/group). *p<0.05. Significance based on ANOVA.
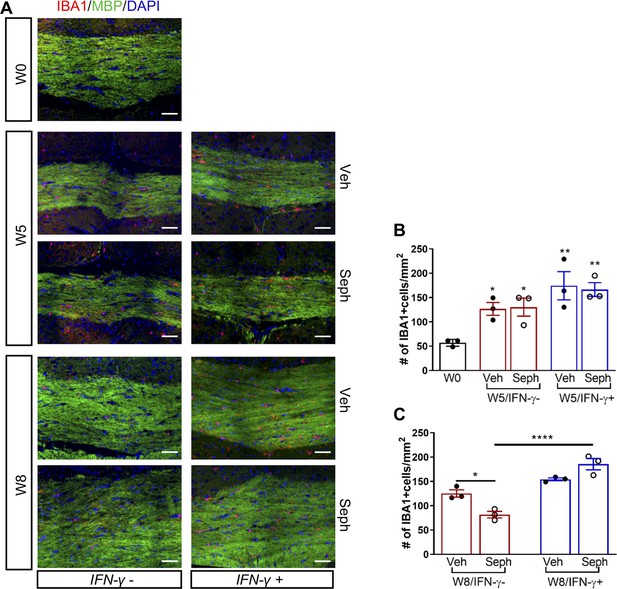
Sephin1 treatment does not affect microglial activation during remyelination in the presence of IFN-γ.
The corpus callosum of GFAP-tTA;TRE-IFN-γ was taken at W0, W5, and W8 with vehicle or Sephin1 treatment. (A) Immunofluorescent staining for IBA1, MBP, and DAPI. Scale bar = 50 µm. (B) Quantification of cells positive for IBA1 in the corpus callosum areas at W0 and W5 in the absence (IFN-γ-) or presence of IFN-γ (IFN-γ+). Data are presented as the mean ± SEM (n = 3 mice/group). *p<0.05, **p<0.01 (*vs W0). Significance based on ANOVA. (C) Quantification of cells positive for IBA1 in the corpus callosum areas at W8 in the absence (IFN-γ-) or presence of IFN-γ (IFN-γ+). Data are presented as the mean ± SEM (n = 3 mice/group). *p<0.05, ****p<0.0001. Significance based on ANOVA.
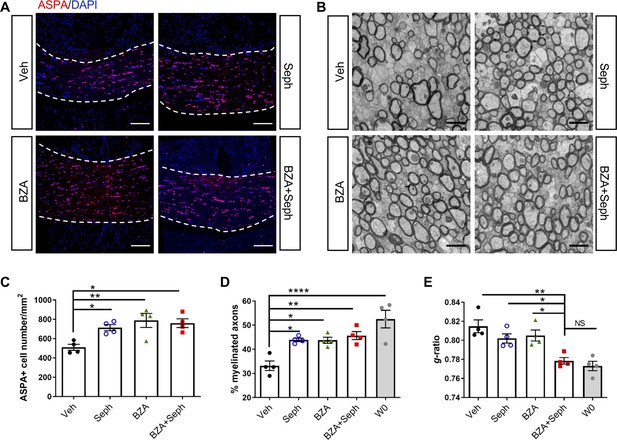
Combined treatment of Sephin1 and BZA accelerates remyelination.
GFAP-tTA;TRE-IFN-γ mice were released from Dox at W0 and given cuprizone chow for 5 weeks. Treatment with vehicle, Sephin1, BZA or combined BZA and Sephin1 was started at W3, and the corpus callosum was harvested and processed at W0 and after 3 weeks of remyelination (W8). (A) Immunofluorescent staining for ASPA and DAPI in the corpus callosum at W8 in the presence of IFN-γ. Scale bar = 100 µm. (B) Representative EM images of axons in the corpus callosum at W8 in the presence of IFN-γ. Scale bar = 1 µm. Quantifications of the number of cells positive for ASPA (C), percentage of remyelinated axons (D) and g-ratios of axons (E) in the corpus callosum areas at W0 and W8 in the presence of IFN-γ. Two males and two females in each treatment group and four males at W0. Data are presented as the mean ± SEM (n = 4 mice/group). *p<0.05, **p<0.01, ****p<0.0001. Significance based on ANOVA.
-
Figure 8—source data 1
The number of ASAP+ cells, myelinated axons and g-ratio of various treatments.
- https://cdn.elifesciences.org/articles/65469/elife-65469-fig8-data1-v1.xlsx
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (M. musculus) | C57Bl/6J | Jackson lab | RRID:IMSR_JAX:000664 | |
| Strain, strain background (M. musculus) | GFAP-tTA mice | Lin et al., 2004 | RRID:IMSR_JAX:005964 | |
| Strain, strain background (M. musculus) | TRE-IFN-γ mice | Lin et al., 2004 | RRID:IMSR_JAX:009344 | |
| Strain, strain background (M. musculus) | Ppp1r15a-/-(GADD34 KO) mice | Gift from David Ron | RRID:MGI:3040935 | |
| Chemical compound, drug | doxycycline | Sigma-Aldrich | Cat # D9891 | |
| Chemical compound, drug | 0.2% cuprizone | Envigo | Cat # TD.160049 | |
| Chemical compound, drug | Sephin1 | Apexbio | Cat # A8708 | |
| Chemical compound, drug | bazedoxifene acetate | Sigma-Aldrich | Cat # PZ0018 | |
| Chemical compound, drug | MOG 35-55peptide | Genemed synthesis | Cat # MOG3555-P-5 | |
| Chemical compound, drug | pertussis toxin | List Biological Laboratories | Cat # 179 | |
| Sequence-based reagent | Gapdh-f | This paper | qPCR primer | TGTGTCCGTCGTGGATCTGA |
| Sequence-based reagent | Gapdh-r | This paper | qPCR primer | TTGCTGTTGAAGTCGCAGGAG |
| Sequence-based reagent | Ifng-f | This paper | qPCR primer | GATATCTGGAGGAACTGGCAAAA |
| Sequence-based reagent | Ifng-r | This paper | qPCR primer | CTTCAAAGAGTCTGAGGTAGAAAGAGATAAT |
| Antibody | Anti-MBP (mouse monoclonal) | Abcam | Cat # ab24567 RRID:AB_448144 | (1:700) |
| Antibody | Anti-ASPA (rabbit polyclonal) | Genetex | Cat # GTX113389 RRID:AB_2036283 | (1:500) |
| Antibody | Anti-Ki67 (rabbit polyclonal) | Abcam | Cat # AB15580 RRID:AB_443209 | (1:100) |
| Antibody | Anti-PDGFR-alpha (mouse monoclonal) | BD Biosciences | Cat # 558774 RRID:AB_397117 | (1:100) |
| Antibody | Anti-Iba1 (rabbit polyclonal) | Wako Pure Chemical | Cat # 019–19741 RRID:AB_839504 | (1:500) |
| Software, algorithm | ImageJ | National Institutes of Health | RRID:SCR_003070 | |
| Software, algorithm | Prism 6.0 | Graphpad | RRID:SCR_002798 |





