Prenatal methadone exposure disrupts behavioral development and alters motor neuron intrinsic properties and local circuitry
Figures
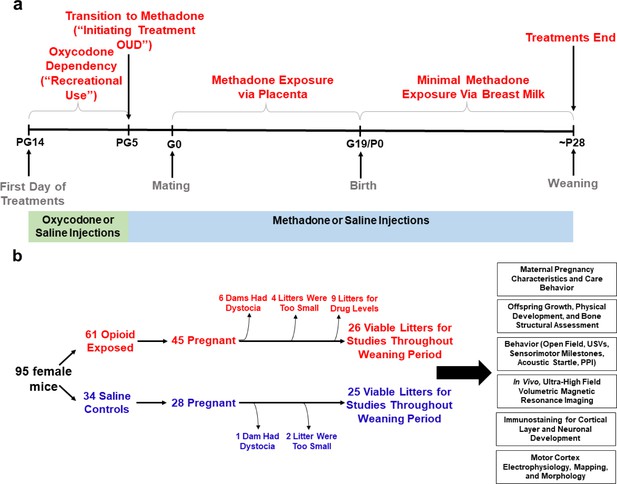
Study overview.
(a) Timeline briefly describing the generation of mice with prenatal methadone exposure (PME). Approximately 15 days prior to mating (pregestational day 14), female mice begin treatment with oxycodone to model recreational opioid use. Following nine days of oxycodone injections, mice began receiving methadone injections to simulate treatment of opioid use disorder (OUD). Following 5 days of methadone administration, the females were mated (gestational day 0) and treatment continued throughout gestation passively exposing the developing embryo and fetus to methadone. Following birth, dam methadone treatment continued which provided minimal, but measurable methadone exposure to offspring (see Figure 2. and Supplementary file 1). Offspring were weaned at approximately postnatal day 28. All doses were given subcutaneously twice daily. Control animals underwent an identical timeline with the exception of receiving saline injections. (b) Flow chart reviewing studies completed in the present study using offspring generated from the timeline in (a). Female mice were randomly assigned to either opioid (oxycodone → methadone) or saline treatment. Of the 45 opioid treated mice which became pregnant, six were removed from the study due to obstructed labor (dystocia), four litters were too small (<3 pups), and nine were chosen at random to assess methadone tissue and plasma concentrations. Of the 28 pregnant control mice, one experienced dystocia and two litters were too small. The offspring from the remaining litters (25–26 per exposure) were pseudo-randomly allocated to the behavioral and biological studies indicated in the black boxes (≥4 litters per exposure with equal representation of both sexes were used for each experiment). See Table 1 for detailed description of litter characteristics. PG, pregestation; G, gestation; P, postnatal, OUD, opioid use disorder; USVs, ultrasonic vocalizations; PPI, prepulse inhibition.
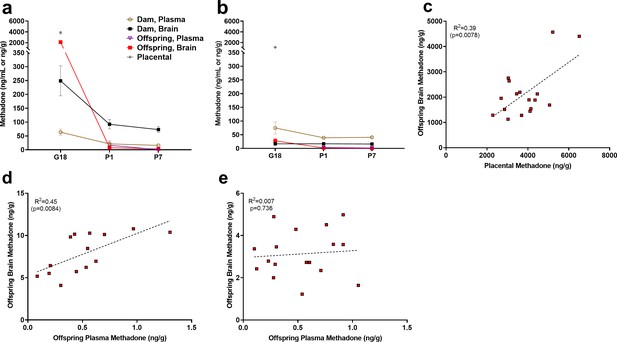
Relationship between placental, plasma, and brain methadone and metabolite levels in dams and offspring during gestation and the postnatal period.
(a) Methadone and (b) EDDP (2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, main metabolite of methadone) concentrations in the plasma, brain, and placenta of dams and offspring on G18 (approximately 1 day before birth), P1 (approximately 1 day after birth), and P7. Methadone highly accumulated in the fetal compartment relative to dam concentrations, but methadone concentrations dropped precipitously following birth. EDDP accumulated in the placenta. Data points indicate mean ± SEM. (c) Placental methadone concentrations predicted fetal brain concentrations on G18 (R2 = 0.39, p=0.0078). (d,e) Offspring plasma methadone concentrations predicted offspring brain methadone on P1 (R2 = 0.45, p=0.0084), but not on P7 (R2 = 0.008, p=0.736). All tissue and blood samples were collected 2.5 hr following the morning administration of methadone. (n = 3 dams + their respective litters per timepoint; n = 17–20 offspring samples at G18, n = 15 offspring at P1, n = 17–18 offspring at P7). Data are collapsed across offspring sex. The limit of quantification for methadone and EDDP detection was 0.1 ng/mL and 0.05 ng/mL in the plasma, respectively, and 0.08 ng/sample and 0.04 ng/sample of placenta and brain for both methadone and EDDP.
-
Figure 2—source data 1
Numerical data to support graphs in Figure 2 describing placental, plasma, and brain methadone and metabolite levels in dams and offspring.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig2-data1-v2.xlsx
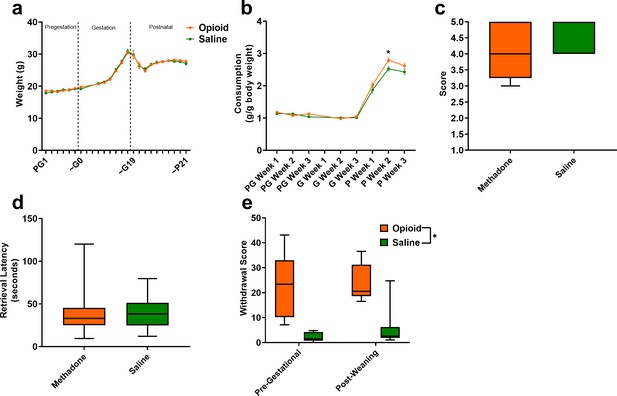
Opioid treatment-induced dependency and reduced gestational weight gain but did not alter maternal care.
(a) Measures of maternal weights over the course of the study revealed no significant effects of opioid treatment (n = 23 opioid, 21 saline mice); however, (b) Maternal food consumption was not significantly affected during pregestation and gestation but was significantly increased in opioid-treated dams following birth (rmANOVA: Interaction, p=0.02; Sidak’s post hoc: P week 2, p=0.004, n = 19 opioid, 16 saline mice). (c,d) Opioid-treated dams demonstrated no differences in (c) nest quality or (d) latency to retrieve pups removed from the nest on P3 suggesting maternal care behavior was grossly intact (n = 12 opioid, 13 saline mice). (e) The nine days of pregestational oxycodone treatment induced maternal opioid dependency and the oxycodone treatment plus the transition to methadone throughout gestation and the pre-weaning period was sufficient to maintain opioid dependence as determined by measures of naloxone-precipitated withdrawal behaviors (ANOVA: Treatment, p<0.0001, n = 7–8 opioid, 7–8 saline mice). *p<0.05. (a,b) Data points indicate mean ± SEM. (c–e) box plots indicate 25th to 75th percentiles with whisker characterizing the minimum and maximum value.
-
Figure 3—source data 1
Numerical data to support graphs in Figure 3 describing maternal characteristics and care behavior.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig3-data1-v2.xlsx
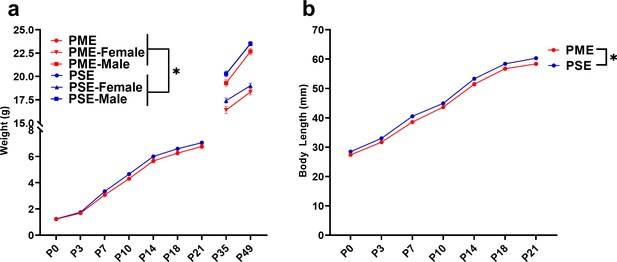
PME impaired offspring physical development.
(a) PME reduced offspring weight during the preweaning period (rmANOVA: Exposure, p=0.049; Interaction, p=0.0087, n = 57 PME (25M:32F), 56 PSE (29M:27F)) and this effect on weight persisted into adolescence in both sexes (rmANOVA: Exposure, p=0.0085, n = 30 PME (16M:14F), 24 PSE (15M:9F)). (b) PME reduced offspring body length during the preweaning period (rmANOVA: Exposure, p=0.0007, n = 57 PME (25M:32F), 56 PSE (29M:27F)). *main effect of exposure p<0.05. From P0-P21, Data were collapsed on sex as initial analyses revealed no main-effects or interaction with sex. Data points indicate mean ± SEM.
-
Figure 4—source data 1
Numerical data to support graphs in Figure 4 describing physical development in offspring.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig4-data1-v2.xlsx
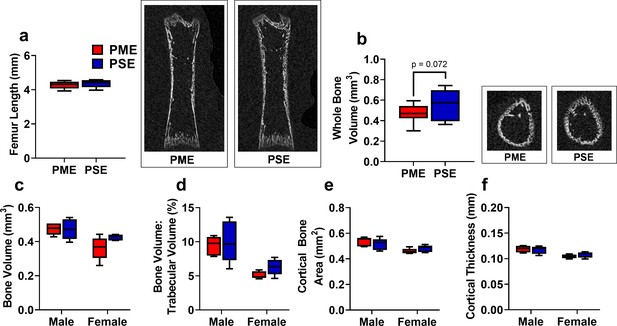
Early life bone density may be reduced in prenatal methadone-exposed offspring but recovered by adolescence.
(a) Femur length in P7 offspring did not differ between exposure groups. Right representative coronal CT image of P7 femur in PME and PSE offspring. (b) Femur whole bone volume was non-significantly reduced in P7 offspring with PME. Right representative CT image of P7 offspring demonstrating transaxial section of femur midshaft in PME and PSE offspring (unpaired t test: p=0.072, n = 13 PME (6M:7F), 12 PSE (6M:6F) at P7). At P35, structural bone measures including (c) distal metaphysis bone volume, (d) trabecular bone volume, (e) cortical bone area, and (f) cortical thickness did not differ between exposure groups. (n = 10 PME (5M:5F), 10 PSE (5M:5F) at P35). For (a,b) data were collapsed on sex as initial analyses revealed no main-effects or interaction with sex. For (c–f), Data were not collapsed on sex as analyses revealed some main effects of sex. Box plots indicate 25–75th percentiles with whisker characterizing the minimum and maximum value.
-
Figure 4—figure supplement 1—source data 1
Numerical data to support graphs in Figure 4—figure supplement 1 describing bone development in offspring.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig4-figsupp1-data1-v2.xlsx
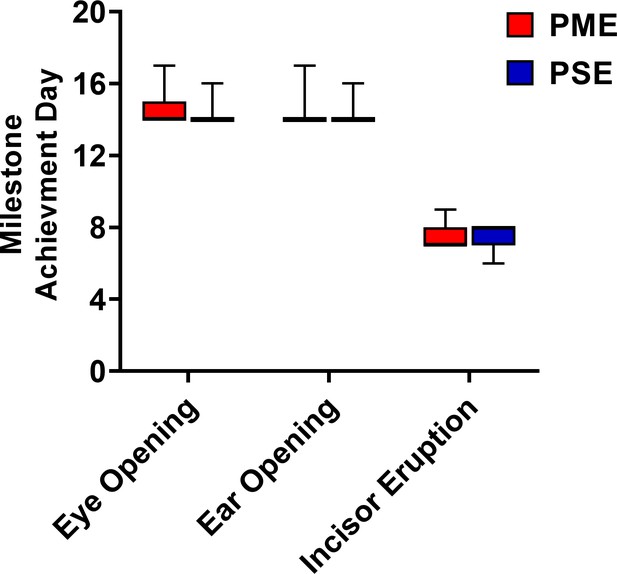
Postnatal craniofacial milestones in offspring is not affected by PME.
Mice were examined daily to examine progress of craniofacial milestone measures. PME did not delay the day when both eyes opened, both ears moved to their final erect position and external auditory canals were patent, or when the bottom incisor teeth erupted (n = 26 (7M:19F) PME, 25 PSE (15M:10F) mice). Data were collapsed on sex as initial analyses revealed no main-effects or interaction with sex. Box plots indicate 25–75th percentiles with whisker characterizing the minimum and maximum value.
-
Figure 4—figure supplement 2—source data 1
Numerical data to support graphs in Figure 4—figure supplement 2 describing craniofacial development in offspring.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig4-figsupp2-data1-v2.xlsx
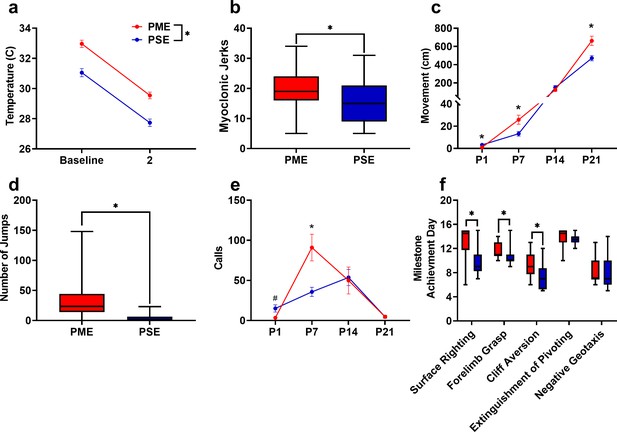
PME disrupted behavioral development in offspring.
(a) Offspring showed significantly higher surface body temperature when removed from the nest (baseline) and continued to maintain a higher surface body temperature following two minutes of isolation at P1 (rmANOVA: Exposure, p<0.0001, n = 25 (7M:18F) PME, 25 PSE (15M:10F) mice). (b) PME offspring show a greater number of twitches/jerks at P1 similar to the myoclonic jerks reportedly observed in human neonates experiencing opioid withdrawal (unpaired t test: p=0.047, n = 26 (7M:19F) PME, 25 PSE (15M:10F) mice). (c) Offspring were repeatedly tested in a modified open field during the first three weeks of life to examine the development of locomotor activity and ultrasonic vocalization (USV) production. (c) PME altered the development of locomotor activity (rmANOVA: Exposure, p=0.027; Interaction, p<0.0001) with PME offspring showing reduced activity at P1 but significantly greater activity at P7 and P21 (Sidak’s post hoc test: p=0.048, p=0.037, p=0.009, respectively, n = 26 (7M:19F) PME, 25 PSE (15M:10F) mice). (d) Subsequent manual scoring of videos on P21 revealed significantly greater number of vertical jumps during the 5-min session in PME mice further supporting a hyperactive phenotype at P21 (Mann-Whitney test: p<0.0001, n = 26 (7M:19F) PME, 25 PSE (15M:10F) mice). (e) PME offspring showed differences in the production of separation-induced USV calls (rmANOVA: Interaction, p=0.032) with less total calls emitted on P1 and significantly more calls emitted on P7 (Sidak’s post hoc test: p=0.079, p=0.015, n = 26 (7M:19F) PME, 25 PSE (15M:10F) mice). (f). Acquisition of the surface righting (unpaired t test: p<0.0001), forelimb grasp, and cliff aversion (Mann-Whitney test: p=0.0029 and p=0.0026, respectively) were significantly delayed in PME offspring (n = 26 (7M:19F) PME, 24 PSE (14M:10F) mice). *p<0.05, # p=0.079. Data were collapsed on sex as initial analyses revealed no main-effects or interaction with sex with the exception of USV results (See Figure 5—figure supplement 1 for results separated by sex). Data points indicate mean ± SEM. Box plots indicate 25–75th percentiles with whisker characterizing the minimum and maximum value.
-
Figure 5—source data 1
Numerical data to support graphs in Figure 5 describing behavioral development in offspring.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig5-data1-v2.xlsx
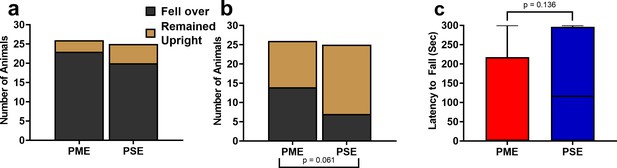
Prenatal methadone exposed offspring displayed a greater propensity to fall over on postnatal day 1.
(a) While the nearly all P1 offspring fell over by the end of the 5-min testing session (chi square test: p=0.41) (b) 14/26 PME compared to 7/25 PSE offspring had fallen over immediately upon placement into the arena (chi square test: p=0.061). (c) Similarly, PME offspring also display a nonsignificant reduction in the latency to fall over during the 5-min session (Mann-Whitney test: p=0.136 n = 26 (7M:19F) PME, 25 PSE (15M:10F) mice). Data were collapsed on sex as initial analyses revealed no main-effects or interaction with sex. Bars indicate proportion of offspring that rolled over or remained upright. Box plots indicate 25–75th percentiles with whisker characterizing the minimum and maximum value.
-
Figure 5—figure supplement 1—source data 1
Numerical data to support graphs in Figure 5—figure supplement 1 describing P1 activity in offspring.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig5-figsupp1-data1-v2.xlsx
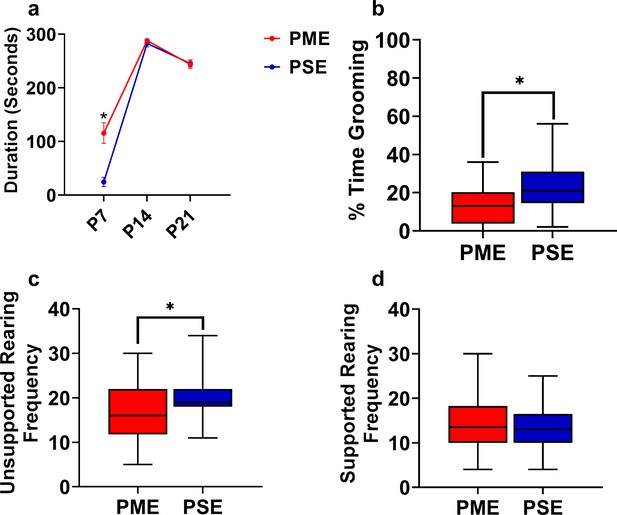
Open-field behaviors are altered in offspring with PME.
(a) PME offspring spent significant more time near the arena walls at P7 (rmANOVA: Exposure, p=0.0001; Interaction, p<0.0001; Sidak’s post hoc test: p=0.0004), however no effect was observed on P14 or P21. As PSE animals were significantly less active at P7, it is likely that PSE animals did not leave the center of the arena where offspring were placed at the beginning of each trial. (b,c,d) Grooming and rearing were further assessed in P21 juvenile mice. (b) The total percentage of time grooming was significant reduced in PME mice (Mann Whitney test: p=0.0035). (c) PME offspring demonstrated significantly less unsupported rearing instances on P21 (unpaired t test: p=0.017), (d) but now differences in rearing supported by the arena walls was observed between exposure groups. (n = 26 (7M:19F) PME, 25 PSE (15M:10F) mice). Data were collapsed on sex as initial analyses revealed no main-effects or interaction with sex. Data points indicate mean ± SEM and box plots indicate 25–75th percentiles with whisker characterizing the minimum and maximum value.
-
Figure 5—figure supplement 2—source data 1
Numerical data to support graphs in Figure 5—figure supplement 2 describing open-field behaviors in offspring.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig5-figsupp2-data1-v2.xlsx
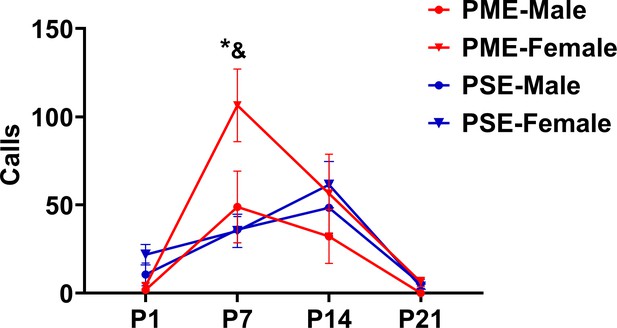
Separation induced ultrasonic vocalizations separated by sex.
While there was a significant time by exposure interaction on the development of USVs (rmANOVA: p=0.032), sex effects were present on the production of USVs (rmANOVA: p=0.045). Although female PME did not vocalize more than male PME offspring on P7 (Sidak’s post hoc test: p=0.60), female PME did emit more USVs than both male and female PSE offspring on P7 (Sidak’s post hoc test: p=0.0045 and p=0.026, respectively, n = 26 (7M:19F) PME, 25 PSE (15M:10F) mice). *p<0.05 for female PME vs female PSE; and p<0.05 for female PME vs male PSE offspring. Data points indicate mean ± SEM.
-
Figure 5—figure supplement 3—source data 1
Numerical data to support graphs in Figure 5—figure supplement 3 describing sex-specific USVs in offspring.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig5-figsupp3-data1-v2.xlsx
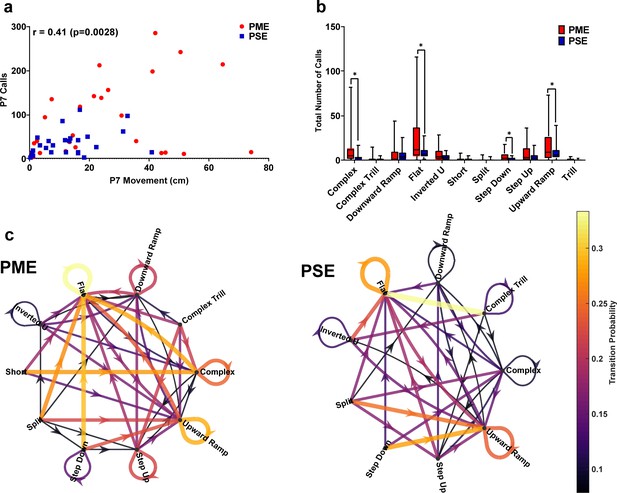
In depth analysis of ultrasonic vocalization revealed differential patterns of call types and syntax at P7.
(a) A moderate correlation exists between total locomotor activity and total USV calls at P7 suggesting a relationship is present between the hyperactivity and increased vocalizations among PME animals. (b) DeepSqueak automated classification of USV call types on P7 revealed an increase in production of all eleven call types in PME offspring but the level of significance was reached for complex, flat, step down, and upward ramp calls (unpaired t tests: p=0.014, p=0.0045, p=0.0086, and p=0.041, respectively). (c) Syntax analysis was also completed with DeepSqueak to determine the probably of call type transitions on P7 in both exposure groups. The probability of call transitions for PME and PSE offspring exhibit qualitative differences in syntax patterns suggesting alterations in maternal-pup communication may be present in offspring with PME. Arrows represent the direction of call transition, and line thickness and color represent the transition probability (n = 26 (7M:19F) PME, 25 PSE (15M:10F) mice). *p<0.05. Data were collapsed on sex for clarity of focusing on prenatal exposure. Box plots indicate 25–75th percentiles with whisker characterizing the minimum and maximum value.
-
Figure 5—figure supplement 4—source data 1
Numerical data to support graphs in Figure 5—figure supplement 4 describing the in-depth analysis of P7 USVs in offspring.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig5-figsupp4-data1-v2.xlsx
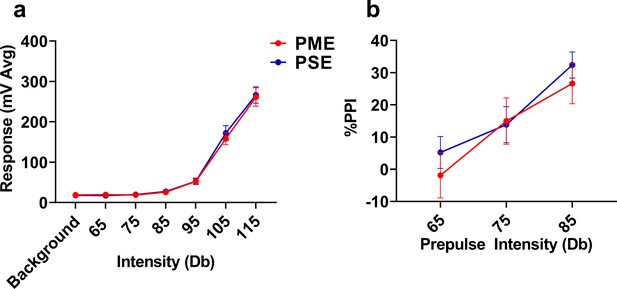
Acoustic startle response and prepulse inhibition was not affected by PME.
PME did not disrupt the (a) acoustic startle response to any startle intensity examined or (b) alter prepulse inhibition (PPI) at any prepulse intensity studied in P28-P29 offspring (n = 19 (10M:9F) PME, 19 PSE (10M:9F) mice). Data were collapsed on sex as initial analyses revealed no main-effects or interaction with sex. Data points indicate mean ± SEM.
-
Figure 5—figure supplement 5—source data 1
Numerical data to support graphs in Figure 5—figure supplement 5 describing PPI and acoustic startle response in offspring.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig5-figsupp5-data1-v2.xlsx
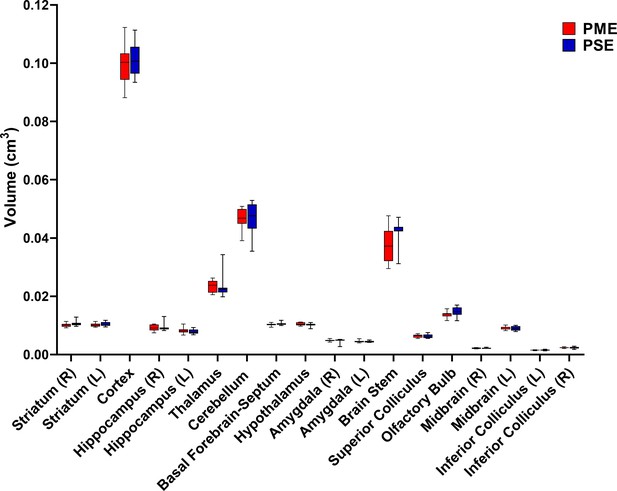
PME did not reduce volumes in brain regions of interest.
Volumetric findings from ultra-high-field MRI of P28-P30 offspring indicate that gross brain structure is primarily unaffected for both cortical and subcortical regions in PME offspring (unpaired t tests, n = 11 (4M:7F) PME, 11 PSE (6M:5F) mice). Data were collapsed on sex due to underpowering. Box plots indicate 25–75th percentiles with whisker characterizing the minimum and maximum value.
-
Figure 6—source data 1
Numerical data to support graphs in Figure 6 and Supplementary file 2 describing MRI gray matter volumes.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig6-data1-v2.xlsx
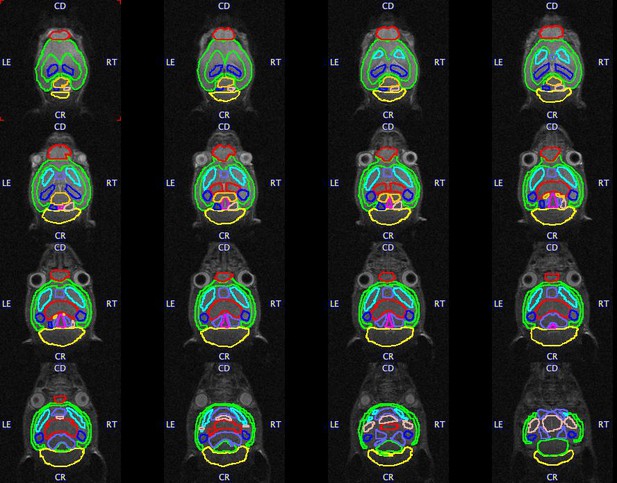
Brain regions examined for volume differences in offspring.
Volumes of interest (VOIs) are overlaid on a representative mouse brain. A total of 18 VOIs segmented including the whole cortex, striatum (L and R), hippocampus (L and R), thalamus, cerebellum, basal-forebrain septum, hypothalamus, amygdala (L and R), brain stem, superior colliculus, olfactory bulbs, midbrain (L and R), and inferior colliculus (L and R). R, Right; L, Left.
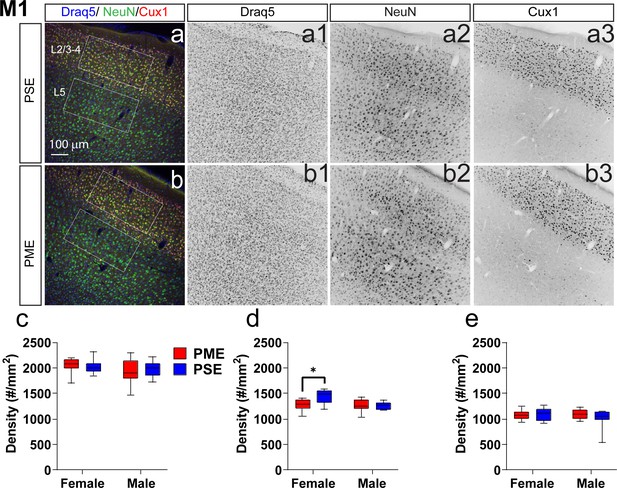
Neuronal density is reduced in the primary motor cortex (M1) of female PME offspring.
Representative slices of the M1 in (a) PSE and (b) PME offspring demonstrating Draq5 (blue, (a1,b1): marker of nuclei), NeuN (green, (a2,b): marker of post-mitotic neurons), and Cux1 (red, (a3,b3): marker of upper cortical layers, typically layer 2/3–4). White boxes represent the areas used for quantification. (c) No effect of Cux1+ cell densities were observed in L2/3-4. (d) A significant decrease in NeuN+ cells was found in layer 2/3-4 of Female PME offspring (ANOVA: Interaction, p=0.044; Sidak’s posthoc test, p=0.0098). (e) However, no differences were observed for NeuN+ cell densities in layer 5 (n = 9 (4M:5F) PME), 8 PSE (4M:4F). *p<0.05. Data were not collapsed on sex as analyses revealed both main-effects of sex and interactions with sex. Box plots indicate 25–75th percentiles with whisker characterizing the minimum and maximum value.
-
Figure 7—source data 1
Numerical data to support graphs in Figure 7 describing cortical lamination and neuronal density in M1.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig7-data1-v2.xlsx
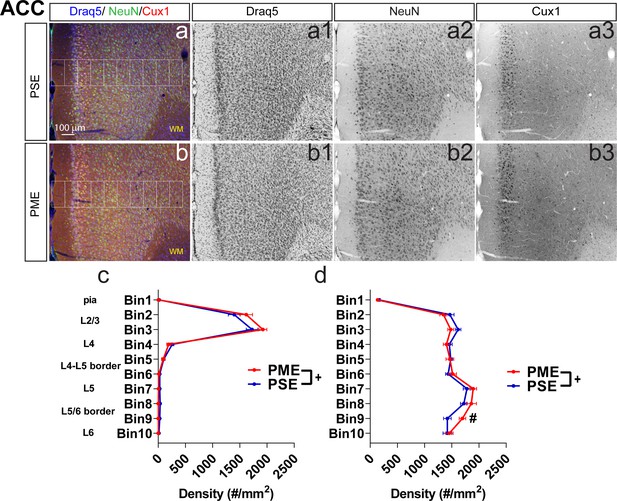
Development of the anterior cingulate cortex (ACC) is minimally affected by PME.
Representative slices of the ACC in (a) PSE and (b) PME offspring demonstrating Draq5 (blue, (a1,b1): marker of nuclei), NeuN (green, (a2,b),: marker of post-mitotic neurons), and Cux1 (red, (a3,b3): marker of upper cortical layers, typically layer 2/3-4). White boxes represent bins 1–10 for quantification with Bin one nearest to the pia and Bin 10 nearest internal white matter. There were subtle but significant effects of PME on (c) Cux1+-cell densities (rmANOVA: Interaction, p=0.028) and (d) NeuN+-cell densities (rmANOVA: Interaction, p=0.028; Sidak’s posthoc text for Bin 9, p=0.05) (n = 9 (4M:5F) PME, 8 PSE (4M:4F)). +Exposure x Bin Interaction, p=0.028 and 0.0070 for Cux1 and NeuN, respectively. #p=0.05. Data were collapsed on sex as initial analyses revealed no main-effects or interaction with sex. Data points indicate mean ± SEM.
-
Figure 7—figure supplement 1—source data 1
Numerical data to support graphs in Figure 7—figure supplement 1 describing cortical lamination and neuronal density in ACC.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig7-figsupp1-data1-v2.xlsx
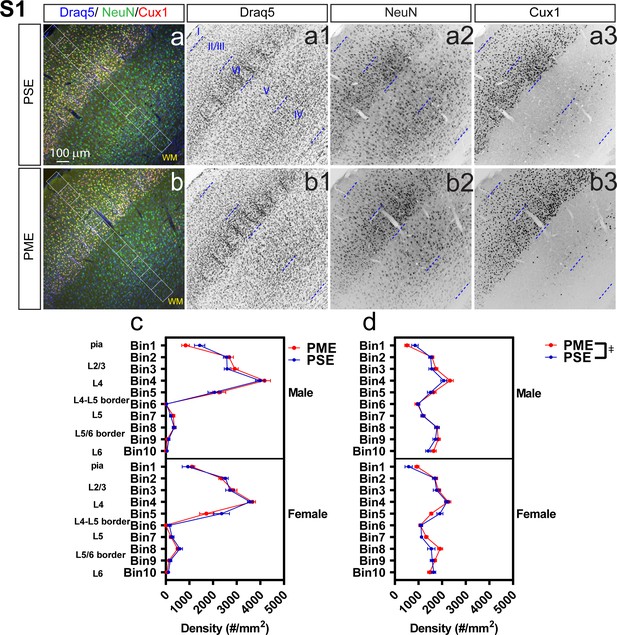
Development of the primary somatosensory cortex (S1) is minimally affected by PME.
Representative slices of the S1 in (a) PSE and (b) PME offspring demonstrating Draq5 (blue, (a1,b1): marker of nuclei), NeuN (green, (a2,b2): marker of post-mitotic neurons), and Cux1 (red, (a3,b3): marker of upper cortical layers, typically layer 2/3-4). White boxes represent bins 1–10 for quantification with Bin one nearest to the pia and Bin 10 nearest internal white matter. No effect of PME was present on (c) Cux1+- cell densities (d) but there was a slight Exposure x Bin x Sex interaction for NeuN+-cell densities (rmANOVA: Interaction, p=0.0051). However, no posthoc tests reached the level of significance (n = 9 (4M:5F) PME, 8 PSE (4M:4F)). Exposure x Bin x Sex interaction, p=0.0051. Data were not collapsed on sex as analyses revealed interactions with sex. Data points indicate mean ± SEM.
-
Figure 7—figure supplement 2—source data 1
Numerical data to support graphs in Figure 7—figure supplement 2 describing cortical lamination and neuronal density in S1.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig7-figsupp2-data1-v2.xlsx
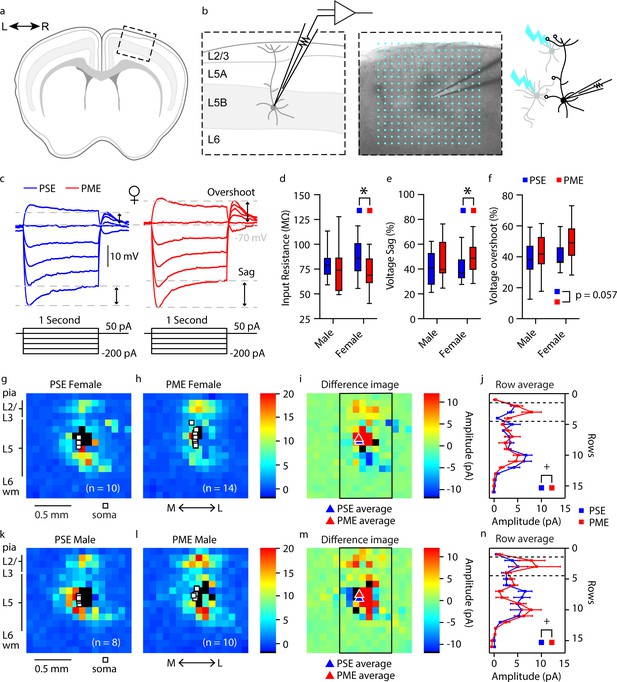
Intrinsic properties and local circuit mapping is altered by PME.
(a) Brains were coronally sectioned to acquire M1 slices for patch clamp electrophysiology. (b) Representation of a M1 neuron recording (left) and example (4X bright field image) of a M1 neuron recording overlaid with the 16 × 16 stimulation grid (100 µm spacing) for local circuit mapping (center). Schematic of laser scanning photo-stimulation of local neurons connected to recorded L5 pyramidal neuron (right). (c) Representative current-clamp traces of sub-threshold responses from L5 M1 neurons from female mice. Step protocols and current values displayed below traces. (d-f) PME decreased input resistance (d), increased voltage sag (e), and increased voltage overshoots (f) (ANOVA: Exposure, p=0.030, p=0.027, and p=0.057; n = 13 PME mice (7M:6F), 41 cells (15M:26F) and n = 11 PSE mice (6M:5F), 28 cells (15M:13F)). (g-h, k-l) Average local input maps from PSE (n = 10 cells, 5 mice) and PME (n = 14 cells, 4 mice) female (g,h) and PSE (n = 8 cells, 5 mice) and PME (n = 10 cells, 5 mice) male (k,l) mice. Each pixel represents the mean amplitude of the response evoked by UV photolysis of MNI-glutamate at that location. (i,m) PME mice demonstrate differences in local circuitry as identified by the difference in PSE average map and PME average map for female (i) and male (m) mice. (j,n) This difference in local circuitry was further supported by a significant Row X Exposure (rmANOVA: Interaction, p=0.0025) indicating PME altered local synaptic input on L5 M1 neurons in a region dependent manner (n = 9 PME mice (5M:4F), 24 cells (10M:14F) and n = 10 PSE mice (5M:5F), 18 cells (8M:10F)). *p<0.05 for main effect of exposure. + p<0.05 for row by exposure interaction. Data were not collapsed on sex as analyses revealed both main-effects of sex and interactions with sex. Box plots indicate 25th to 75th percentiles with whisker characterizing the minimum and maximum value. Row average data points indicate mean ± SEM where rows are the average input from rows 1:16, columns 6:13.
-
Figure 8—source data 1
Numerical data to support graphs in Figure 8d–f describing excitability and intrinsic properties.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig8-data1-v2.xlsx
-
Figure 8—source data 2
Numerical data to support graphs in Figure 8g–n describing local circuit mapping in L5 M1 neurons.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig8-data2-v2.xlsx
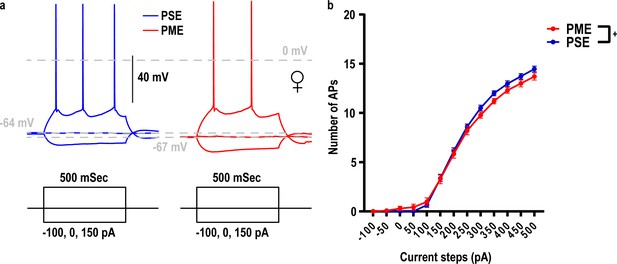
Current threshold for action potential (AP) firing in L5 M1 neurons is impacted by PME.
(a) Representative current-clamp traces of action potential firing from L5 motor cortex neurons from PSE (blue traces) and PME (red traces) female mice. (b) The number of action potentials in response to injected current in L5 M1 neurons revealed PME mice exhibit altered excitability (rmANOVA: Interaction, p=0.047; n = 40 (15M:25F) PME, 28 PSE (15M:13F)). +: Current x Exposure Interaction, p=0.047. Data were collapsed on sex as initial analyses revealed no main-effects or interaction with sex. Data points indicate mean ± SEM.
-
Figure 8—figure supplement 1—source data 1
Numerical data to support graphs in Figure 8—figure supplement 1 describing excitability of M1 L5 neurons.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig8-figsupp1-data1-v2.xlsx
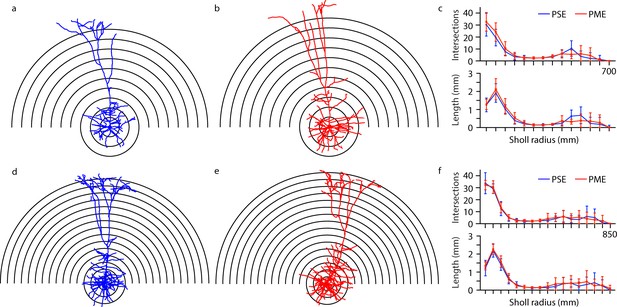
Morphological analysis of L5 M1 neurons revealed no effect of PME.
(a-b, d-e) Representative morphological reconstruction and Sholl radii of motor cortex neurons filled with biocytin during whole-cell patch-clamp recordings in acute brain slices from PSE and PME female (a–b) and male (d-e) mice (concentric circles increase by 50 µm from 50 µm). (c) Analysis of PSE (n = 9) and PME (n = 10) intersections (top) and length (mm, bottom) in female mice by Sholl radius (µm, 50:50:700) revealed no significant differences related to exposure. (f) Analysis of PSE (n = 5) and PME (n = 7) intersections (top) and length (mm, bottom) in male mice by Sholl radius (µm, 50:50:850) also revealed no significant differences related to exposure. Data were not collapsed on sex as analyses revealed both main-effects or interaction with sex. Data points indicate mean ± SEM.
-
Figure 9—source data 1
Numerical data to support graphs in Figure 9 and Figure 9—figure supplement 1 describing morphology of L5 M1 Neurons.
- https://cdn.elifesciences.org/articles/66230/elife-66230-fig9-data1-v2.xlsx
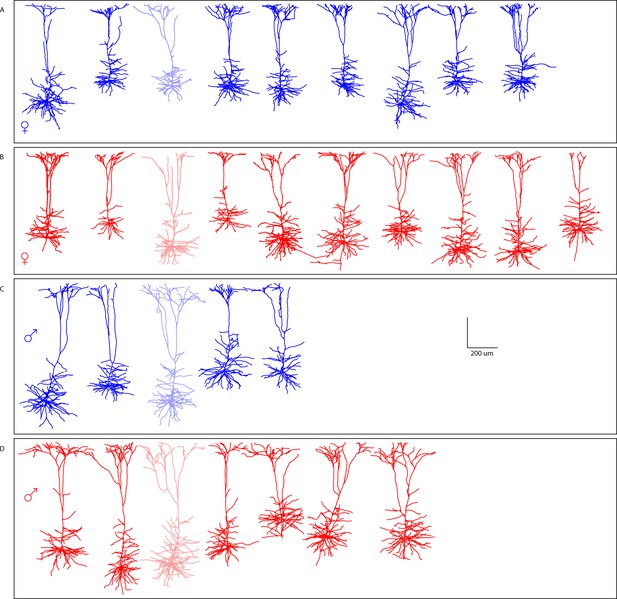
Reconstruction of M1 L5 neurons in offspring.
Morphological reconstructions confirm thick-tufted layer V M1 neurons recorded from (a) female PSE, (b) female PME, (c) male PSE, and (d) male PME mice. Partially opaque neurons are represented in Supplementary file 1.
Tables
Pregnancy and litter characteristics.
All data were analyzed using Chi-Square tests except litter size which was analyzed using an unpaired, independent t test (value represents mean ± SEM). Sex was not determined in earlier cohorts of animals until nipple marks began to appear (~P7). However, given the potential sex differences in survival, for later cohorts we began determining sex at P0 and P7 which led to a lower total sample size of litters at the P0 date of sex determination. Postnatal deaths occur within approximately 72 hr after birth. As not all litters survived to ~P7 when sex was determined in offspring, the total sample size of litters from which postnatal deaths were examined is larger than the total sample size of litters for P7 sexes. PME, Prenatal methadone exposure; PSE, Prenatal saline exposure, P0, P7, Postnatal day 0,7.
| PME | PSE | p-value | |
|---|---|---|---|
| Total pregnancies (%) | 45/61 mated (73.8%) | 28/34 mated (82.4%) | p=0.34 |
| Obstructed labor (%) | 6/42 pregnancies (14.3%) | 1/27 pregnancies (3.6%) | p=0.14 |
| Litter size | 7.50 ± 0.34 (n = 36 litters) | 6.85 ± 0.31 (n = 27 litters) | p=0.18 |
| P0 Male:Female Ratio (% Male) | 96M:66F from 21 litters (59.3%) | 73M:49F from 18 litters (59.8%) | p=0.58 |
| P7 Male:Female Ratio (% Male) | 90M:90F from 26 litters (50.0%) | 92M:64F from 25 litters (59.0%) | p=0.10 |
| Postnatal deaths (%) | 50/248 from 33 litters (20.2%) | 29/158 from 27 litters (18.4%) | p=0.65 |
| Male:Female Postnatal Death Ratio (% Male Deaths) | 18M:5F from 21 litters (78.3%) | 6M:8F from 18 litters (42.9%) | p=0.03 |
-
Table 1—source data 1
Numerical data to support graphs in Table 1 describing pregnancy and litter characteristics.
- https://cdn.elifesciences.org/articles/66230/elife-66230-table1-data1-v2.xlsx
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus male and female) | C57BL/6J | Jackson Laboratories | B6/J | |
| Antibody | Rabbit anti-Cux1 (polyclonal) | Santa Cruz Biotechnology | Cat# SC-13024, RRID:AB_2261231 | (1:250 dilution) |
| Antibody | Mouse anti-NeuN (monoclonal) | Millipore | Cat# MAB377, RRID:AB_2298772 | (1:1000 dilution) |
| Antibody | Goat anti-mouse IgG (H+L), Alexa Fluor 488 conjugate | Thermo Fisher Scientific | Cat# A-11001, RRID:AB_2534069 | (1:1000 dilution) |
| Antibody | Goat anti-rabbit IgG (H+L), Alexa Fluor 555 conjugate | Thermo Fisher Scientific | Cat# A-32732; RRID:AB_2633281 | (1:1000 dilution) |
| Antibody | Streptavidin, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# S32354; RRID:AB_2315383 | (1:1000 dilution) |
| Chemical compound, drug | Draq5 | Cell Signaling | Cat# 4084 | |
| Chemical compound, drug | Methadone HCl | National Institute on Drug Abuse | ||
| Chemical compound, drug | Oxycodone HCl | National Institute on Drug Abuse | ||
| Chemical compound, drug | MNI-caged glutamate | R and D Systems Inc (Tocris) | Cat# 295325-62-1 | |
| Chemical compound, drug | (R)-CPP | R and D Systems Inc (Tocris) | Cat# 126453-07-4 | |
| Software, algorithm | Prism 8.2 | GraphPad | ||
| Software, algorithm | Ethovision XT | Noldus | ||
| Software, algorithm | DeepSqueak | Coffey et al., 2019 | ||
| Software, algorithm | Ephus software | Vidrio Technologies | ||
| Software, algorithm | ImageJ | NIH | ||
| Software, algorithm | Matlab | MathWorks | ||
| Software, algorithm | Neurolucida 360 | MBF Bioscience | ||
| Software, algorithm | Neurolucida Explorer | MBF Bioscience | ||
| Software, algorithm | PMOD Ma-Benveniste-Mirrione mouse brain atlas | PMOD Technologies |
Additional files
-
Supplementary file 1
Dam and offspring methadone and metabolite concentrations.
n = 3 dams + their respective litters per timepoint; n = 17–20 offspring samples at G18, n = 15 offspring at P1, n = 17–18 offspring at P7. All tissue and blood samples were collected 2.5 hr following the morning administration of methadone. Data are collapsed across offspring sex. All data are mean ± SEM. EDDP: 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine. The limit of quantification for methadone and EDDP detection was 0.1 ng/mL and 0.05 ng/mL in the plasma, respectively, and 0.08 ng/sample and 0.04 ng/sample of placenta and brain for both methadone and EDDP.
- https://cdn.elifesciences.org/articles/66230/elife-66230-supp1-v2.docx
-
Supplementary file 2
Test statistics for volumetric MRI analysis.
Unpaired t tests, n = 11 (4M:7F) PME, 11 PSE (6M:5F) mice. See Figure 8—figure supplement 1 for visual representation of the data. R, Right; L, Left
- https://cdn.elifesciences.org/articles/66230/elife-66230-supp2-v2.docx
-
Supplementary file 3
Intrinsic properties of L5 M1 neurons.
Resting membrane potential evaluated with no applied current, all other properties evaluated with current applied to hold the membrane potential near minus 70 mV. Data were not collapsed on sex as analyses revealed some main-effects of sex. Data presented as mean ± SEM. F statistics (df = 1,65) are presented in the final column with significant results bolded (*p<0.05). n = 10 PME mice (6M:4F), 30 cells (13M:17F) and n = 9 PSE mice (5M:4F), 23 cells (11M:12F).
- https://cdn.elifesciences.org/articles/66230/elife-66230-supp3-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/66230/elife-66230-transrepform-v2.docx





