Ubiquitination and degradation of NF90 by Tim-3 inhibits antiviral innate immunity
Figures
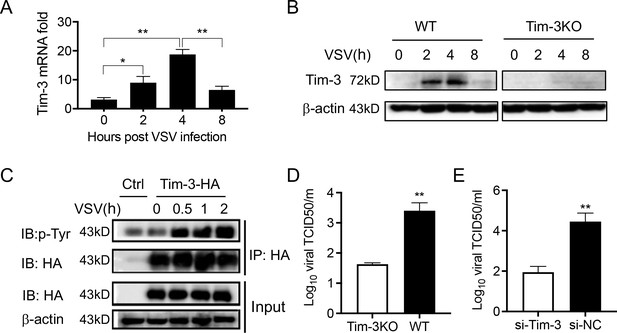
Tim-3 inhibits VSV replication in macrophages.
(A) Peritoneal macrophages were isolated from wild-type (WT) mice and infected with vesicular stomatitis virus (VSV) for the indicated hours. Then the Tim-3 mRNA expression was examined by quantitative reverse transcription PCR (qPCR). (B) Peritoneal macrophages were isolated from WT and Tim-3 gene (Havcr2) knockout (Tim-3KO) mice and were infected with VSV for the indicated times. Then the expression of Tim-3 was analyzed by western blot analysis. (C) VSV infection induces tyrosine phosphorylation of Tim-3. HA-Tim-3 plasmid was transfected into HEK293T cells for 24 hr, and the cells were infected with VSV for the indicated hours. Cell lysates were immunoprecipitated with Human influenza hemagglutinin(HA) antibody and analyzed by immunoblotting for the indicated proteins. (D and E) Peritoneal macrophages obtained from WT and Tim-3KO mice and RAW264.74 macrophages silenced of Tim-3 (si-Tim-3) and RAW264.7 macrophages (si-NC) were challenged by VSV for 6 hr, and then the cells were harvested for VSV load analysis by 50% tissue cell infectious dose (TCID50) assay. The results shown in all panels were performed three times. *p<0.05, **p<0.01.
-
Figure 1—source code 1
Flox gene amplication.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig1-code1-v3.zip
-
Figure 1—source code 2
Cre gene amplication.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig1-code2-v3.zip
-
Figure 1—source code 3
Genotype identification.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig1-code3-v3.zip
-
Figure 1—source code 4
Phenotype identification.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig1-code4-v3.zip
-
Figure 1—source data 1
Tim-3 mRNA expression in macrophages in response to VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig1-data1-v3.zip
-
Figure 1—source data 2
Tim-3 protein expression in macrophage in response to VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig1-data2-v3.zip
-
Figure 1—source data 3
VSV induces Tim-3 phosphorylation .
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig1-data3-v3.zip
-
Figure 1—source data 4
Tim-3 signaling promotes VSV replication in macrophage .
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig1-data4-v3.zip
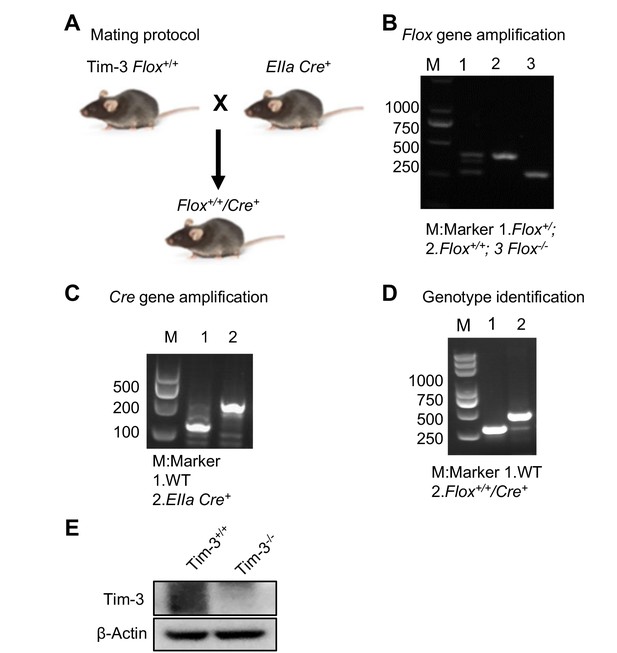
Development and identification of Tim-3 knockout mice.
(A) Mating protocol. (B and C) Mice tails were cut and DNA was extracted. Flox gene (B) and Cre gene (C) segments were PCR amplified and determined by electrophoresis. (D) Genotype examination to select the mice expressing both the Tim-3-flox and EIIa-Cre genes. (E) Western blot analysis of Tim-3 expression in peritoneal macrophages from wild-type (WT) and Tim-3KO mice. The results shown in panels (B–D) were performed three times.
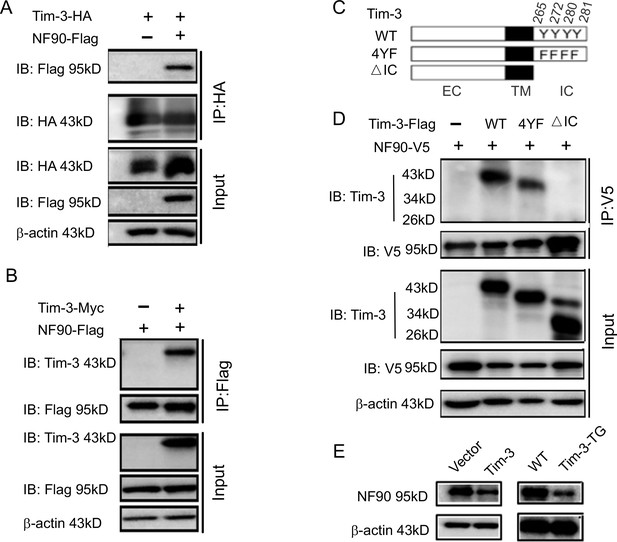
Tim-3 interacts with and inhibits NF90.
(A and B) Protein complex of Tim-3 and NF90 overexpressed in cells. HEK293T cells were transfected with plasmids encoding HA-Tim-3, Flag-NF90, and Myc-Tim-3 for 24 hr, immunoprecipitated with HA or Flag antibody, respectively, and detected by western blot for the indicated antibodies. (C and D) Interaction of Tim-3 intracellular domain with NF90. Schematic structure of Tim-3 and the derivatives used are shown (C). Whole-cell lysis of HEK293T cells transfected with Flag-Tim-3 (WT), Flag-Tim-3 (Del), Flag-Tim-3 (4YF), and V5-NF90 was used for immunoprecipitation and immunoblotting, as indicated (D). (E) Immunoblot analysis of NF90. HEK293T cells were transfected with Tim-3 plasmid for 24 hr, and lysates were detected for NF90 expression by western blot (left). Peritoneal macrophages from wild-type (WT) and Tim-3-TG mice were lysed and NF90 protein was detected by western blot (right). The results shown in panels (A, B, D, and E) were performed three times.
-
Figure 2—source code 1
Tim-3-WT were transfected into HEK293T cells.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig2-code1-v3.zip
-
Figure 2—source code 2
Candidates interacts with Tim-3.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig2-code2-v3.zip
-
Figure 2—source data 1
Tim-3 interacts with NF90.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig2-data1-v3.zip
-
Figure 2—source data 2
NF90 interacts with Tim-3.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig2-data2-v3.zip
-
Figure 2—source data 3
Tim-3 interacts with NF90 via intracellular tail.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig2-data3-v3.zip
-
Figure 2—source data 4
Tim-3 inhibits NF90.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig2-data4-v3.zip
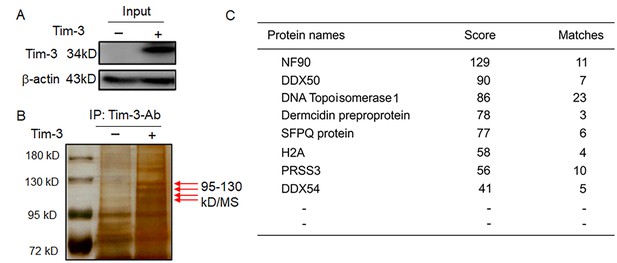
MS data showing Tim-3-interacting proteins in macrophages.
(A and B) Co-immunoprecipitation and mass spectrometry for screening of proteins interacting with Tim-3. Tim-3-WT were transfected into HEK293T cells for 24 hr, cell lysates were immunoprecipitated with antibody to Tim-3, and precipitates were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS/PAGE) and visualized by silver staining. Differential bands were subjected to trypsin digestion and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis (B, arrows). (C) NF90 (ILF3), a new protein, associates with Tim-3. 11 peptides derived from a 95- to 130-kD protein ladder matched with amino acid sequence of the NF90 protein (defined by Mascot score). The results shown in all panels were performed three times. All the matched peptides can be found using the following link: file:///Users/hangencheng/Library/Containers/com.tencent.xinWeChat/Data/Library/Application%20Support/com.tencent.xinWeChat/2.0b4.0.9/60a5a94034905dff41ba3805bd9c0182/Message/MessageTemp/6c186828c1bcceb1559f238406f1ac30/File/110.htm.
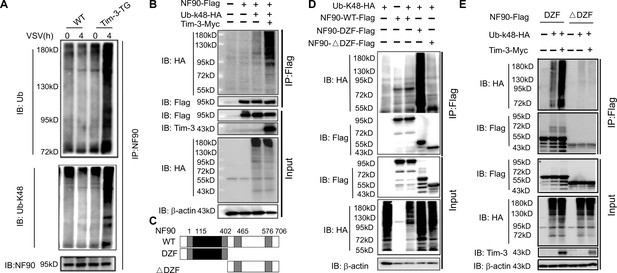
Tim-3 promotes the ubiquitination of NF90 at the DZF domain.
(A) Tim-3 enhances the ubiquitination of NF90 in macrophages in response to vesicular stomatitis virus (VSV) challenges. Peritoneal macrophages in wild-type (WT) and Tim-3-TG mice were infected with VSV for 4 hr and cells were treated with MG132 (20 ug/ml) for 6 hr before harvesting protein lysates, followed by western blot analysis of the total Ub and K48-linked Ub of NF90 immunoprecipitated with antibody to ILF3 (NF90). (B) Tim-3 promotes the ubiquitination of NF90. HEK293T cells were transfected with plasmids encoding Flag-NF90 or HA-Ub-K48, Tim-3-Myc for 24 hr, treated with MG132 (20 ug/ml) for 6 hr, immunoprecipitated with Flag antibody, and then detected by western blot for the indicated antibodies. (C) Schematic structure of NF90 and the derivatives used are shown. (D–E) Tim-3 promotes the K48-Ub modification of NF90 at the DZF domain. HEK293T cells were transfected with the indicated plasmids for 24 hr and treated with MG132 (20 ug/ml) for 6 hr. The cells were then lysed, immunoprecipitated with Flag antibody, and detected by western blot using the indicated antibodies. Three independent experiments were conducted for all panels.
-
Figure 3—source code 1
Tim-3 mediates NF90 ubiquitination.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig3-code1-v3.zip
-
Figure 3—source data 1
Tim-3 promotes ubiquitination of NF90.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig3-data1-v3.zip
-
Figure 3—source data 2
Tim-3 promotes k48-linked ubiquitination of NF90.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig3-data2-v3.zip
-
Figure 3—source data 3
NF90 canbe ubiquitinated at the DZF domain.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig3-data3-v3.zip
-
Figure 3—source data 4
Tim-3 promotes NF90 ubiquitination at the DZF domain.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig3-data4-v3.zip
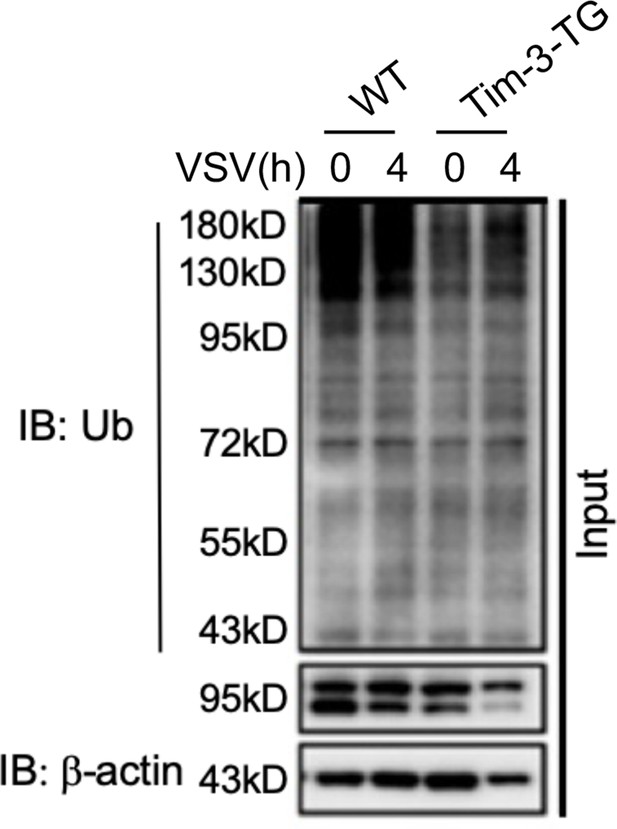
Peritoneal macrophages from WT and Tim-3-TG mice were infected with VSV for 4 hr and treated with MG132 (20 ug/ml) for 6 hr.
The cells were lysed, immunoprecipitated with NF90 (ILF3) antibody, and detected by western blot using the indicated antibodies.
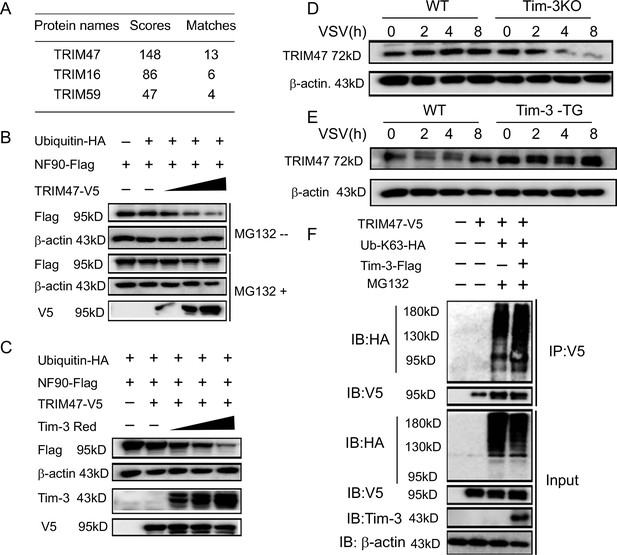
Involvement of TRIM47 in Tim-3-mediated NF90 degradation.
(A) E3 ligases identified by mass spectrometry for top peptide hits (defined by Mascot score) associated with NF90 ubiquitination. (B) TRIM47 promotes NF90 degradation in a proteasome-dependent manner. Plasmids encoding Flag-NF90, HA-ubiquitin, along with increasing amounts of V5-TRIM47 (0.5, 1.0, and 2.0 ug), were transfected into HEK293T cells for 24 hr, cells were treated with and without MG132 (20 ug /ml), respectively, followed by western blot to examine the NF90 protein level. (C) Tim-3 accelerates TRIM47-mediated NF90 degradation in a dose-dependent manner. HEK293T cells were transfected with plasmids encoding Flag-NF90, HA-ubiquitin, and V5-TRIM47 and an increasing dose of plasmid encoding Red-Tim-3 (0.5, 1.0, and 2 ug) for 24 hr. The protein level of NF90 was examined in cells. (D and E) Tim-3 upregulates TRIM47 in protein levels. TRIM47 protein levels were analyzed by immunoblotting in lysates from wild-type (WT) or Tim-3KO and WT or Tim-3-TG macrophages infected with vesicular stomatitis virus (VSV) for the indicated time. (F) Tim-3 facilitates K63-linked ubiquitination mediated by TRIM47. Plasmids encoding HA-Ub-K63, V5-TRIM47, and Flag-Tim-3 were transfected into HEK293T cells. Cells were treated with MG132 (20 ug/ml) for 6 hr, and cell lysates were immunoprecipitated with Flag antibody and detected by western blot for K48-Ub levels. Three independent experiments were conducted for all panels.
-
Figure 4—source code 1
Silence of TRIM47 in macrophages.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig4-code1-v3.zip
-
Figure 4—source code 2
TRIM47 promotes NF90 degradation.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig4-code2-v3.zip
-
Figure 4—source data 1
TRIM47 promotes NF90 degradation.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig4-data1-v3.zip
-
Figure 4—source data 2
Tim-3 synthesizes with TRIM47 to promote NF90 degradation.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig4-data2-v3.zip
-
Figure 4—source data 3
Tim-3 promotes TRIM47 expression.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig4-data3-v3.zip
-
Figure 4—source data 4
Tim-3 promotes TRIM47 expression.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig4-data4-v3.zip
-
Figure 4—source data 5
Tim-3promotes k63-linked ubiquitination of TRIM47.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig4-data5-v3.zip
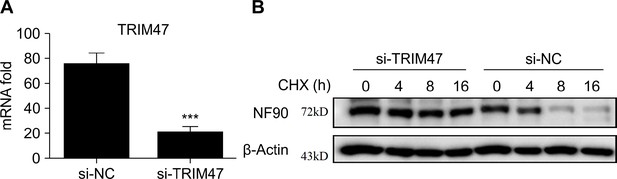
TRIM47 knockdown increases expression of NF90.
(A) Quantitative reverse transcription PCR (qPCR) analysis of TRIM47 mRNA expression in RAW264.7 macrophages 48 hr after transfection with TRIM47 siRNA. (B) Western blot analysis of the protein degradation of NF90 in RAW264.7 macrophages 48 hr after transfection with control siRNA or TRIM47 siRNA pre-infected for 2 hr with vesicular stomatitis virus (VSV) before cycloheximide (CHX) (10 μg/ml) treatment for indicated hours. At least three independent experiments were conducted for all panels.
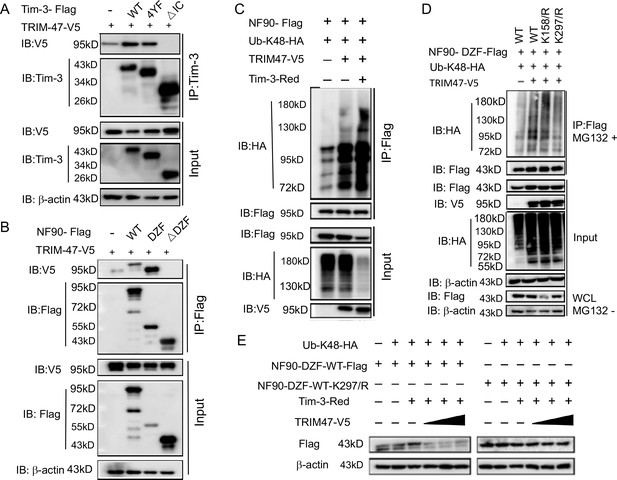
Tim-3 recruits TRIM47 to DZF domain of NF90 within which Lys297 is a critical site for TRIM47-mediated K48-linked ubiquitination and degradation of NF90.
(A and B) The intracellular domain of Tim-3 and the DZF domain of NF90 interact with TRIM47 respectively. HEK293T cells were transfected with the indicated plasmids for 24 hr and treated with MG132 (20 ug/ml) for 6 hr. The cells were then lysed, immunoprecipitated with Tim-3 or Flag antibody, and detected by western blot using the indicated antibodies. (C) Tim-3 promotes NF90 degradation mediated by E3 ligase TRIM47. HEK293T cells were transfected with plasmids encoding HA-Ub-K48, V5-TRIM47, Flag-NF90, and Red-Tim-3, and treated with MG132 (20 ug/ml) for 6 hr. Cell lysates were then immunoprecipitated with Flag antibody and analyzed by western blot using the indicated antibodies. (D) Residue K297 of NF90 is the major site of TRIM47-mediated K48-linked ubiquitination. Flag-NF90-DZF (WT) or KR mutants, HA-Ub-K48 and V5-TRIM47, were transfected into HEK293T cells for 24 hr. Cells were then treated with MG132 (20 ug/ml) for 6 hr. Cell lysates were analyzed by western blot for K48-linked ubiquitination of NF90. (E) Residue K297 is the decisive site in TRIM47-mediated degradation of NF90. Plasmids encoding Flag-NF90-DZF (WT) or K297R mutants, PDsRed-Tim-3, HA-Ub-K48, and V5-TRIM47, were transfected into HEK293T cells, and cell lysates were examined by western blot for the indicated proteins. Three independent experiments were conducted for all panels.
-
Figure 5—source code 1
Lys297 is a critical site for ubiquitination of NF9.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig5-code1-v3.zip
-
Figure 5—source data 1
Tim-3 interacts with TRIM47 via its intracellular tail.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig5-data1-v3.zip
-
Figure 5—source data 2
NF90 interacts with TRIM47 via its DZF domain.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig5-data2-v3.zip
-
Figure 5—source data 3
Tim-3 promotes TRIM47 mediated ubiquitination of NF90.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig5-data3-v3.zip
-
Figure 5—source data 4
Lys297 is a critical site for TRIM47-mediated ubiquitination of NF90.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig5-data4-v3.zip
-
Figure 5—source data 5
Lys297 is a critical site for TRIM47-mediated ubiquitination of NF90-DZF.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig5-data5-v3.zip
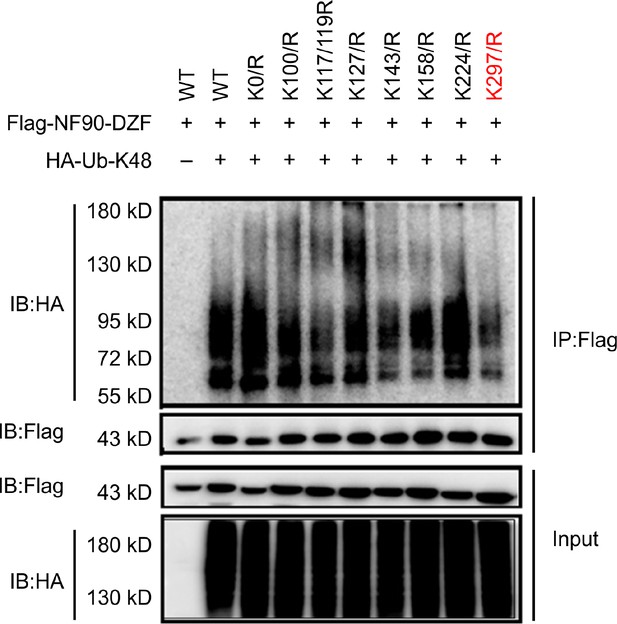
Lys297 of NF90 is a critical site in TRIM47-mediated K48-linked ubiquitination and degradation of NF90.
Co-immunoprecipitation analysis of the polyubiquitination of NF90-DZF (WT) and its mutants in HEK293T cells transfected with plasmids encoding HA–ubiquitin (K48). Data are representative of three independent experiments.
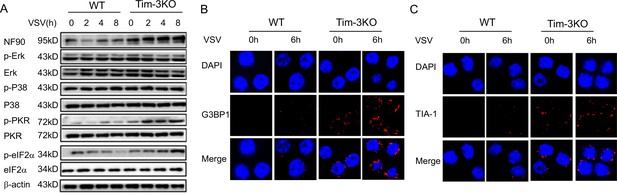
Tim-3 selectively inhibits the phosphorylation of PKR and eIF2a and decreases the expression of SG markers G3BP1 and TIA-1 in macrophages.
(A) Lysates from wild-type (WT) and Tim-3 gene (Havcr2) knockout (Tim-3KO) peritoneal macrophages infected with vesicular stomatitis virus (VSV) were analyzed by immunoblotting for the indicated proteins. (B and C) Peritoneal macrophages isolated for WT or Tim-3KO mice were infected with or without VSV for 6 hr, and then the cells were immunostained with the indicated antibodies and analyzed by fluorescence microscopy. Three independent experiments were conducted for all panels.
-
Figure 6—source code 1
TRIM47 knock down increases TIA-1,G3BP1 expression and decrease VSV expression.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig6-code1-v3.zip
-
Figure 6—source data 1
Tim-3 selectively inhibits the phosphorylation of PKR and eIF2a.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig6-data1-v3.zip
-
Figure 6—source data 2
Tim-3 increases the expression of SGs markers G3BP1 and TIA-1 in macrophages.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig6-data2-v3.zip
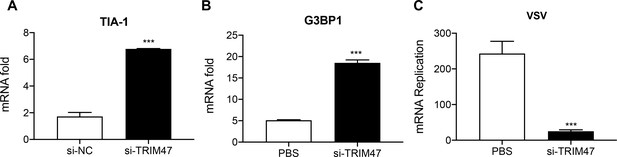
TRIM47 knockdown enhances antiviral immunity in macrophages.
TRIM47 was silenced in RAW264.7 macrophages as in Figure 2—figure supplement 1. (A and B) Quantitative reverse transcription PCR (qPCR) analysis of TIA-1 and G3BP1 expression in RAW264.7 macrophages or in RAW264.7 macrophages silenced of TRIM47. (C) TRIM47 promotes vesicular stomatitis virus (VSV) production. qPCR analysis of VSV mRNA expression in 48 hr after transfection with control siRNA or TRIM47 siRNA, infected with VSV. The results shown are representative of three independent experiments.
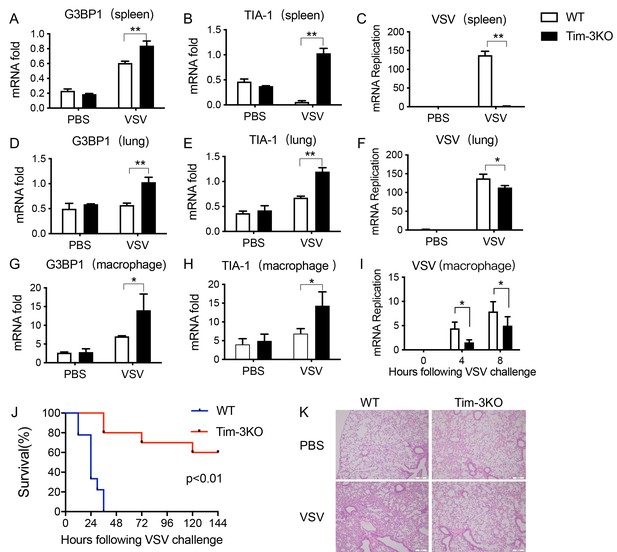
Tim-3 deficiency upregulates G3BP1 and TIA-1 and protects mice from VSV infection.
(A, B, D, E, G, and H) Detection of mRNA transcription of G3BP1 and TIA-1 in organs and peritoneal macrophages by quantitative reverse transcription PCR (qPCR) after wild-type (WT) and Tim-3KO mice (n = 5 per group) were intraperitoneally injected with vesicular stomatitis virus (VSV) for 24 hr. (C, F, and I) qPCR analysis of VSV loads in organs and peritoneal macrophages after WT and Tim-3KO mice (n = 5 per group) were intraperitoneally injected with VSV for 24 hr. (J) WT and Tim-3KO mice (~7 weeks old) were intraperitoneally injected with VSV (1 × 108 pfu/g) (n = 10 per group) followed by recording survival of both groups. p<0.01. (K) The lung tissues from WT and Tim-3KO mice (C, F, and I) were stained with hematoxylin and eosin, and their pathology analyzed in response to VSV. The results shown are representative of three independent experiments. *p<0.05; **p<0.01.
-
Figure 7—source data 1
Tim-3 knowdown increases G3BP1expression in spleen with VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data1-v3.zip
-
Figure 7—source data 2
Tim-3 knowdown increases TIA-1 expression in spleen with VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data2-v3.zip
-
Figure 7—source data 3
Tim-3 knowdown decreases VSV expression in spleen with infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data3-v3.zip
-
Figure 7—source data 4
Tim-3 knowdown increases G3BP1 expression in lung with VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data4-v3.zip
-
Figure 7—source data 5
Tim-3 knowdown increases TIA-1 expression in lung with VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data5-v3.zip
-
Figure 7—source data 6
Tim-3 knowdown decreases VSV expression in lung with VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data6-v3.zip
-
Figure 7—source data 7
Tim-3 knowdown increases G3BP1 expression in peritoneal macrophages with VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data7-v3.zip
-
Figure 7—source data 8
Tim-3 knowdown increases TIA-1 expression in peritoneal macrophages with VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data8-v3.zip
-
Figure 7—source data 9
Tim-3 knowdown decreases VSV expression in peritoneal macrophages with VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data9-v3.zip
-
Figure 7—source data 10
Tim-3 deficiency protects mice from VSV infection.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data10-v3.zip
-
Figure 7—source data 11
Tim-3 knowdown attenuated tissue damage.
- https://cdn.elifesciences.org/articles/66501/elife-66501-fig7-data11-v3.zip
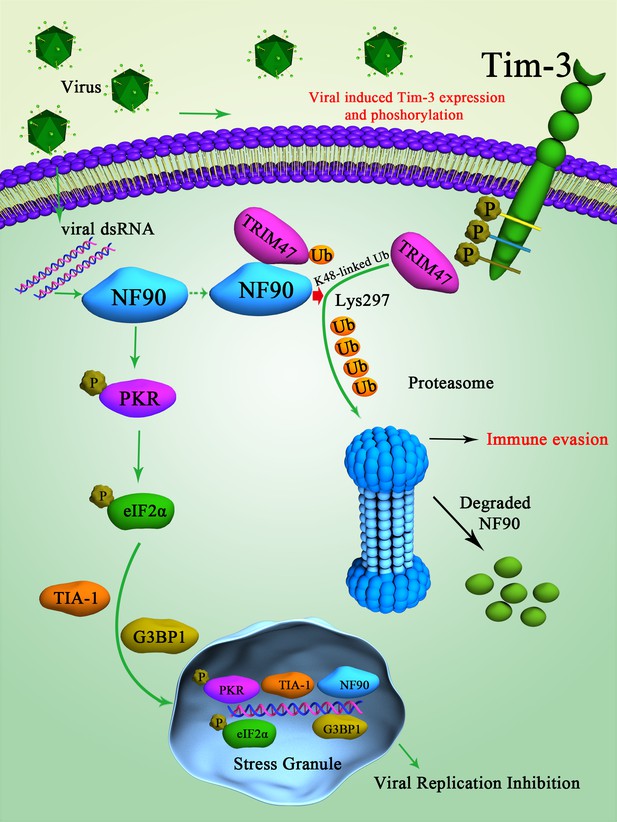
Schematic diagram of how Tim-3 inhibits NF90-SG pathway in macrophages during infection.
Upon vesicular stomatitis virus (VSV) infection, Tim-3 is activated and upregulated. The activation of Tim-3 in turn recruited the E3 ubiquitin ligase TRIM47 to the zinc finger domain of NF90 and initiated a proteasome-dependent degradation of NF90 via K48-linked ubiquitination at Lys297. The negative regulation of NF90 by Tim-3 blocked the RNA virus and triggered NF90-SG-mediated antiviral immunity and finally led to virus immune evasion.
Additional files
-
Supplementary file 1
Sequences of the primers used for PCR.
- https://cdn.elifesciences.org/articles/66501/elife-66501-supp1-v3.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/66501/elife-66501-transrepform-v3.pdf





