Metabolic flexibility via mitochondrial BCAA carrier SLC25A44 is required for optimal fever
Figures
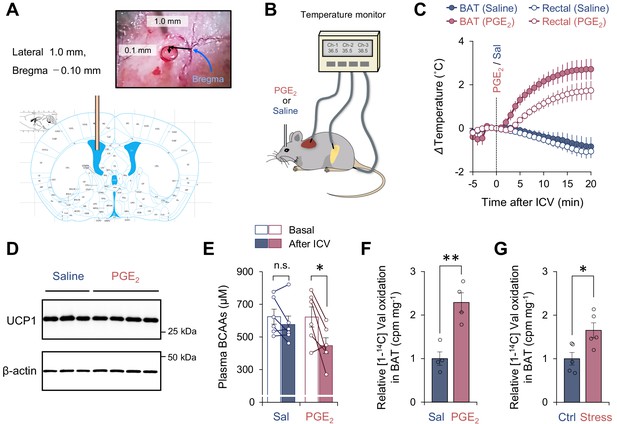
Activation of branched chain-amino acid (BCAA) catabolism and thermogenesis in the brown adipose tissue (BAT) following fever stimuli.
(A) The injection site of prostaglandin E2 (PGE2) in the left lateral ventricle. The image was adapted from ‘The mouse brain in stereotaxic coordinates’ (Franklin and Paxinos, 2008). (B) Schematic illustration of the experiment. Mice received intracerebroventricular (ICV) administration of PGE2 (1.4 μg/mouse) or saline (control). Tissue temperature and rectal temperature were simultaneously recorded by the live monitoring system. (C) Real-time temperature changes in the interscapular BAT (iBAT) and rectum of mice in (B). n = 6 per group. (D) Immunoblotting of UCP1 in BAT of mice in (C). BAT was harvested after 2 hr of an ICV administration of PGE2. β-Actin was used as a loading control. n = 3 for saline, n = 4 for PGE2. (E) Plasma BCAA concentration before and after an ICV administration of PGE2. Plasma samples were harvested after 2 hr of an ICV administration of PGE2. n = 6 per group. (F) Relative [1-14C] Val oxidation in isolated iBAT of mice. The iBAT tissues of mice were dissected after 2 hr of an ICV administration of PGE2. n = 4 per group. (G) Relative [1-14C] Val oxidation in isolated iBAT of rats exposed to social defeat stress. The iBAT of rats was dissected 2 hr after social defeat stress. n = 5 per group. *p<0.05, **p<0.01, n.s., not significant. Data were analyzed by using paired t-test (E) or unpaired Student’s t-test (F, G).
-
Figure 1—source data 1
Activation of branched chain-amino acid (BCAA) catabolism and thermogenesis in the brown adipose tissue (BAT) following fever stimuli.
- https://cdn.elifesciences.org/articles/66865/elife-66865-fig1-data1-v2.xlsx
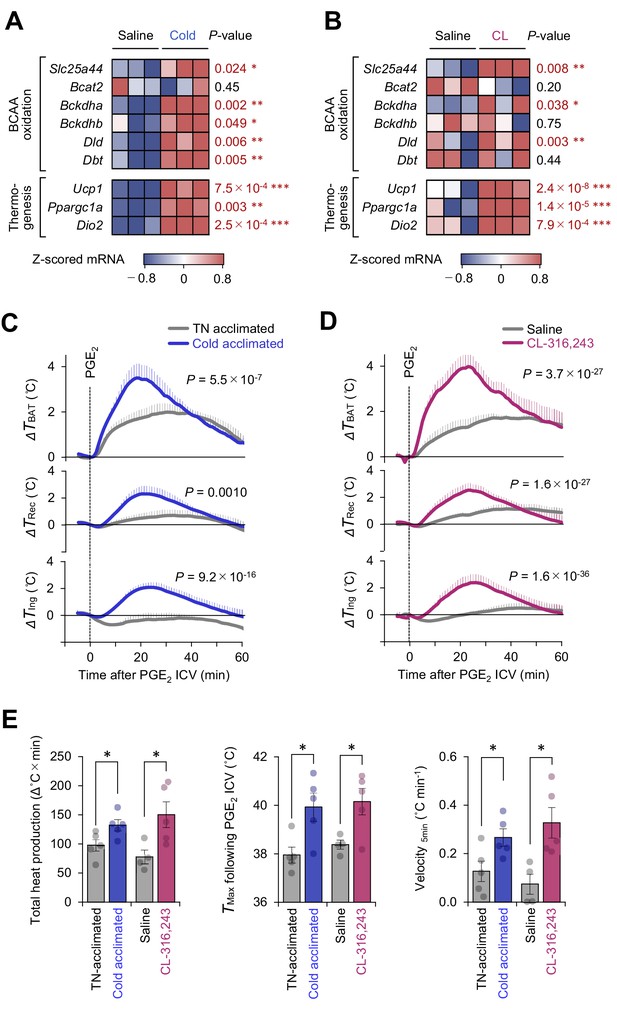
Mitochondrial branched chain-amino acid (BCAA) catabolism enhances prostaglandin E2 (PGE2)-induced fever.
(A) mRNA expression of indicated BCAA catabolism genes and thermogenic genes in brown adipose tissue (BAT) following cold exposure. n = 3 per group. Data were obtained from a previous microarray dataset from GEO under the accession # GSE51080 (Rosell et al., 2014). (B) mRNA expression of indicated BCAA catabolism genes and thermogenic genes in BAT following β3-AR agonist (CL-316243; CL) treatment. n = 3 per group. Data were obtained from a previous RNA-sequence dataset from ArrayExpress under the accession # E-MTAB-7445 (Tajima et al., 2019). (C) Real-time temperature changes in the interscapular BAT (iBAT) (ΔTBAT), rectum (ΔTRec), and ing-WAT (ΔTIng) of mice acclimated to thermoneutrality (TN, 30°C) or cold (15°C) for 2 weeks. n = 5 per group. (D) Real-time temperature changes in the iBAT (ΔTBAT), rectum (ΔTRec), and ing-WAT (ΔTIng) of mice treated with β3-AR agonist (CL-316243; CL) for 1 week. Saline, n = 4; CL, n = 5. (E) PGE2-stimulated total heat production, maximal temperature (TMax), and velocity of tissue temperature in the iBAT of mice in (C) and (D). Data were analyzed by using unpaired Student’s t-test (A, B, E) or two-way repeated measures ANOVA (C, D).
-
Figure 2—source data 1
Mitochondrial branched chain-amino acid (BCAA) catabolism enhances prostaglandin E2 (PGE2-)-induced fever.
- https://cdn.elifesciences.org/articles/66865/elife-66865-fig2-data1-v2.xlsx
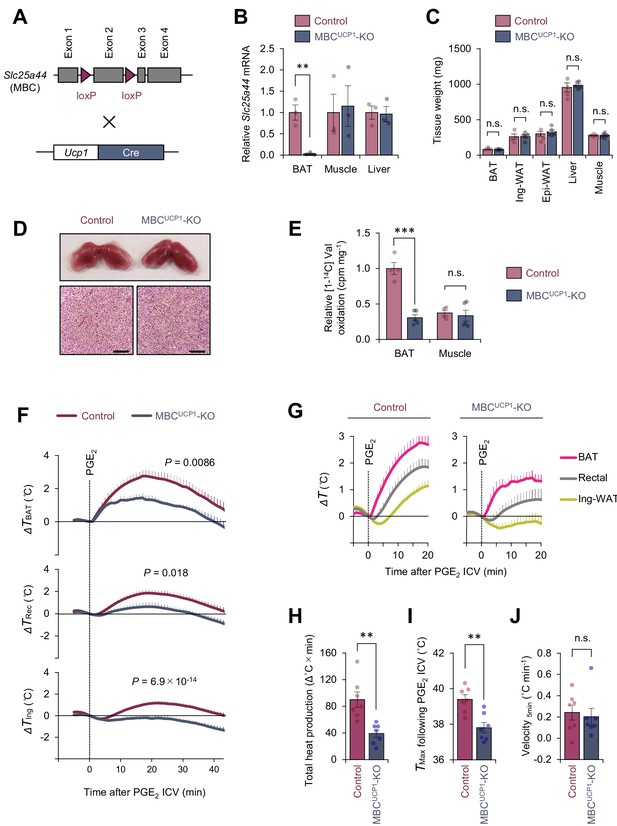
Mitochondrial branched chain-amino acid (BCAA) carrier SLC25A44/mitochondrial BCAA carrier (MBC) is required for prostaglandin E2 (PGE2)-induced fever.
(A) Generation of brown adipose tissue (BAT)-specific MBC knock-out (KO) mice (Ucp1-Cre; Slc25a44flox/flox; MBCUCP1 KO). (B) mRNA expression of Slc25a44 in the interscapular BAT (iBAT), skeletal muscle, and liver of BAT-specific MBC KO mice and the littermate control mice. n = 3 per group. (C) Tissue weight of the iBAT, ing-WAT, epi-WAT, liver, and gastrocnemius skeletal muscle of BAT-specific MBC KO mice (MBCUCP1 KO) and the littermate control mice. Control, n = 4; MBCUCP1 KO, n = 5. (D) Morphology and hematoxylin and eosin (H&E) staining of the iBAT of BAT-specific MBC KO mice and the littermate control mice. Scale bars, 50 μm. (E) Relative [1-14C] Val oxidation in the iBAT and gastrocnemius skeletal muscle of BAT-specific MBC KO mice (MBCUCP1 KO) and the littermate control mice. Control, n = 4; MBCUCP1 KO, n = 5. (F) Real-time temperature changes in the iBAT (ΔTBAT), rectum (ΔTRec), and ing-WAT (ΔTIng) of BAT-specific MBC KO mice (MBCUCP1 KO) and the littermate control mice. n = 7 per group. (G) Temperature changes during the first 20 min after intracerebroventricular (ICV) administration of PGE2 in (F). (H) Total heat production in the iBAT following ICV administration of PGE2 in (F). (I) Tmax of iBAT temperature following ICV administration of PGE2 in (F). (J) Velocity of iBAT temperature increase following ICV administration of PGE2 in (F). **p<0.01, ***p<0.001, n.s., not significant. Data were analyzed by using unpaired Student’s t-test (B, C, E, H–J) or two-way repeated measures ANOVA (F).
-
Figure 3—source data 1
Mitochondrial branched chain-amino acid (BCAA) carrier SLC25A44/mitochondrial BCAA carrier (MBC) is required for prostaglandin E2 (PGE2-)-induced fever.
- https://cdn.elifesciences.org/articles/66865/elife-66865-fig3-data1-v2.xlsx
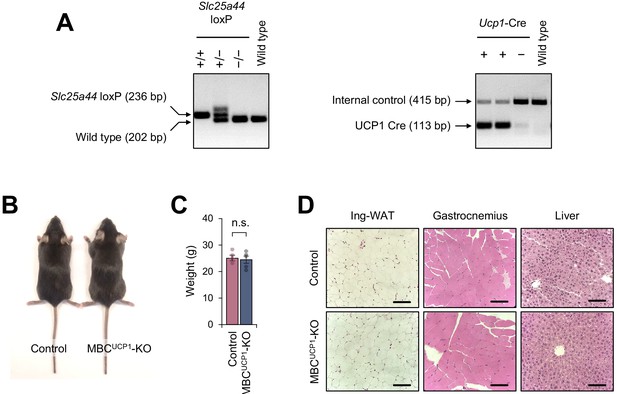
Characterization of brown adipose tissue (BAT)-specific mitochondrial BCAA carrier (MBC) knock-out (KO) mice.
(A) Representative genotyping images of BAT-specific MBC KO mice (Ucp1-Cre x Slc25a44flox/flox; MBCUCP1 KO) and the littermate control mice (Slc25a44flox/flox). (B) A representative picture of BAT-specific MBC KO mouse and littermate control mouse. (C) Body weight of BAT-specific MBC KO mice and littermate control mice on a regular chow diet. Control, n = 4; MBCUCP1 KO, n = 5. n.s., not significant by unpaired Student’s t-test. (D) H&E staining of the ing-WAT, gastrocnemius skeletal muscle, and liver of BAT-specific MBC KO mice and littermate control mice in (C).
-
Figure 3—figure supplement 1—source data 1
Characterization of brown adipose tissue (BAT)-specific mitochondrial BCAA carrier (MBC) knock-out (KO) mice.
- https://cdn.elifesciences.org/articles/66865/elife-66865-fig3-figsupp1-data1-v2.xlsx
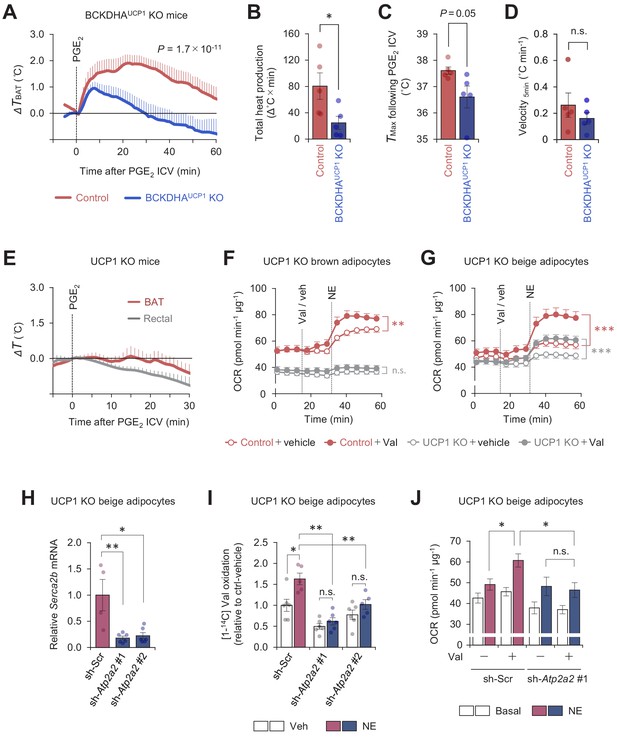
Mitochondrial branched chain-amino acid (BCAA) oxidation triggers thermogenesis via UCP1-dependent and UCP1-independent mechanisms.
(A) Real-time temperature changes in the interscapular BAT (iBAT) of BCKDHAUCP1knock-out (KO) and the littermate control mice following intracerebroventricular (ICV) administration of prostaglandin E2 (PGE2). n = 5 per group. (B) Total heat production in the iBAT of indicated mice following ICV administration of PGE2 in (A). (C) Tmax of iBAT temperature following ICV administration of PGE2 in (A). (D) Velocity of iBAT temperature increase following ICV administration of PGE2 in (A). (E) Real-time temperature changes in the iBAT and rectum of UCP1 KO mice following ICV injection of PGE2. n = 5 per group. (F) Oxygen consumption rate (OCR) in brown adipocytes derived from wild-type mice (control) or UCP1 KO mice. Differentiated adipocytes in the BCAA-free medium were treated with valine (Val) or vehicle (Veh), and subsequently stimulated with norepinephrine (NE) at indicated time points. OCR values were normalized by total protein (µg). n = 10 per group. (G) OCR in beige adipocytes derived from wild-type mice (control) or UCP1 KO mice. Differentiated adipocytes in the BCAA-free medium were treated with valine (Val) or vehicle (Veh), and subsequently stimulated with NE at indicated time points. OCR values were normalized by total protein (µg). Control + vehicle, n = 9; Control + Val n=8; UCP1 KO + vehicle, n = 6; UCP1 KO + Val, n = 9. (H) mRNA expression of Serca2b in UCP1 KO beige adipocytes expressing a scrambled control (Scr, n = 4) or two independent shRNAs targeting Atp2a2 (#1 and #2, n = 6 per group). (I) Relative [1-14C] Val oxidation in UCP1 KO beige adipocytes in (H). Differentiated adipocytes were cultured in the presence of NE or vehicle. Valine oxidation was measured using [1-14C] valine and normalized by total protein (μg). Vehicle n = 6, NE n = 5, except sh-Atp2a2 #1 treated with NE n = 6. (J) OCR was measured in UCP1 KO beige adipocytes in (H). Differentiated adipocytes were incubated in the BCAA-free media supplemented with Val (sh-Scr, n = 9; sh-Atp2a2, n = 6) or vehicle (sh-Scr, n = 6; sh-Atp2a2, n = 7), and subsequently stimulated with NE. OCR values were normalized by total protein (μg). *p<0.05, **p<0.01, ***p<0.001, n.s., not significant. Data were analyzed by unpaired Student’s t-test (B–D), one- (H) or two-way (I) factorial ANOVA followed by Tukey’s post hoc test, or two-way repeated measures ANOVA (A, F, G) followed by Tukey’s post hoc test (J).
-
Figure 4—source data 1
Mitochondrial branched chain-amino acid (BCAA) oxidation triggers thermogenesis via UCP1-dependent and UCP1-independent mechanisms.
- https://cdn.elifesciences.org/articles/66865/elife-66865-fig4-data1-v2.xlsx
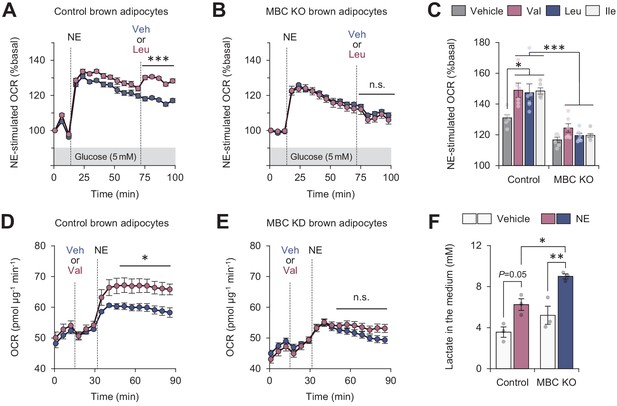
Mitochondrial branched chain-amino acid (BCAA) catabolism required for sustained thermogenesis.
(A) Oxygen consumption rate (OCR) normalized to total protein (in μg) in control brown adipocytes. Differentiated adipocytes in BCAA-free medium with 5 mM glucose were stimulated with norepinephrine (NE), and subsequently supplemented with Leu or vehicle. n = 9 per group. (B) OCR normalized to total protein (in μg) in mitochondrial BCAA carrier (MBC) knock-out (KO) brown adipocytes. Differentiated adipocytes cultured in BCAA-free medium with glucose (5 mM) were stimulated with NE, and subsequently supplemented with Leu or vehicle. n = 9 per group. (C) NE-stimulated OCR in control and MBC KO brown adipocytes. Differentiated adipocytes in BCAA-free medium, containing 5 mM glucose, supplemented with Val, Leu, or Ile were stimulated with NE for 30 min. n = 6 per group. (D) OCR normalized to total protein (in μg) in control brown adipocytes. Differentiated adipocytes in BCAA-free medium with glucose (20 mM) were supplemented with Leu or vehicle, and subsequently stimulated with NE. n = 5 per group. (E) OCR normalized to total protein (in μg) in MBC KO brown adipocytes. Differentiated adipocytes in BCAA-free medium with glucose (20 mM) were supplemented with Leu or vehicle, and subsequently stimulated with NE. n = 5 per group. (F) Lactate production in control and MBC KO brown adipocytes. Differentiated adipocytes were stimulated with vehicle or NE for 30 min, and lactate content in the medium was measured. n = 3 per group. *p<0.05, **p<0.01, ***p<0.001, n.s., not significant. Data were analyzed by two-way repeated measures ANOVA (A, B, D, E) or two-way factorial ANOVA followed by Tukey’s post hoc test (C, F).
-
Figure 5—source data 1
Mitochondrial branched chain-amino acid (BCAA) catabolism required for sustained thermogenesis.
- https://cdn.elifesciences.org/articles/66865/elife-66865-fig5-data1-v2.xlsx
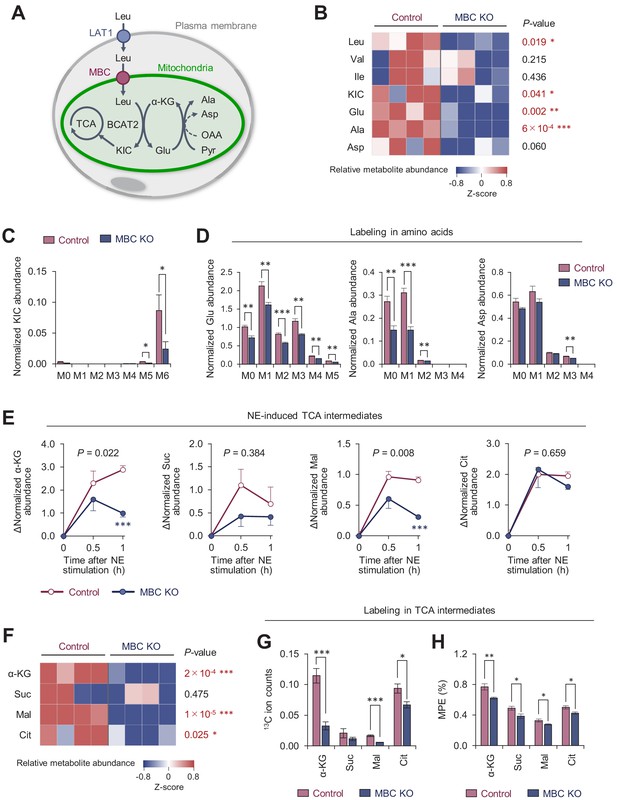
Mitochondrial BCAA carrier (MBC) is required for the synthesis of the mitochondrial amino acids and TCA intermediates.
(A) Schematic illustration of mitochondrial branched chain-amino acid (BCAA) fate to the TCA cycle and amino acid synthesis. (B) Amino acid relative abundance in wild-type (WT) control and MBC knock-out (KO) brown adipocytes as determined by 13C-Leu metabolomics. Cells were harvested at a basal state. n = 4 per group. (C) Leu-derived 13C labeling in KIC in WT control and MBC KO brown adipocytes in (B). (D) Labeling in indicated amino acids derived from Leu in WT control and MBC KO brown adipocytes in (B). (E) Norepinephrine (NE)-induced changes in TCA cycle intermediates in WT control and MBC KO brown adipocytes. Cells were harvested at the indicated time points following NE treatment in (B). (F) The relative abundance of indicated TCA cycle intermediate in WT control and MBC KO brown adipocytes at 1 hr following NE treatment in (B). (G) Leu-derived 13C labeling in TCA cycle intermediates in WT control and MBC KO brown adipocytes. Cells were harvested at 1 hr following NE treatment in (B). (H) Mole percentage enrichment (MPE) of TCA cycle intermediates derived from [U-13C6] Leu in control and MBC KO brown adipocytes. Cells were harvested at 1 hr following NE treatment. n = 4 per group. Data in 6B-H was normalized to a norvaline internal standard. *p<0.05, **p<0.01, ***p<0.001. Data were analyzed by unpaired Student’s t-test (B–D, F–H) or two-way factorial ANOVA followed by post hoc unpaired t-test (E).
-
Figure 6—source data 1
Mitochondrial BCAA carrier (MBC) is required for the synthesis of the mitochondrial amino acids and TCA intermediates.
- https://cdn.elifesciences.org/articles/66865/elife-66865-fig6-data1-v2.xlsx
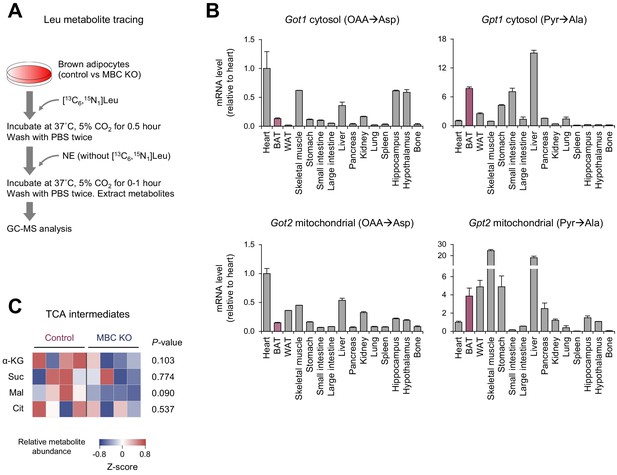
Metabolic fate of Leu in mitochondrial BCAA carrier (MBC) knock-out (KO) brown adipocytes.
(A) Schematic protocol of Leu metabolite tracing experiment. (B) Tissue distribution of indicated genes that are involved in amino acid synthesis from branched chain-amino acid (BCAA). Glutamate-pyruvate transaminase (GPT), the cytoplasmic form (Gpt1) and the mitochondrial form (Gpt2), catalyze the reversible conversion of Ala and α-KG to Glu and pyruvate. Aspartate aminotransferase (GOT), the cytoplasmic form (Got1) and the mitochondrial form (Got2), catalyze the interconversion of Asp and oxaloacetate (OAA). The data are derived from BioGPS (Wu et al., 2009). (C) The relative abundance of indicated TCA cycle intermediate in wild-type (WT) control and MBC KO brown adipocytes at a basal condition. n = 4 per group.
-
Figure 6—figure supplement 1—source data 1
Metabolic fate of Leu in mitochondrial BCAA carrier (MBC) knock-out (KO) brown adipocytes.
- https://cdn.elifesciences.org/articles/66865/elife-66865-fig6-figsupp1-data1-v2.xlsx
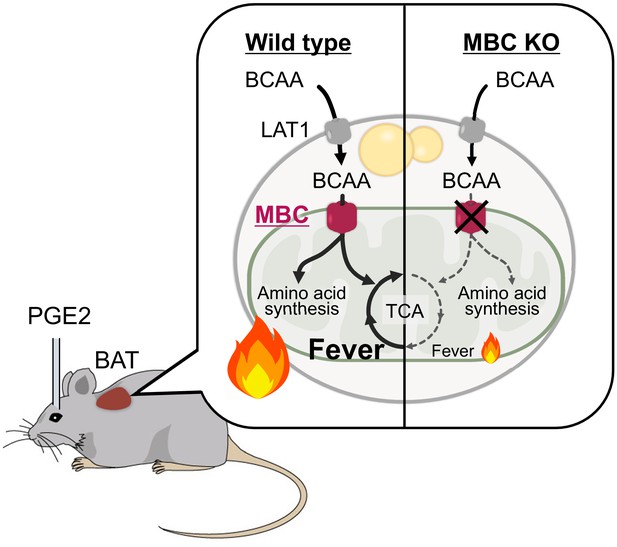
A model of mitochondrial branched chain-amino acid (BCAA) catabolism and brown adipose tissue (BAT) thermogenesis in response to fever stimuli.
In response to intracerebroventricular (ICV) administration of prostaglandin E2 (PGE2) or psychological stress, mitochondrial BCAA catabolism in the BAT is activated along with enhanced thermogenesis. The fever-induced mitochondrial BCAA catabolism is required for sustaining thermogenesis. Mitochondrial BCAA uptake via mitochondrial BCAA carrier (MBC) is required for BCAA deamination and the synthesis of mitochondrial amino acid pool and TCA intermediates.
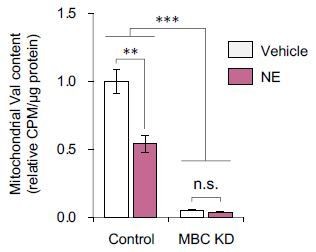
Effects of norepinephrine (NE) on mitochondrial BCAA content in brown adipocytes.
Differentiated brown adipocytes were treated with NE for 20 min and harvested to isolate mitochondria of brown adipocytes with shRNA knockdown of MBC (KD) or control cells. Subsequently, isolated mitochondria were incubated with [U-14C] Val at 37°C for 1 h. After washing with PBS 3 times, [U-14C] Val content in mitochondria was quantified by using a scintillation counter. n = 3/group. ANOVA with Tukey’s HSD test.
Additional files
-
Supplementary file 1
Primer sequences.
- https://cdn.elifesciences.org/articles/66865/elife-66865-supp1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/66865/elife-66865-transrepform-v2.docx





