Inverse regulation of Vibrio cholerae biofilm dispersal by polyamine signals
Figures
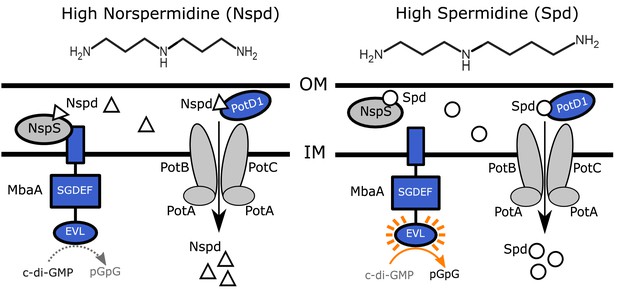
Polyamine sensing in V. cholerae.
Schematic showing the previously proposed polyamine detection and import mechanisms in V. cholerae. Norspermidine (Nspd, triangles) promotes biofilm formation and spermidine (Spd, circles) represses biofilm formation. The primarily eukaryotic polyamine spermine (not pictured) also signals through the NspS-MbaA pathway. See text for details. OM: outer membrane; IM: inner membrane.
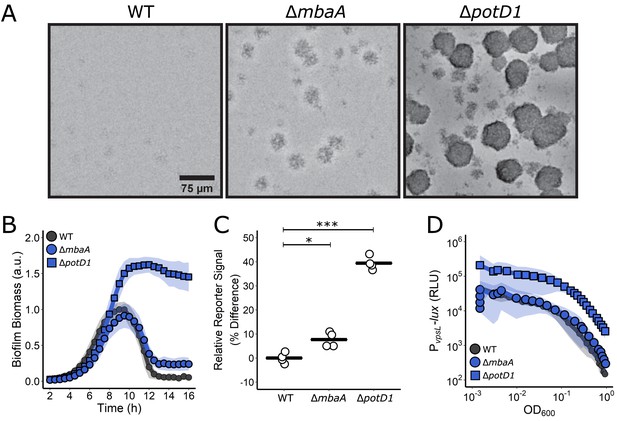
Polyamine signaling regulates V. cholerae biofilm dispersal.
(A) Representative images of the designated V. cholerae strains at 16 hr. (B) Quantitation of biofilm biomass over time measured by time-lapse microscopy for WT V. cholerae and the designated mutants. In all cases, N = 3 biological and N = 3 technical replicates, ± SD (shaded). a.u.: arbitrary unit. (C) Relative c-di-GMP reporter signals for the indicated strains. Values are expressed as the percentage difference relative to the WT strain. N = 4 biological replicates. Each black bar shows the sample mean. Unpaired t-tests were performed for statistical analysis, with p values denoted as *p<0.05; ***p<0.001. (D) The corresponding PvpsL-lux outputs for the strains and growth conditions in (B). For vpsL-lux measurements, N = 3 biological replicates, ± SD (shaded). RLU: relative light units.
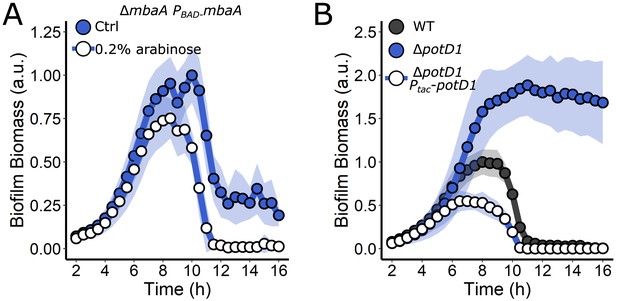
mbaA and potD1 complement the ΔmbaA and ΔpotD1 mutants, respectively.
(A) Quantitation of biofilm biomass over time measured by time-lapse microscopy for the ΔmbaA PBAD-mbaA strain following addition of water (Ctrl) or 0.2% arabinose. (B) Quantitation of biofilm biomass over time measured by time-lapse microscopy for the WT, ΔpotD1, and ΔpotD1 Ptac-potD1 strains. Data are represented as means normalized to the peak biofilm biomass of the Ctrl (left panel) or WT strain (right panel). N = 3 biological and N = 3 technical replicates, ± SD (shaded). a.u.: arbitrary unit.
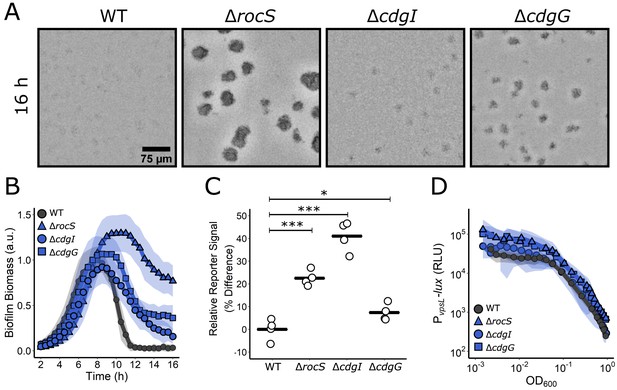
c-di-GMP signaling controls biofilm dispersal.
(A) Representative brightfield images of biofilms at 16 hr for the designated strains. (B) Quantitation of biofilm biomass over time measured by time-lapse microscopy for WT and the designated mutants. Data are represented as means normalized to the peak biofilm biomass of the WT strain. N = 3 biological and N = 3 technical replicates, ± SD (shaded). a.u.: arbitrary unit. (C) Relative c-di-GMP reporter signals for the indicated strains. Values are expressed as the percentage difference relative to the WT strain. N = 4 biological replicates. Each black bar shows the sample mean. Unpaired t-tests were performed for statistical analysis, with p values denoted as *p<0.05; ***p<0.001. (D) The corresponding PvpsL-lux reporter output for the strains and growth conditions in (B) over the growth curve. RLU: relative light units. N = 3 biological replicates, ± SD (shaded).
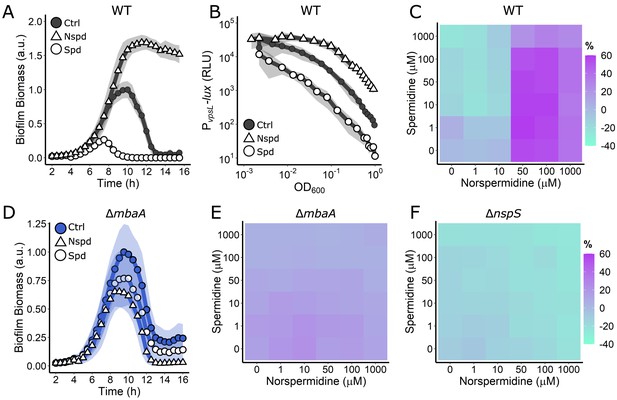
Periplasmic detection of polyamines controls V. cholerae biofilm dispersal.
(A) Quantitation of biofilm biomass over time measured by time-lapse microscopy following addition of water (Ctrl), 100 µM norspermidine, or 100 µM spermidine to WT V. cholerae. (B) Light output from the PvpsL-lux reporter for the treatments in (A) over the growth curve. (C) c-di-GMP reporter output at the indicated polyamine concentrations for WT V. cholerae. Relative reporter signal (% difference) is displayed as a heatmap (teal and purple represent the lowest and highest reporter output, respectively). (D) As in (A) for the ΔmbaA mutant. (E) As in (C) for the ΔmbaA mutant. (F) As in (C) for the ΔnspS mutant. Biofilm biomass data are represented as means normalized to the peak biofilm biomass of the Ctrl condition. In all biofilm biomass measurements, N = 3 biological and N = 3 technical replicates, ± SD (shaded). a.u.: arbitrary unit. In vpsL-lux measurements, N = 3 biological replicates, ± SD (shaded). RLU: relative light units. For the c-di-GMP reporter assays, values are expressed as the percentage difference relative to the untreated WT strain, allowing comparisons to be made across all heatmaps in all figures in this article. The same color bar applies to all heatmaps in this article. For each condition, N = 3 biological replicates. Numerical values and associated SDs are available in Supplementary file 1.
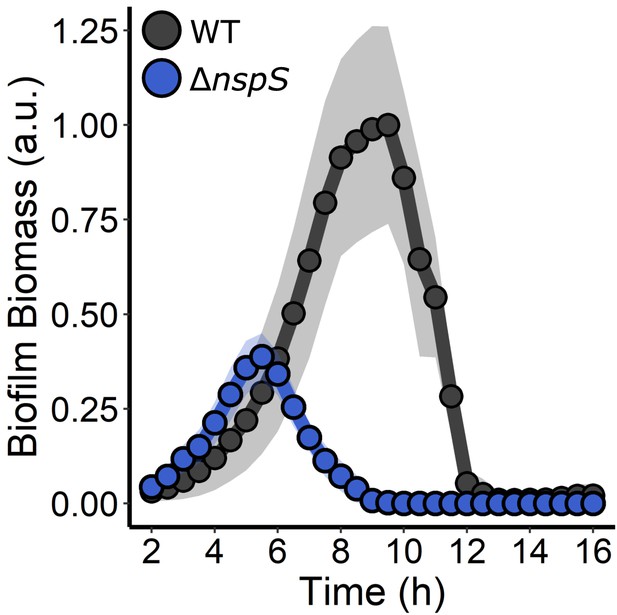
NspS is required for V. cholerae biofilm formation.
Quantitation of biofilm biomass over time measured by time-lapse microscopy for WT V. cholerae and the ΔnspS strain. For each strain, N = 3 biological and N = 3 technical replicates, ± SD (shaded). a.u.: arbitrary unit.
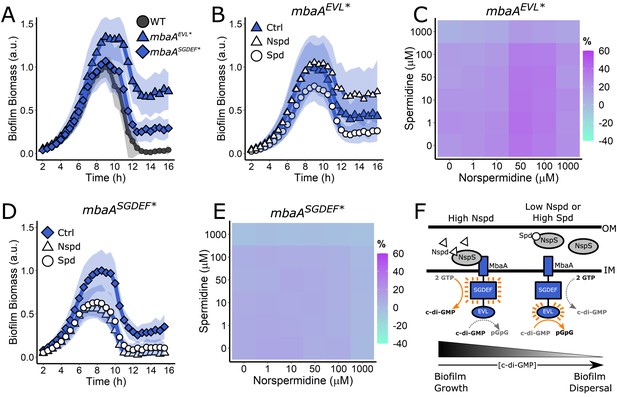
Both the MbaA SGDEF and EVL domains are required for regulation of V. cholerae biofilm dispersal.
(A) Quantitation of biofilm biomass over time for the V. cholerae strains carrying mbaA-3xFLAG, mbaAEVL*−3xFLAG, and mbaASGDEF*−3xFLAG. (B) Quantitation of biofilm biomass over time measured by time-lapse microscopy for V. cholerae carrying mbaAEVL*−3xFLAG following addition of water (Ctrl), 100 µM norspermidine, or 100 µM spermidine. (C) c-di-GMP reporter output at the indicated polyamine concentrations for V. cholerae carrying mbaAEVL*−3xFLAG. Relative reporter signal (% difference) is displayed as a heatmap (teal and purple represent the lowest and highest reporter output, respectively). (D) As in (B) for the mbaASGDEF*−3xFLAG mutant. (E) As in (C) for the mbaASGDEF*−3xFLAG mutant. (F) Schematic representing the proposed MbaA activities in response to norspermidine and spermidine. Biofilm biomass data are represented as means normalized to the peak biofilm biomass of the WT strain or Ctrl condition. In all cases, N = 3 biological and N = 3 technical replicates, ± SD (shaded). a.u.: arbitrary unit. In the c-di-GMP reporter assays, values are expressed as the percentage difference relative to the untreated WT strain, allowing comparisons to be made across all heatmaps in all figures in this article. The same color bar applies to all panels in this article. For each condition, N = 3 biological replicates. Numerical values and associated SDs are available in Supplementary file 1. OM: outer membrane; IM: inner membrane.
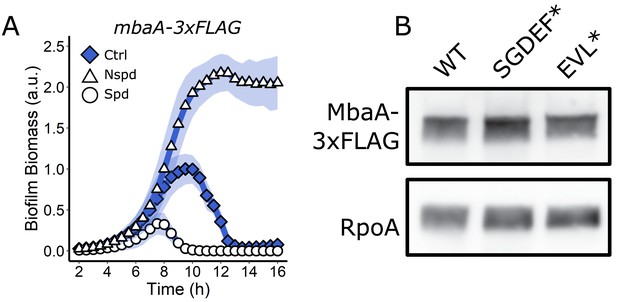
MbaA active site mutations do not alter protein levels.
(A) Quantitation of biofilm biomass over time measured by time-lapse microscopy for V. cholerae carrying MbaA-3xFLAG following addition of water (Ctrl), 100 µM norspermidine, or 100 µM spermidine. Biofilm biomass data are represented as means normalized to the peak biofilm biomass of the WT strain or Ctrl condition. In all cases, N = 3 biological and N = 3 technical replicates, ± SD (shaded). a.u.: arbitrary unit. (B) Top panel: western blot showing 3xFLAG for the WT MbaA, MbaA SGDEF*, and MbaA EVL* proteins. Bottom panel: RpoA is the loading control. Data are representative of three biological replicates.
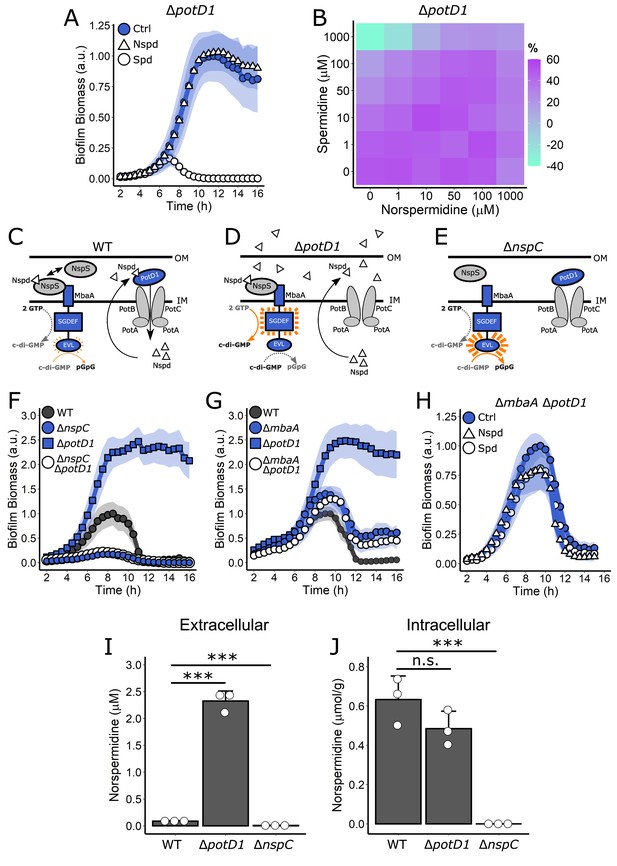
Polyamine import is not required for MbaA regulation of V. cholerae biofilm dispersal but is required to reduce external norspermidine levels.
(A) Quantitation of biofilm biomass over time measured by time-lapse microscopy following addition of water (Ctrl), 100 µM norspermidine, or 100 µM spermidine to the ΔpotD1 mutant. (B) c-di-GMP reporter output at the indicated polyamine concentrations for the ΔpotD1 strain. Relative reporter signal (% difference) is displayed as a heatmap (teal and purple represent the lowest and highest reporter output, respectively). Values are expressed as the percentage difference relative to the untreated WT strain, allowing comparisons to be made across all heatmaps in all figures in this article. The same color bar applies to all c-di-GMP reporter heatmaps in this article and for each condition, N = 3 biological replicates. Numerical values and associated SDs are available in Supplementary file 1. (C) Schematic of NspS-MbaA periplasmic detection of polyamines and polyamine import by PotD1 in WT V. cholerae. OM: outer membrane; IM: inner membrane. (D) Schematic of NspS-MbaA periplasmic detection of norspermidine together with the accumulation of elevated extracellular norspermidine in the V. cholerae ΔpotD1 mutant. (E) Schematic of NspS-MbaA activity in the ΔnspC mutant that is incapable of norspermidine biosynthesis. (F) Biofilm biomass over time for WT, the ΔnspC mutant, the ΔpotD1 mutant, and the ΔnspC ΔpotD1 double mutant. (G) As in (F) for WT, the ΔmbaA mutant, the ΔpotD1 mutant, and the ΔmbaA ΔpotD1 double mutant. (H) As in (A) for the ΔmbaA ΔpotD1 double mutant. All biofilm biomass data are represented as means normalized to the peak biofilm biomass of the WT strain or Ctrl condition, and in all cases, N = 3 biological and N = 3 technical replicates, ± SD (shaded). a.u.: arbitrary unit. (I) Concentration of norspermidine in cell-free culture fluids for the WT, ΔpotD1, and ΔnspC strains grown to OD600 = 2.0, measured by mass spectrometry. (J) Intracellular norspermidine levels normalized to wet cell pellet mass for the strains in (I). The strains used in (I) and (J) also contained a ΔvpsL mutation to abolish biofilm formation. Thus, all strains existed in the same growth state, which enabled comparisons between strains and, moreover, eliminated any possibility of polyamine sequestration by the biofilm matrix. For (I) and (J), N = 3 biological replicates, error bars represent SDs, and unpaired t-tests were performed for statistical analysis. n.s.: not significant; ***p<0.001.
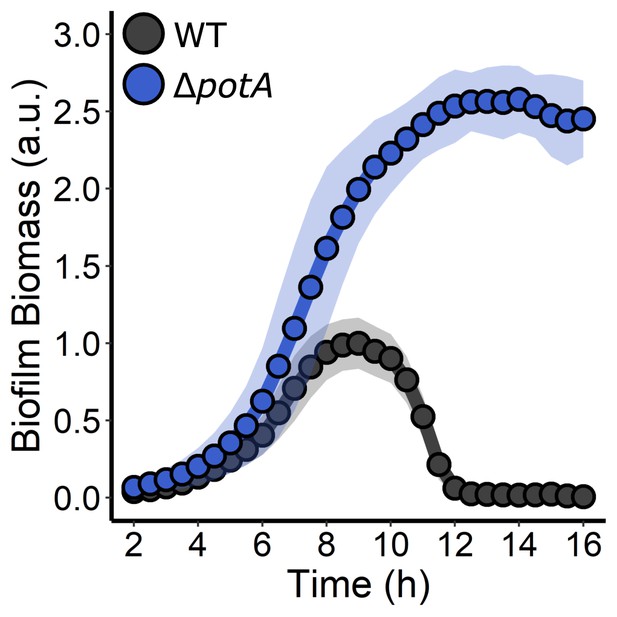
PotA is required for V. cholerae biofilm dispersal.
Quantitation of biofilm biomass over time measured by time-lapse microscopy for WT V. cholerae and the ΔpotA strain. For each strain, N = 3 biological and N = 3 technical replicates, ± SD (shaded). a.u.: arbitrary unit.
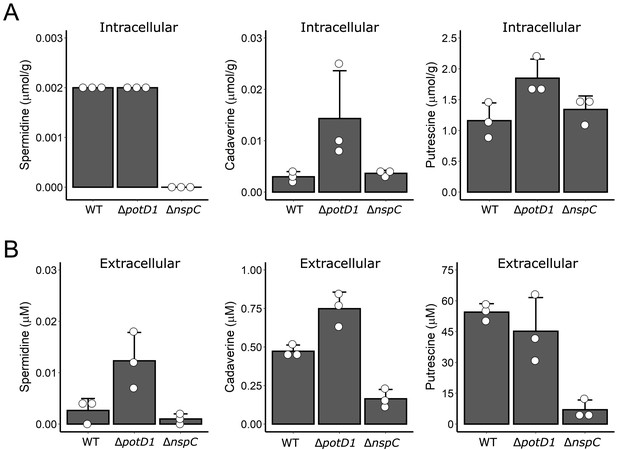
Polyamine levels for WT, ΔpotD1, and ΔnspC strains.
(A) Intracellular spermidine (left panel), cadaverine (middle panel), and putrescine (right panel) levels normalized to wet cell pellet mass for the samples from Figure 5I, J. (B) Molar concentrations of polyamines in the cell-free culture fluids of the strains from (A). The strains used in Figure 5 I, J also contained a ΔvpsL mutation to abolish biofilm formation. Thus, all strains existed in the same growth state, which enabled comparisons between strains and, moreover, eliminated any possibility of polyamine sequestration by the biofilm matrix. N = 3 biological replicates. Error bars represent SDs.
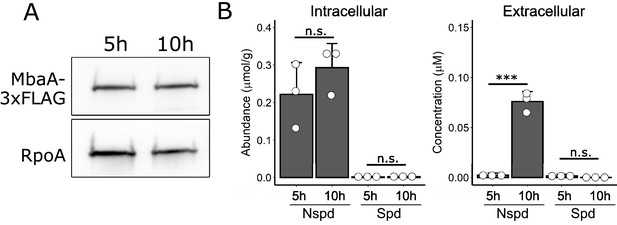
MbaA and intracellular polyamine levels remain constant throughout the biofilm lifecycle.
(A) Top panel: western blot showing MbaA-3xFLAG levels at 5 hr and 10 hr post inoculation. Bottom panel: the RpoA loading control. Data are representative of three biological replicates. (B) Measurements of intracellular (left panel) and extracellular (right panel) norspermidine (Nspd) and spermidine (Spd) levels at 5 hr and 10 hr post inoculation. Intracellular levels were normalized to wet cell pellet mass. N = 3 biological replicates, error bars represent SDs, and unpaired t-tests were performed for statistical analysis. n.s.: not significant; ***p<0.001.
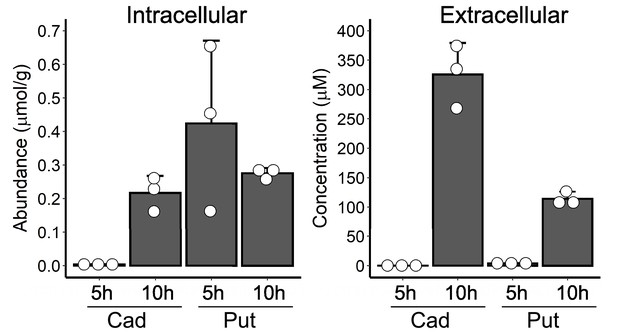
Polyamine levels in WT V. cholerae prior to and following biofilm dispersal.
Measurements of intracellular (left panel) and extracellular (right panel) cadaverine (Cad) and putrescine (Put) levels at 5 hr and 10 hr post inoculation for the samples shown in Figure 6B. Intracellular levels were normalized to wet cell pellet mass. N = 3 biological samples. Error bars represent SDs.
Videos
Representative time-lapse images of the biofilm lifecycles of the WT, ΔmbaA, ΔpotD1, and ΔmbaA ΔpotD1 V. cholerae strains following treatment with water (Ctrl), 100 µM norspermidine (Nspd), or 100 µM spermidine (Spd).
Representative time-lapse images of the biofilm lifecycles of the mbaA-3xFLAG, mbaAEVL*−3xFLAG, and mbaASGDEF*−3xFLAG V. cholerae strains following treatment with water (Ctrl), 100 µM norspermidine (Nspd), or 100 µM spermidine (Spd).
Additional files
-
Supplementary file 1
c-di-GMP reporter output averages and standard deviations.
- https://cdn.elifesciences.org/articles/65487/elife-65487-supp1-v2.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/65487/elife-65487-transrepform-v2.docx






