An excitatory lateral hypothalamic circuit orchestrating pain behaviors in mice
Figures
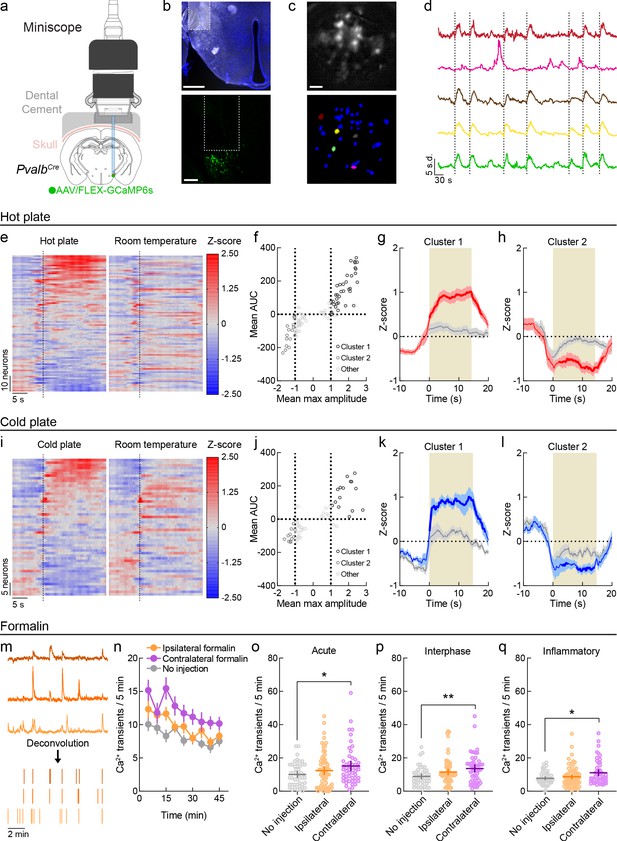
In vivo functional imaging of LHPV neurons.
(a) Schematic configuration for deep-brain functional imaging from LHPV neurons in freely moving mice. Permission to publish miniscope drawing granted by Doric Lenses Inc. (b) Top: representative GRIN lens placement for functional imaging of LHPV neurons. Scale bar: 500 µm. Bottom: depiction of GRIN lens above GCaMP6s-expressing LHPV neurons. Scale bar: 200 µm. (c) Top: sample background-subtracted frame from a recording session. Bottom: spatial footprints of extracted neural segments. Scale bar: 100 μm. (d) Representative filtered traces from individual LHPV neurons. Dotted lines represent contacts with hot plate. (e) Z-scored Ca2+ traces of LHPV neurons (87 neurons, three mice) averaged across exposures to a 51°C hot plate or a room temperature control surface. Dotted line represents contact with plate or control surface. (f) Clustering of 87 units by mean max amplitude and mean area under the curve (AUC) following hot plate surface contact. Dotted lines indicate the thresholds for inclusion into cluster 1 (mean max amplitude ≥ 1 and mean AUC ≥ 0) or cluster 2 (mean max amplitude ≤ 1 and mean AUC ≤ 0). (g) Neurons in cluster 1 (n = 35/87) displayed time-locked increases in activity in response to the hot plate as compared to the control room temperature surface. Two-way repeated-measures ANOVA on average Z-score per second revealed a significant time × stimulus interaction (F(29, 986) = 10.47, p<0.0001). Bonferroni multiple comparisons post-test significant between-stimulus differences are represented by the bolded red line. Red and gray shaded areas represent s.e.m. Tan shaded region represents average contact time with hot plate stimulus. (h) Neurons in cluster 2 (n = 16/87) displayed average decreases in activity in response to the hot plate as compared to the control room temperature surface. Two-way repeated-measures ANOVA on average Z-score per second revealed a significant time × stimulus interaction (F(29, 435) = 7.61, p<0.0001). Bonferroni multiple comparisons post-test significant between-stimulus differences are represented by the bolded red line. Red and gray shaded areas represent s.e.m. Tan shaded region represents average contact time with hot plate stimulus. (i) Z-scored Ca2+ traces of LHPV neurons (53 neurons, three mice) averaged across exposures to a 4°C cold plate or a room temperature control surface. Dotted line represents contact with plate or control surface. (j) Clustering of 53 units by mean max amplitude and mean AUC following cold plate surface contact. Dotted lines indicate the thresholds for inclusion into cluster 1 (mean max amplitude ≥ 1 and mean AUC ≥ 0) or cluster 2 (mean max amplitude ≤ 1 and mean AUC ≤ 0). (k) Neurons in cluster 1 (n = 15/53) displayed time-locked increases in activity in response to the cold plate as compared to the control room temperature surface. Two-way repeated-measures ANOVA on average Z-score per second revealed a significant time × stimulus interaction (F(29, 406) = 5.94, p<0.0001). Bonferroni multiple comparisons post-test significant between-stimulus differences are represented by the bolded blue line. Blue and gray shaded areas represent s.e.m. Tan shaded region represents average contact time with cold plate stimulus. (l) Neurons in cluster 2 (n = 11/53, top) displayed average decreases in activity in response to the hot plate as compared to the control room temperature surface. Two-way repeated-measures ANOVA on average Z-score per second revealed a significant time × stimulus interaction (F(29, 290) = 2.05, p=0.0016). Bonferroni multiple comparisons post-test significant between-stimulus differences are represented by the bolded blue line. Blue and gray shaded areas represent s.e.m. Tan shaded region represents average contact time with cold plate stimulus. (m) Illustration of fluorescent trace deconvolution to estimated periods of neuronal firing. (n) Average deconvolved events per 5 min period following no injection (n = 46 neurons) or formalin injection in the hindpaw ipsilateral (n = 67 neurons) or contralateral (n = 51 neurons) to the brain hemisphere implanted with a GRIN lens. (o–q) Formalin induced fluctuations in LHPV neuronal activity in each phase of the formalin test. Mann–Whitney U-tests with Holm–Sidak correction for multiple comparisons revealed significantly higher Ca2+ event frequency following contralateral formalin injection in the (o) acute (p=0.048), (p) interphase (p=0.0078), and (q) inflammatory phases (p=0.048) relative to no injection, whereas no significant differences were found between ipsilateral formalin and no injection (acute p=0.80, interphase p=0.18, inflammatory p=0.86). Lines and error bars indicate mean ±95% CI. See also Figure 1—figure supplements 1 and 2.
-
Figure 1—source data 1
LHPV neuronal responses to acute thermal stimuli.
- https://cdn.elifesciences.org/articles/66446/elife-66446-fig1-data1-v1.xlsx
-
Figure 1—source data 2
LHPV calcium transient frequency during formalin tests.
- https://cdn.elifesciences.org/articles/66446/elife-66446-fig1-data2-v1.xlsx
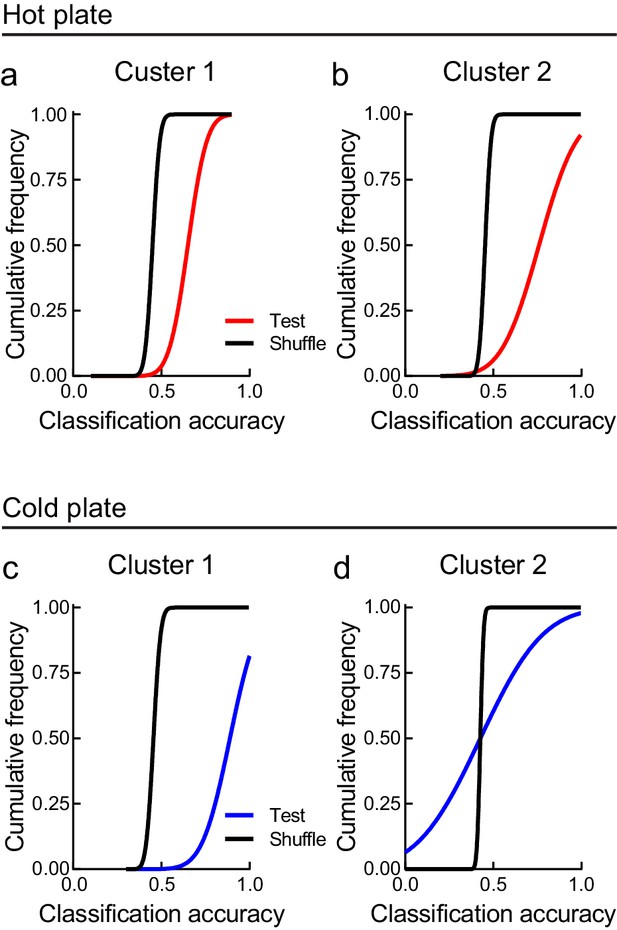
Classification accuracy of noxious and neutral stimuli from LHPV neuronal activity.
(a) Hot plate cluster 1 neuronal responses decoded hot from neutral stimulus trials above chance levels (extra sum-of-squares F-test; F(1, 14) = 2990, p<0.0001). (b) Hot plate cluster 2 neuronal responses decoded hot from neutral stimulus trials above chance levels (extra sum-of-squares F-test; F(1, 14) = 319.8, p<0.0001). (c) Cold plate cluster 1 neuronal responses decoded cold from neutral stimulus trials above chance levels (extra sum-of-squares F-test; F(1, 12) = 136.7, p<0.0001). (d) Cold plate cluster 2 neuronal responses did not decode cold from neutral stimulus trials above chance levels (extra sum-of-squares F-test; F(1, 18) = 0.146, p=0.71).
-
Figure 1—figure supplement 1—source data 1
Responses of cluster 1 and cluster 2 LHPV neurons to acute thermal stimuli used for trial-type decoding.
- https://cdn.elifesciences.org/articles/66446/elife-66446-fig1-figsupp1-data1-v1.xlsx
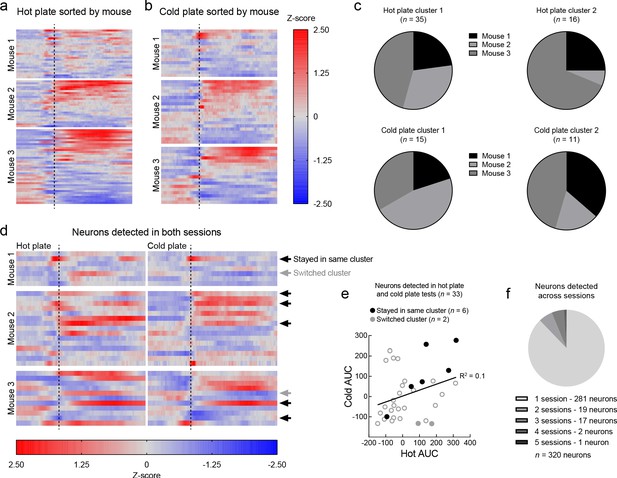
Responses of LHPV neurons to acute thermal stimuli in individual mice and cell registration across sessions.
(a) Z-scored Ca2+ traces of LHPV neurons from the hot plate test in Figure 1e and (b) the cold plate test in Figure 1i, broken down by individual mouse and sorted by post-stimulus response. Each row represents data from one neuron. (c) Proportions of neurons contributed by individual mice to clusters 1 and 2 in the hot plate and cold plate tests. (d) Results of cell registration across hot plate and cold plate tests, broken down by individual mouse. 33 total neurons were detected in both sessions. The rows of each heatmap represent the same neuron in both tests. Black arrows indicate cells that remained in the same cluster designation between tests; gray arrows indicate cells that switched cluster designation. (e) Correlation of the AUC of Z-scored Ca2+ traces to the hot or cold stimuli of the 33 neurons detected in both hot plate and cold plate sessions. No significant correlation was observed (R2 = 0.1, p=0.074). (f) Results of cell registration across hot plate, cold plate, ipsilateral formalin, contralateral formalin, and ‘no injection’ tests. Of 320 total recorded neurons, only three neurons were observed in more than three test sessions.
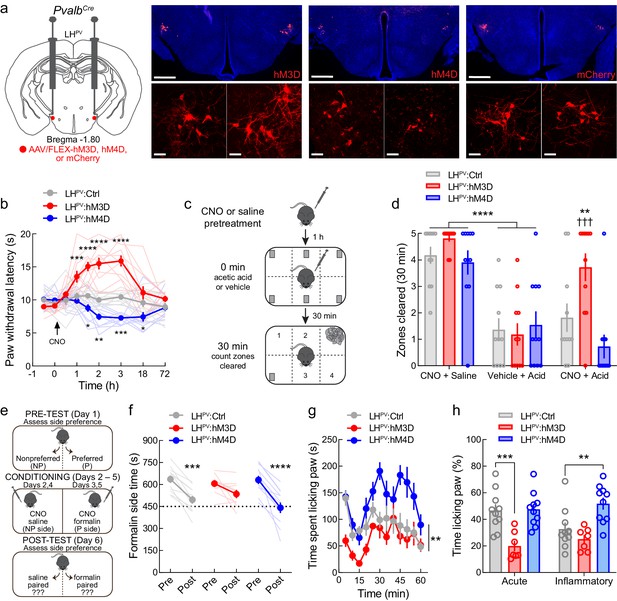
Chemogenetic modulation of LHPV neurons regulates pain-suppressed behavior and alters pain-associated negative affect.
(a) Representative images of hM3D, hM4D, or mCherry expression in LHPV neurons. Scale bars: 500 μm, widefield; 50 μm, zoom. (b) Chemogenetic activation and inhibition of LHPV neurons evoked long-lasting significant increases and decreases in thermal pain thresholds, respectively (n = 11 mice per group; two-way mixed-model ANOVA group × time interaction, F(16, 240)=14.15, p<0.0001). Significant differences from LHPV:mCherry mice were determined by Bonferroni multiple comparisons tests and are represented graphically, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. (c) Schematic for pain-suppressed nesting assay. (d) Chemogenetic activation of LHPV neurons prevented the reductions in nesting behavior induced by i.p. injection of 0.6% acetic acid (10 ml/kg). Two-way mixed-model ANOVA revealed a significant group × test interaction (n = 11 mice per group; F(4, 60) = 4.17, p=0.0048). Bonferroni multiple comparisons post-tests revealed no differences in normal nesting behavior from clozapine-N-oxide (CNO) injections (p=0.92), and that acetic acid injection decreased nesting behavior across groups when administered without CNO (p<0.0001). Administration of CNO before acetic acid increased nesting behavior in LHPV:hM3D mice relative to LHPV:mCherry control mice (p=0.008; Cohen’s d = 1.09) and tests without CNO (p=0.0002). (e) Schematic of the formalin place conditioning experiment. (f) Chemogenetic modulation of LHPV neurons altered the effects of formalin on place conditioning. Two-way mixed-model ANOVA revealed a significant group × test interaction (n = 11 mice per group; F(2,28) = 3.89, p=0.032). Bonferroni multiple comparisons post-tests showed significant shift in chamber preference in LHPV:mCherry (p=0.0001) and LHPV:hM4D mice (p<0.0001) but not LHPV:hM3D mice (p=0.094). (g) Time spent paw licking was altered by LHPV neuronal modulation (n = 10 LHPV:mCherry, 7 LHPV:hM3D, and 10 LHPV:hM4D mice; two-way mixed-model ANOVA group × time interaction, F(22, 264) = 1.99, p=0.0064). (h) Acute and inflammatory phase paw licking were differentially altered by LHPV neuronal activation and inhibition (n listed above; two-way mixed-model ANOVA group × phase interaction, F(2, 24) = 4.33, p=0.025). Activation of LHPV neurons in LHPV:hM3D mice decreased acute (p=0.0004, Cohen’s d = 2.07) but not inflammatory phase paw licking (p=0.50), whereas LHPV neuronal inhibition in LHPV:hM4D mice increased inflammatory (p=0.0049, Cohen’s d = 1.29) but not acute phase paw licking (p>0.99).
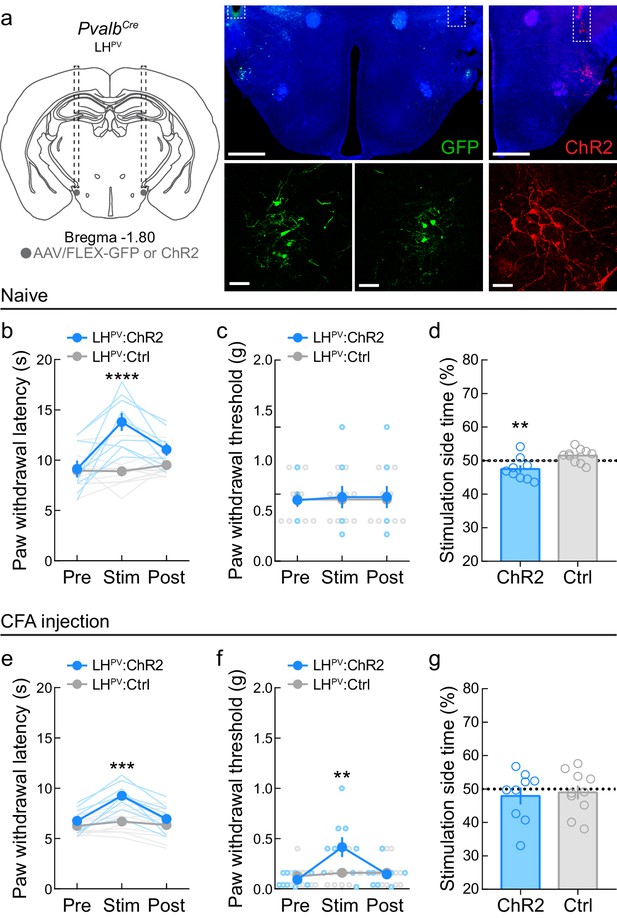
Optogenetic activation of LHPV neurons attenuates thermal and mechanical nociception following the induction of inflammatory pain.
(a) Representative images of ChR2 or GFP expression in LHPV neurons and optical fiber implants above the lateral hypothalamus (LH). Scale bars: 500 μm, widefield; 50 μm, zoom. (b) Optogenetic activation of LHPV neurons in naive mice triggers thermal antinociception (n = 9 ChR2 mice and 10 Ctrl mice). Two-way mixed-model ANOVA revealed a significant group × epoch interaction (F(2, 34) = 14.01, p<0.0001), and Bonferroni multiple comparisons post-test showed that LHPV:ChR2 mice had significantly higher PWLHP during the photostimulation epoch than LHPV:Ctrl mice, p<0.0001; Cohen’s d = 2.22. (c) Optogenetic activation of LHPV neurons in naive mice does not affect mechanical nociception (n = 9 ChR2 mice and 10 Ctrl mice). Two-way mixed-model ANOVA interaction, p=0.87. (d) Naive LHPV:ChR2 mice displayed significant real-time place avoidance to photostimulation relative to controls (n = 9 ChR2 mice and 10 Ctrl mice, t(17) = 3.15, p=0.0058, Cohen’s d = 1.43). (e) Optogenetic activation of LHPV neurons in mice 5 days following complete Freund’s adjuvant (CFA) injection triggers increases in PWLHP (n = 9 ChR2 mice and 10 Ctrl mice). Two-way mixed-model ANOVA revealed a significant group × epoch interaction (F(2, 34) = 15.05, p<0.0001), and Bonferroni multiple comparisons post-test showed that LHPV:ChR2 mice had significantly higher PWLHP during the photostimulation epoch than LHPV:Ctrl mice (p=0.0001; Cohen’s d = 2.08). (f) Optogenetic activation of LHPV neurons in mice 6 days following CFA injection triggers increases in PWTVF (n = 9 ChR2 mice and 10 Ctrl mice). Two-way mixed-model ANOVA revealed a significant group × epoch interaction (F(2, 34) = 11.28, p=0.0002), and Bonferroni multiple comparisons post-test showed that LHPV:ChR2 mice had significantly higher PWLHP during the photostimulation epoch than LHPV:Ctrl mice (p=0.003; Cohen’s d = 1.11). (g) LHPV:ChR2 mice did not display significant real-time place avoidance to photostimulation relative to controls 7 days post-CFA, p=0.75 (n = 9 ChR2 mice and 10 Ctrl mice). See also Figure 3—figure supplement 1.
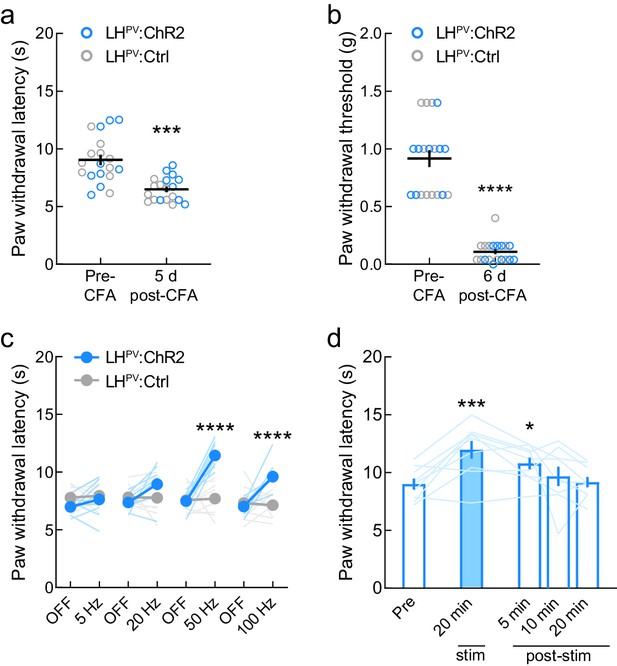
Effects of optogenetic activation of LHPV neurons following complete Freund’s adjuvant (CFA) inflammatory pain induction.
(a) CFA injection significantly decreased thermal nociception thresholds by day 5 post-administration (n = 19 mice; t(18) = 4.71, p=0.0002) and (b) mechanical nociception thresholds by day 6 post-administration (t(18) = 11.43, p<0.0001). (c) LHPV-mediated thermal antinociception post-CFA injection was dependent on the photostimulus frequency (n = 9 ChR2 and 10 Ctrl mice); two-way mixed-model ANOVA group × epoch interaction (F(7, 119) = 10.92, p<0.0001). Bonferroni multiple comparisons post-tests revealed significant between-group differences at 50 and 100 Hz (p<0.0001). Further, in LHPV:ChR2 mice 50 Hz photostimulation evoked greater antinociception than the other frequencies tested (50 vs. 100 Hz, p=0.0032, 50 vs. 20 Hz and 50 vs. 5 Hz, p<0.0001). (d) LHPV activation-induced thermal antinociception post-CFA injection was not strictly photostimulus-bound. 20 min photostimulation (50 Hz, 50 pulses delivered every other second) evoked antinociception that persisted after photostimulation ceased. Repeated-measures one-way ANOVA (n = 9 ChR2 mice; F(4, 32) = 7.074, p=0.0003). Bonferroni’s multiple comparisons post-tests showed significantly increased PWLHP from baseline during photostimulation (p=0.004) and 5 min post-photostimulation (p=0.0451).
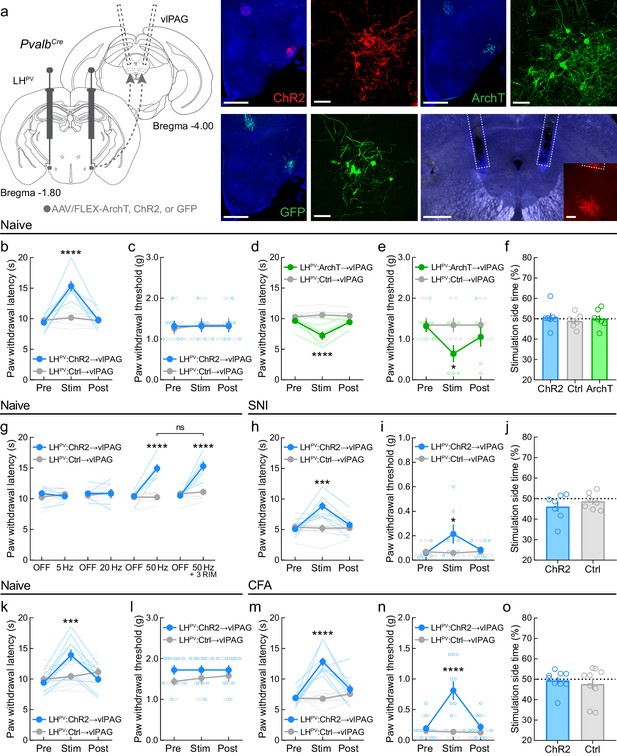
LHPV→vlPAG pathway mediates nociception in models of chronic neuropathic and inflammatory pain.
(a) Representative images of ChR2, ArchT, or GFP expression in LHPV neurons and optical fiber implants above the ventrolateral periaqueductal gray area (vlPAG). Inset shows axons from LHPV neurons under the optical fiber. Scale bars: 500 μm, widefield; 50 μm, zoom; 100 μm, inset. (b) In naive mice, optogenetic activation of LHPV axonal projections in the vlPAG evokes thermal antinociception (n = 7 mice per group; two-way mixed-model ANOVA group × epoch interaction, F(2, 24) = 25.19, p<0.0001, Bonferroni multiple comparisons post-test during photostimulation epoch, p<0.0001; Cohen’s d = 2.85) but not (c) mechanical antinociception (p=0.38). (d) In naive mice, optogenetic inhibition of LHPV axonal projections in the vlPAG decreases both thermal (n = 7 mice per group; two-way mixed-model ANOVA group × epoch interaction, F(2, 24) = 11.96, p=0.0002, Bonferroni multiple comparisons post-test during photostimulation epoch, p<0.0001; Cohen’s d = 2.13) and (e) mechanical thresholds (two-way mixed-model ANOVA group × epoch interaction, F(2, 24) = 12.10, p<0.0002, Bonferroni multiple comparisons post-test during photostimulation epoch, p=0.038; Cohen’s d = 1.36). (f) Optogenetic activation or inhibition of the LHPV→vlPAG pathway did not affect real-time place preference behavior in naive mice (n = 7 mice per group; one-way ANOVA, F(2, 18) = 0.28, p=0.76). (g) LHPV→vlPAG activation-induced antinociception is dependent on photostimulus frequency but is not attenuated by the CB1 receptor antagonist rimonabant (3 mg/kg, i.p.; ‘3 RIM’). Two-way mixed-model ANOVA revealed a significant group × epoch interaction (n = 6 ChR2 mice and 7 Ctrl mice; F(7, 77) = 14.27, p<0.0001). Bonferroni multiple comparisons post-tests revealed between-group differences during the ‘50 Hz’ and ‘50 Hz + 3 RIM’ epochs (p<0.0001), but no within-group differences between these epochs (p>0.99). (h) Optogenetic activation of the LHPV→vlPAG pathway evokes increases in PWLHP on day 5 post-spared nerve injury (SNI) (n = 7 mice per group; two-way mixed-model ANOVA group × epoch interaction, F(2, 24) = 12.86, p<0.0001, Bonferroni multiple comparisons post-test during photostimulation epoch, p=0.0002; Cohen’s d = 2.04) and (i) PWTVF on day 6 post-SNI (two-way mixed-model ANOVA group × epoch interaction, F(2, 24) = 5.24, p<0.013, Bonferroni multiple comparisons post-test during photostimulation epoch, p=0.019; Cohen’s d = 1.03). (j) On day 7 post-SNI, optogenetic activation of the LHPV→vlPAG pathway did not affect real-time place preference behavior (n = 7 mice per group; p=0.39). (k) In a new cohort of naive mice, optogenetic activation of the LHPV→vlPAG pathway evoked thermal (n = 10 mice per group; two-way mixed-model ANOVA group × epoch interaction, F(2, 36) = 23.64, p<0.0001, Bonferroni multiple comparisons post-test during photostimulation epoch, p=0.0009) but not (l) mechanical antinociception (p=0.31). (m) Optogenetic activation of the LHPV→vlPAG pathway evokes increases in PWLHP on day 5 post-complete Freund’s adjuvant (CFA) injection (n = 10 mice per group; two-way mixed-model ANOVA group × epoch interaction, F(2, 36) = 19.65, p<0.0001, Bonferroni multiple comparisons post-test during photostimulation epoch, p<0.0001; Cohen’s d = 3.66) and (n) PWTVF on day 6 post-SNI (two-way mixed-model ANOVA group × epoch interaction, F(2, 36) = 24.63, p<0.0001, Bonferroni multiple comparisons post-test during photostimulation epoch, p<0.0001; Cohen’s d = 1.88). (o) On day 7 post-CFA injection, optogenetic activation of the LHPV→vlPAG pathway did not affect real-time place preference behavior (n = 9 mice per group; p=0.59). See also Figure 4—figure supplements 1–3.
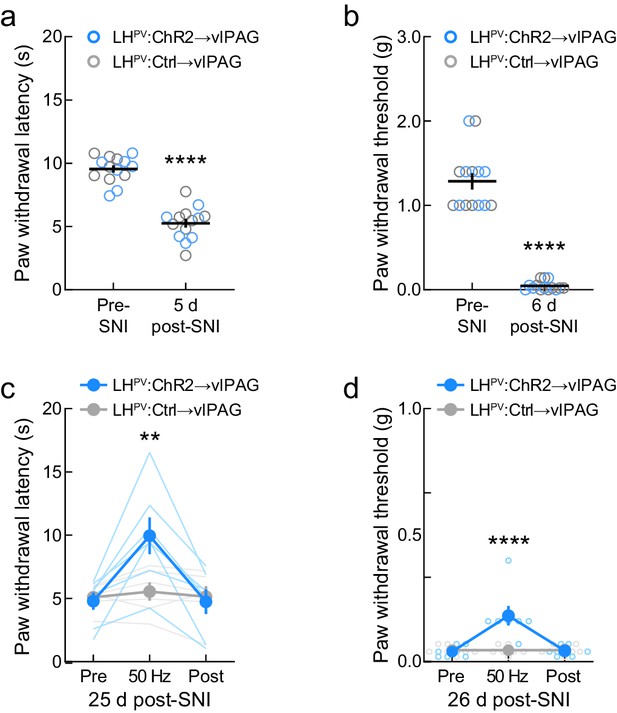
Effects of optogenetic activation of LHPV axonal projections in the ventrolateral periaqueductal gray area (vlPAG) following spared nerve injury (SNI) neuropathic pain induction.
(a) SNI significantly decreased thermal nociception thresholds by day 5 post-surgery (n = 7 mice per group; t(13) = 9.70, p<0.0001) and (b) mechanical nociception thresholds by day 6 post-surgery (t(13) = 13.09, p<0.0001). (c) Activation of the LHPV→vlPAG pathway evoked thermal antinociception on day 25 post-SNI (n = 7 ChR2 mice and 6 Ctrl mice; two-way mixed-model ANOVA group × epoch interaction, F(2, 22) = 12.26, p=0.0003, Bonferroni multiple comparisons post-test during photostimulation, p=0.007). (d) Activation of the LHPV→vlPAG pathway evoked mechanical antinociception on day 26 post-SNI (n = 7 mice per group; group × epoch interaction, F(2, 24) = 17.21, p<0.0001, Bonferroni multiple comparisons test during photostimulation, p=0.0001).
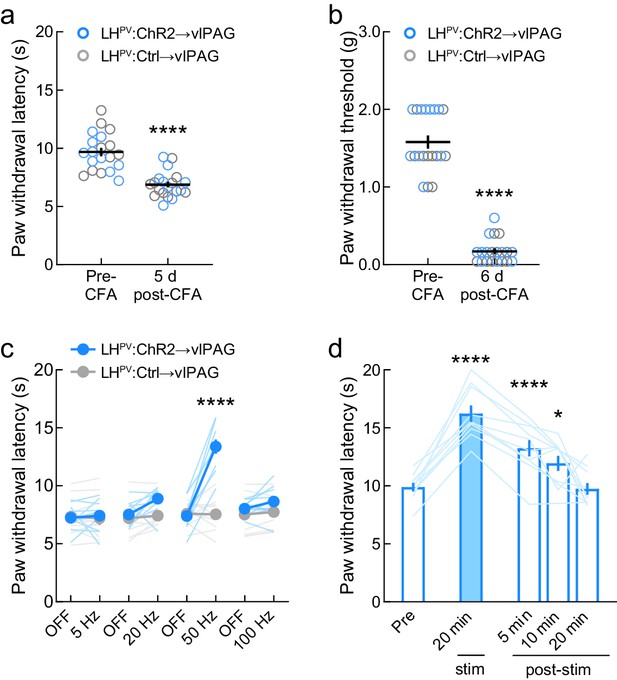
Effects of optogenetic activation of LHPV axonal projections in the ventrolateral periaqueductal gray area (vlPAG) following complete Freund’s adjuvant (CFA) inflammatory pain induction.
(a) CFA injection significantly decreased thermal nociception thresholds by day 5 post-administration (n = 20 mice; t(19) = 7.24, p<0.0001) and (b) mechanical nociception thresholds by day 6 post-administration (t(19) = 16.06, p<0.0001). (c) LHPV→vlPAG pathway-mediated thermal antinociception post-CFA injection was photostimulus frequency-dependent (n = 10 ChR2 mice and 10 Ctrl mice; two-way mixed-model ANOVA group × epoch interaction, F(7, 126) = 17.08, p<0.0001). Bonferroni multiple comparisons post-tests revealed significant between-group differences at 50 Hz (p<0.0001). (d) LHPV→vlPAG pathway activation-induced thermal antinociception post-CFA injection was not strictly photostimulus-bound. 20 min photostimulation (50 Hz, 50 pulses delivered every other second) evoked antinociception that persisted after photostimulation ceased (n = 10 ChR2 mice; repeated-measures one-way ANOVA, F(4, 36) = 36.13, p<0.0001). Bonferroni’s multiple comparisons post-tests showed significantly increased PWLHP from baseline during photostimulation (p<0.0001), 5 min post-photostimulation (p<0.0001), and 10 min post-photostimulation (p=0.01).
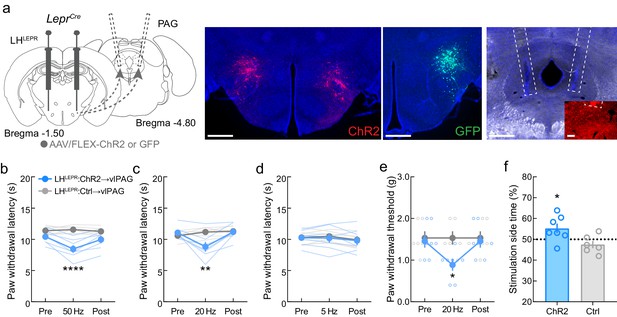
Behavioral outputs evoked by optogenetic activation of GABAergic LHLEPR axonal projections in the ventrolateral periaqueductal gray area (vlPAG).
(a) Representative images of ChR2 or GFP expression in LHLEPR neurons and optical fiber implants above the vlPAG. Inset shows axons from LHPV neurons under the optical fiber. Scale bars: 500 μm, widefield; 100 μm, inset. (b) Photostimulation of LHLEPR axonal projections in the vlPAG evoked decreases in thermal nociceptive thresholds at 50 Hz photostimulation (n = 7 ChR2 mice and 6 Ctrl mice; two-way mixed-model ANOVA group × epoch interaction, F(2, 22) = 15.68, p<0.0001, Bonferroni multiple comparisons post-test during photostimulation, p<0.0002), and (c) 20 Hz photostimulation (group × epoch interaction, F(2, 22) = 12.64, p=0.0012, Bonferroni multiple comparisons post-test during photostimulation, p=0.005) but not at (d) 5 Hz photostimulation (p=0.89). (e) Photostimulation of LHLEPR axonal projections in the vlPAG evoked decreases in mechanical nociceptive thresholds at 20 Hz photostimulation (n = 7 ChR2 mice and 6 Ctrl mice; group × epoch interaction, F(2, 22) = 42.31, p<0.0001, Bonferroni multiple comparisons post-test during photostimulation, p=0.018). (f) Optogenetic activation (20 Hz) of the LHLEPR→vlPAG pathway was rewarding as it evoked significant real-time place preference (n = 7 ChR2 mice and 6 Ctrl mice; p=0.025).
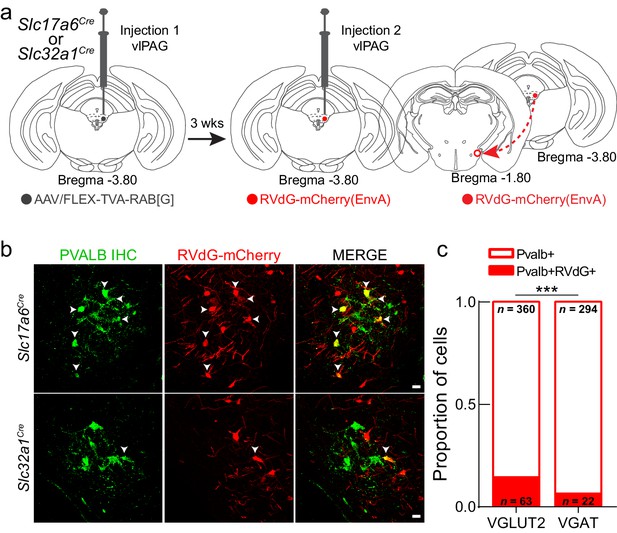
LHPV neurons preferentially target glutamatergic neurons in the ventrolateral periaqueductal gray area (vlPAG).
(a) Schematic for modified rabies viral tracing strategy. (b) Images from Slc17a6Cre (top row) and Slc32a1Cre (bottom row) brain slices showing the overlap of RVdG-mCherry(EnvA) with LHPV neurons. Scale bars: 20 μm. (c) Proportion of LHPV neurons that express or do not express RVdG-mCherry(EnvA) in Slc17a6Cre or Slc32a1Cre mice. LHPV neurons were connected to a greater proportion of vlPAGVGLUT2 neurons than vlPAGVGAT neurons (chi-square = 11.18, p=0.0008).
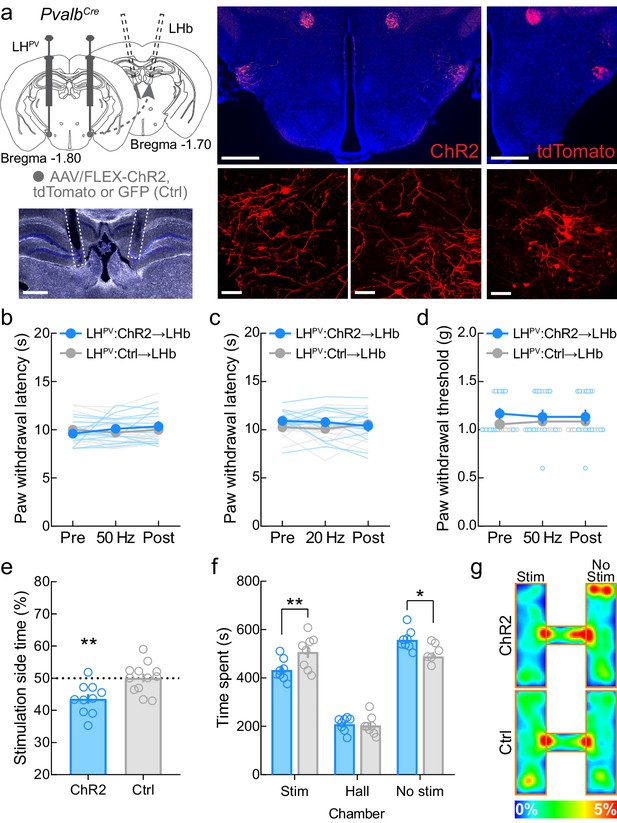
Activation of the LHPV→LHb pathway triggers aversion but not antinociception.
(a) Representative images of ChR2 and tdTomato expression in LHPV neurons and optical fiber implants above the lateral habenula (LHb). Scale bars: 500 μm, widefield; 50 μm, zoom. (b) Optogenetic activation of LHPV axonal projections in the LHb does not alter thermal nociception at 50 Hz photostimulus frequency (p=0.16) or (c) 20 Hz photostimulus frequency (p=0.23) in healthy mice (n = 13 ChR2 mice and 14 Ctrl [GFP/tdTomato] mice). (d) Optogenetic activation of LHPV axonal projections in the LHb does not alter mechanical nociception at 50 Hz (p=0.15) in healthy mice (n = 13 ChR2 mice and 14 Ctrl mice). (e) Optogenetic activation of the LHPV→LHb pathway evokes significant real-time place aversion (p=0.0041; Cohen’s d = 1.38) in a standard rectangular one-chamber testing apparatus (n = 10 ChR2 mice and 12 Ctrl mice). (f) Optogenetic activation of the LHPV→LHb pathway also evokes real-time place aversion in a three-chamber testing apparatus (n = 8 mice per group); two-way mixed-model ANOVA group × chamber interaction, F(2, 28) = 6.22, p=0.0058, Bonferroni’s multiple comparisons post-test revealed the LHPV:ChR2→LHb group spent less time in the photostimulation chamber (p=0.0089; Cohen’s d = 1.28) and more time in the no photostimulation chamber (p=0.016) than LHPV:Ctrl→LHb control mice, but no differences were observed in hall zone occupancy (p>0.99). (g) Representative heatmaps of LHPV:ChR2→LHb and LHPV:Ctrl→LHb mice in a three-chamber real-time place preference session. See also Figure 6—figure supplement 1.
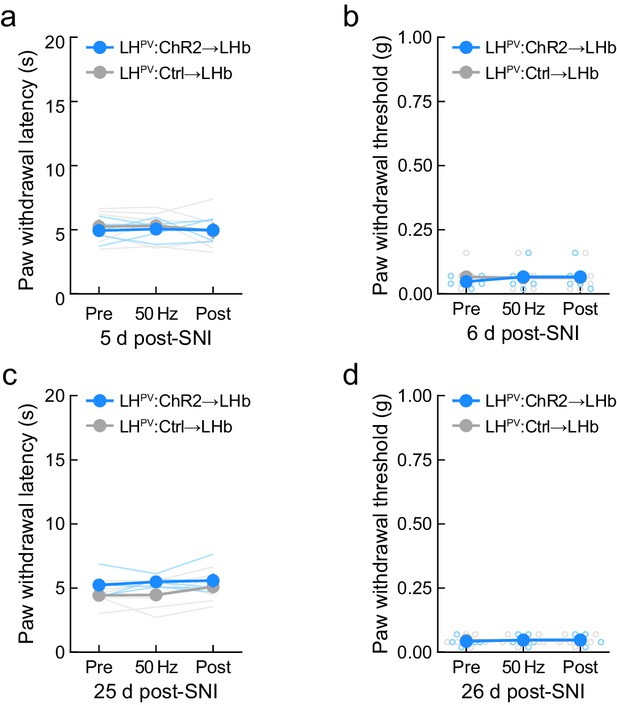
Effects of optogenetic activation of LHPV axonal projections in the lateral habenula (LHb) following complete Freund’s adjuvant (CFA) inflammatory pain induction.
(a) Activation of the LHPV→LHb pathway did not influence thermal (n = 5 ChR2 mice and 6 Ctrl mice; p=0.83) or (b) mechanical nociception (p=0.22) when tested on day 5 or day 6 post-spared nerve injury (SNI), respectively. (c) LHPV→LHb activation did not change thermal (n = 5 ChR2 mice and 6 Ctrl mice; p=0.65) or (d) mechanical nociception (p=0.32) when tested on day 25 or day 26 post-SNI, respectively.
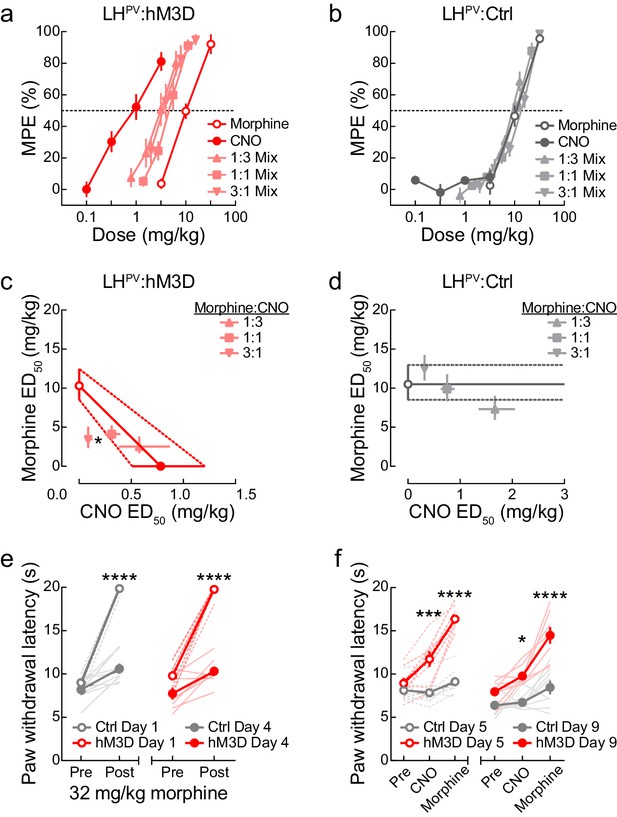
Antinociceptive interactions between LHPV neuronal activation and morphine.
(a) Dose-response curves of clozapine-N-oxide (CNO) and morphine alone or in combinations of different fixed proportions in LHPV:hM3D and (b) LHPV:Ctrl mice in the hot plate test (n = 8 mice per group). (c, d) Isobolograms constructed from the data shown in panels (a) and (b). Each point represents the ED50 ± 95% CI of each drug alone or in a mixture; ordinates represent the ED50 value of morphine and abscissae represent the ED50 value of CNO. In LHPV:hM3D mice, the 3:1 morphine:CNO mixture was significantly more potent than predicted by the hypothesis of additivity (paired Student’s t-test, t(7) = 2.92, p=0.022). (e) Both groups of mice developed significant antinociceptive tolerance to 32 mg/kg morphine when administered twice per day for 3 days. Three-way mixed-model ANOVA revealed a significant morphine × test interaction (n = 8 mice per group; F(1, 14) = 134.7, p<0.0001), and Bonferroni multiple comparisons post-tests showed the antinociceptive effects of 32 mg/kg morphine were significantly lower on day 4 than day 1 (both p<0.0001). (f) Activation of LHPV neurons restored morphine potency, and further tolerance did not develop to combination treatment. Three-way mixed-model ANOVA revealed a significant treatment × group interaction (n = 8 mice per group; F(2, 28) = 42.10, p<0.0001). Bonferroni multiple comparisons post-tests revealed that there were between-group differences in PWLHP evoked on day 5 by CNO (p=0.0006) and morphine (p<0.0001) and on day 9 by CNO (p=0.016) and morphine (p<0.0001). However, no within-group differences were observed between day 5 and 9 in LHPV:hM3D mice during CNO (p>0.99) or morphine treatment (p>0.99).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (Mus musculus; male/female) | PvalbCre | The Jackson Laboratory | RRID:IMSR_JAX:008069 | C57BL/6J background |
| Genetic reagent (M. musculus; male/female) | Slc32a1Cre | The Jackson Laboratory | RRID:IMSR_JAX:028862 | C57BL/6J background |
| Genetic reagent (M. musculus; male/female) | Slc17a6Cre | The Jackson Laboratory | RRID:IMSR_JAX:028863 | C57BL/6J background |
| Genetic reagent (M. musculus; male/female) | LeprCre | M.G. Myers Jr., University of Michigan Medical School | RRID:IMSR_JAX:032457 | C57BL/6J background |
| Antibody | Anti-DsRed, rabbit polyclonal | Takara Bio, Inc | Cat # 632496 RRID:AB_10013483 | (1:1000) |
| Antibody | Anti-parvalbumin (PVALB), guinea pig polyclonal | Swant | Cat # GP72; RRID:AB_2665495 | (1:300) |
| Antibody | Anti-rabbit Alexa Fluor 488, goat polyclonal | Thermo Fisher Scientific | Cat # A11034 RRID:AB_2576217 | (1:500) |
| Antibody | Anti-guinea pig Alexa Fluor 488, donkey polyclonal | Jackson ImmunoResearch Laboratories | Cat # 706-545-148; RRID:AB_2340472 | (1:500) |
| Antibody | Anti-guinea pig Alexa Fluor 647, donkey polyclonal | Jackson ImmunoResearch Laboratories | Cat # 706-605-148; RRID:AB_2340476 | (1:500) |
| Recombinant DNA reagent | rAAV2/9-CAG-FLEX-GCaMP6s-WPRE-SV40 | Addgene | RRID:Addgene_10084; Addgene viral prep 100842-AAV9 | 5.0 × 1012 GC/ml |
| Recombinant DNA reagent | rAAV2/rh10-hSYN-DIO-hM3D(Gq)-mCherry | University of North Carolina (UNC) Vector Core | RRID:Addgene_44361 | 2.0 × 1012 GC/ml |
| Recombinant DNA reagent | rAAV2/rh10-hSYN-DIO-hM4D(Gi)-mCherry | UNC Vector Core | RRID:Addgene_44362 | 2.0 × 1012 GC/ml |
| Recombinant DNA reagent | rAAV2/9-hSYN-DIO-mCherry | Addgene | RRID:Addgene_50459; Addgene viral prep 50459-AAV9 | 2.1 × 1013 GC/ml |
| Recombinant DNA reagent | rAAV2/1-CAG-FLEX-rev-ChR2-tdTomato | Addgene | RRID:Addgene_18917; Addgene viral prep 18917-AAV1 | 6.9 × 1012 GC/ml |
| Recombinant DNA reagent | rAAV2/9-CAG-FLEX-ArchT-GFP | UNC Vector Core | RRID:Addgene_29777 | 4.7 × 1012 GC/ml |
| Recombinant DNA reagent | rAAV2/9-CAG-FLEX-GFP | University of Pennsylvania (U Penn) Vector Core | RRID:Addgene_51502 | 3.3 × 1013 GC/ml |
| Recombinant DNA reagent | rAAV2/1-CAG-FLEX-tdTomato | U Penn Vector Core | RRID:Addgene_51503 | 4.5 × 1013 GC/ml |
| Recombinant DNA reagent | rAAV2/9-CAG-FLEX-tdTomato | U Penn Vector Core | RRID:Addgene_51503 | 4.1 × 1013 GC/ml |
| Recombinant DNA reagent | rAAV2/8-hSYN-FLEX-TVA-Rabies B19G (TVA+) | Michigan Diabetes Research Center Molecular Genetics Core, University of Michigan | 4 × 1012 GC/ml | |
| Recombinant DNA reagent | EnvA-∆G-Rabies-mCherry | Michigan Diabetes Research Center Molecular Genetics Core, University of Michigan | 1 × 1010 pfu/ml | |
| Chemical compound, drug | Clozapine N-oxide (CNO) | Tocris Bioscience | Cat # 4936; PUBCHEM:135445691 | |
| Chemical compound, drug | Acetic acid | Sigma-Aldrich | Cat # 320099; PUBCHEM:176 | |
| Chemical compound, drug | Formalin | Macron Fine Chemicals | Cat # 5016–02; PUBCHEM:712 | |
| Chemical compound, drug | Complete Freund’s adjuvant (CFA) | Sigma-Aldrich | Cat # F5881 | |
| Chemical compound, drug | Morphine | National Institute on Drug Abuse Drug Supply Program | PUBCHEM:5288826 | |
| Software, algorithm | ANY-maze video tracking system v5 | Stoelting Co. | RRID:SCR_014289 | |
| Software, algorithm | Doric Neuroscience Studio v5.1 | Doric Lenses Inc | RRID:SCR_018569 | |
| Software, algorithm | FIJI/ImageJ v1.52p | https://imagej.net/Fiji | RRID:SCR_002285 | |
| Software, algorithm | Prism 8 | GraphPad | RRID:SCR_002798 | |
| Software, algorithm | Miniscope Analysis Pipeline | Etter, 2021 | ||
| Software, algorithm | CellReg | Sheintuch et al., 2021 | ||
| Software, algorithm | MATLAB | MathWorks | RRID:SCR_001622 | R2019a & R2020a |
| Other | Snap-in Imaging Cannula Model L-V | Doric Lenses Inc | GRIN lenses | |
| Other | Basic Fluorescence Snap-In Microscopy System – Deep Brain | Doric Lenses Inc | In vivo imaging system |





