Constructing an adult orofacial premotor atlas in Allen mouse CCF
Figures
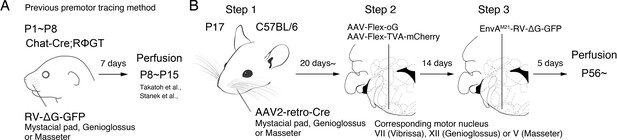
Monosynaptic rabies virus tracing strategy for labeling adult orofacial premotor circuits.
(A) Schematic of previously used monosynaptic premotor transsynaptic tracing method in neonatal mice. (B) Schematic of the three-step monosynaptic premotor tracing strategy in adult mice developed in this study.
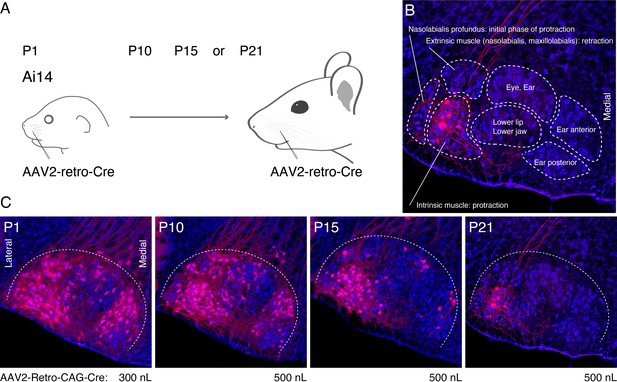
Optimization of the timing of AAV2-retro-Cre injection in the peripheral muscle.
(A) Schematic of AAV2-retro-Cre injection. AAV2-retro-Cre is injected into the mystacial pad of Ai14 mice at postnatal day (P)1, P10, P15, or P21. (B) Subnuclei of VII. Each subnucleus is circled by dotted lines. Peripheral muscle targets are shown in each subnucleus. Motoneurons in the lateral part of VII (red) are labeled by AAV2-retro-Cre injection at P21. (C) Labeling patterns from P1, P10, P15, or P21 injected animals. Injection volumes of AAV2-retro-Cre are shown under each panel. Sections were counterstained with fluorescent Nissl (blue).
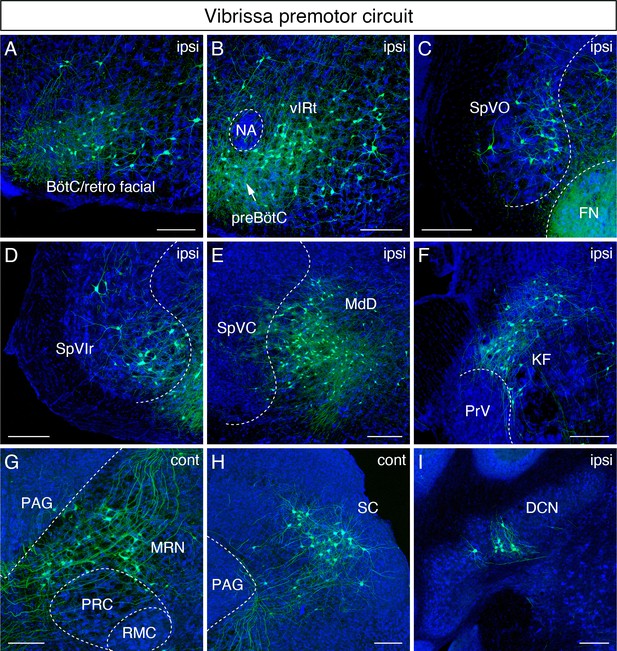
Monosynaptic tracing results of vibrissa premotor neurons in adult mice.
Representative images of traced vibrissa premotor neurons on coronal sections. Sections were counterstained with fluorescent Nissl (blue). Labeled neurons shown in the ipsilateral Bötzinger complex (BötC)/retrofacial area (A), preBötC (arrow) and vibrissal intermediate reticular formation (vIRt) (B), spinal trigeminal nucleus oralis (SpVO) (C), rostral part of spinal trigeminal nucleus interpolaris (SpVIr) (D), medullary reticular nucleus dorsal (MdD) located medial to spinal trigeminal nucleus caudalis (SpVC) (E), Kölliker-Fuse (KF) (F), contralateral midbrain reticular nucleus (MRN) located dorsal to the red nucleus parvicellular region (RPC) (G), contralateral superior colliculus (SC) (H), and ipsilateral deep cerebellar nucleus (DCN) (I). Scale bars, 200 µm.
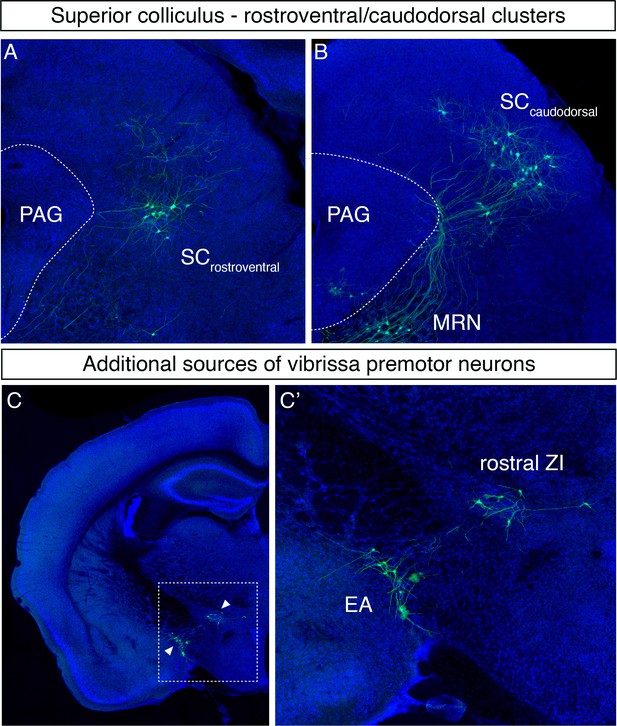
Additional vibrissa premotor inputs.
(A, B) Labeled neurons in the rostroventral (A) and caudodorsal superior colliculus (B). (C) Labeled neurons are observed in the rostral zona incerta (ZI) and extended amygdala (EA) (a magnified image of the boxed area is shown in C').
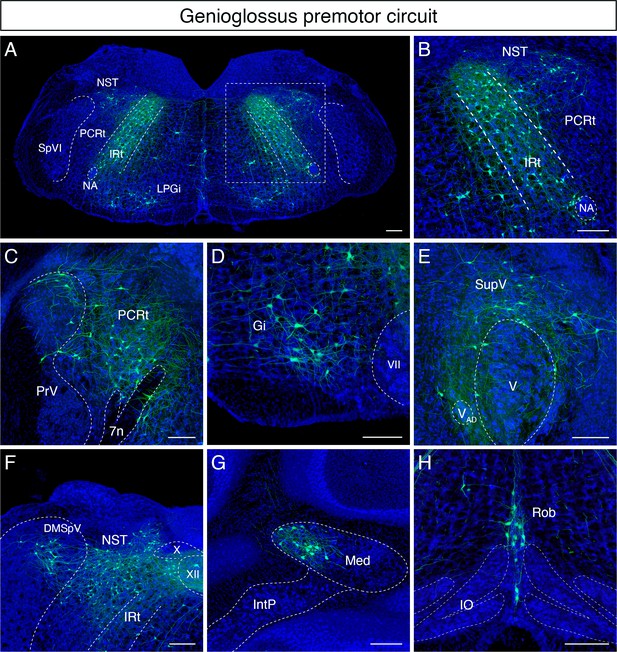
Monosynaptic tracing results of tongue-protruding genioglossus premotor neurons in adult mice.
Representative images of traced genioglossus premotor neurons on coronal sections. Sections were counterstained with fluorescent Nissl (blue). Labeled neurons are shown in the dorsal intermediate reticular nucleus (IRt), nucleus of solitary tract (NST), lateral paragigantocellular nucleus (LPGi) at the anterior-posterior level between VII and XII (A, magnified view of the boxed area in A is shown in B), parvicellular reticular nucleus (PCRt), dorsal region of the principal trigeminal nucleus (PrV) (C), gigantocellular reticular nucleus (Gi) (D), supratrigeminal region (SupV) (E), dorsomedial part of spinal trigeminal nucleus (DMSpV), rostral NST at the anterior-posterior level of the anterior part of XII (F), the medial subnucleus of the deep cerebellar nucleus (DCN) (G), and raphe obscurus nucleus (Rob) (H). Scale bars, 200 µm.
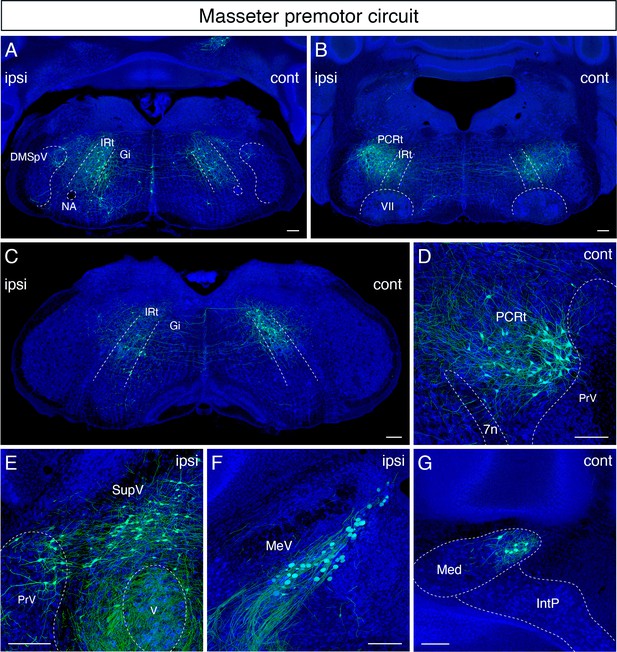
Monosynaptic tracing results of jaw-closing masseter premotor neurons in adult mice.
Representative images of traced masseter premotor neurons on coronal sections. Sections were counterstained with fluorescent Nissl (blue). Labeled neurons are observed bilaterally in the dorsal intermediate reticular nucleus (dorsal IRt), in dorsomedial part of spinal trigeminal nucleus (DMSpV) at the anterior-posterior level between VII and XII (A), bilaterally in the dorsal IRt, parvicellular reticular nucleus (PCRt) at the anterior-posterior level of VII (B), contralaterally in the dorsal IRt at the anterior-posterior level of the anterior part of XII (C), PCRt (D), supratrigeminal region (SupV), dorsal principal trigeminal nucleus (PrV) (E), ipsilateral mesencephalic nucleus (MeV) (F), PCRt, dorsal PrV (C), gigantocellular reticular nucleus (Gi) (D), SupV (E), and the contralateral medial subnucleus of deep cerebellar nucleus (DCN) (G). Scale bars, 200 µm.
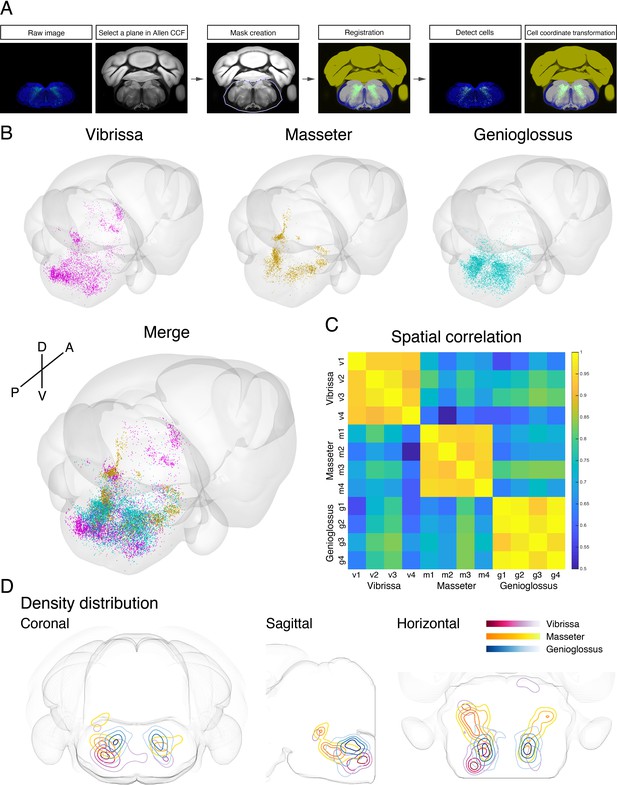
Co-registration and comparison of the spatial distributions of vibrissa, genioglossus, and masseter premotor circuits in Allen common coordinate framework (CCF).
(A) Procedure for mapping orofacial premotor neurons to Allen CCF. (B) Reconstructed representative vibrissa (magenta), masseter (gold), and genioglossus (cyan) premotor circuits in Allen CCF (top). Merged image (bottom). (C) Cross-correlation analysis of the spatial distribution patterns of individual animals; vibrissa (v1–v4; four mice), masseter (m1–m4; four mice), and genioglossus (g1–g4; four mice) premotor circuits. (D) Two-dimensional contour density analysis of representative vibrissa (magenta), masseter (yellow), and genioglossus (blue) premotor circuits.
-
Figure 5—source data 1
Coordinates of all labeled premotor neurons.
Vibrissa premotor neurons from four mice (v1, v2, v3, v4), genioglossus premotor neurons from four mice (g1, g2, g3, g4), and masseter premotor neurons from four mice (m1, m2, m3, m4). Coordinates are relative to Bregma (in millimeters). Those coordinates can be converted into Allen common coordinate framework (CCF) coordinates by the following equations: AP in CCF = –1 × AP coordinates × 1000 + 5400; DV in CCF = DV coordinates × 1000; ML in CCF = ML coordinates × 1000 + 5700.
- https://cdn.elifesciences.org/articles/67291/elife-67291-fig5-data1-v2.zip
-
Figure 5—source data 2
Coordinates of all starter cells.
Vibrissa starter cells from four mice (VII_v1, VII_v2, VII_v3, VII_v4), genioglossus starter cells from four mice (XII_g1, XII_g2, XII_g3, XII_g4), and masseter starter cells from four mice (V_m1, V_m2, V_m3, V_m4). Coordinates are relative to Bregma (in millimeters). Those coordinates can be converted into Allen common coordinate framework (CCF) coordinates by the following equations: AP in CCF = –1 × AP coordinates × 1000 + 5400; DV in CCF = DV coordinates × 1000; ML in CCF = ML coordinates × 1000 + 5700.
- https://cdn.elifesciences.org/articles/67291/elife-67291-fig5-data2-v2.zip
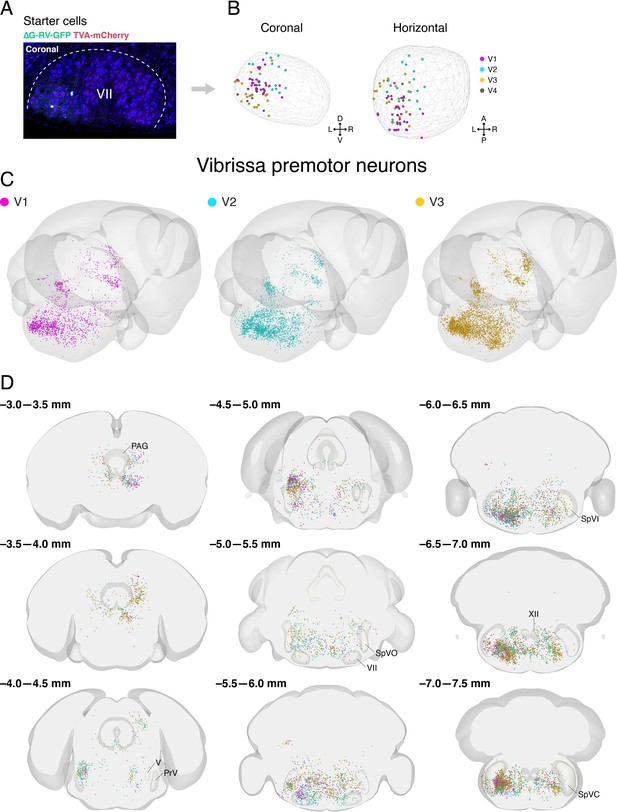
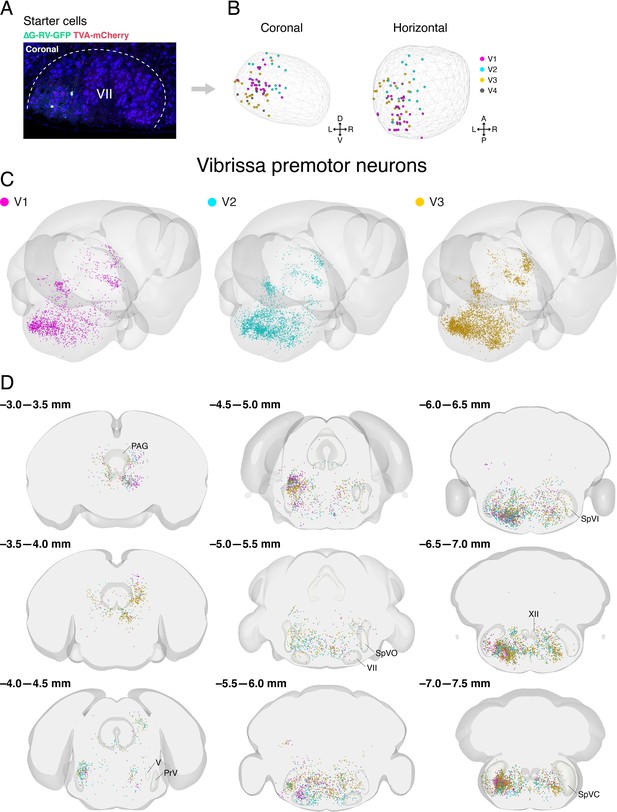
Distribution of starter cells in the facial motor nucleus and labeled vibrissa premotor neurons from individual animals in Allen mouse brain common coordinate framework.
(A) A representative image of starter cells in the facial motor nucleus (VII) that co-express TVA-mCherry (red) and GFP (green) from ∆G-RV-GFP. (B) Reconstructed locations of starter cells in VII projected onto coronal (left) and horizontal (right) planes. The contour of VII is shown by the wireframe. (C) Three-dimensional reconstruction of labeled vibrissa premotor neurons with posterior oblique view from three different mice (V1, magenta; V2, cyan; V3, gold). (D) Coronal views of reconstructed vibrissa premotor neurons from all three mice in the same coordinates. Anterior-posterior levels (referenced to Bregma) are shown on the top left of each panel.
Distribution of starter cells in the hypoglossal nucleus and labeled genioglossus premotor neurons from individual animals in Allen mouse brain common coordinate framework.
(A) A representative image of starter cells in the hypoglossal nucleus (XII) that co-express TVA-mCherry (red) and GFP (green) from ∆G-RV-GFP. (B) Reconstructed locations of starter cells in XII projected onto coronal (left) and horizontal (right) planes. The contour of XII is shown by the wireframe. (C) Three-dimensional reconstruction of labeled genioglossus premotor neurons with the posterior oblique view from three different mice (G1, magenta; G2, cyan; G3, gold). (D) Coronal views of reconstructed genioglossus premotor neurons from all three mice in the same coordinates. Anterior-posterior levels (referenced to Bregma) are shown on the top left of each panel.
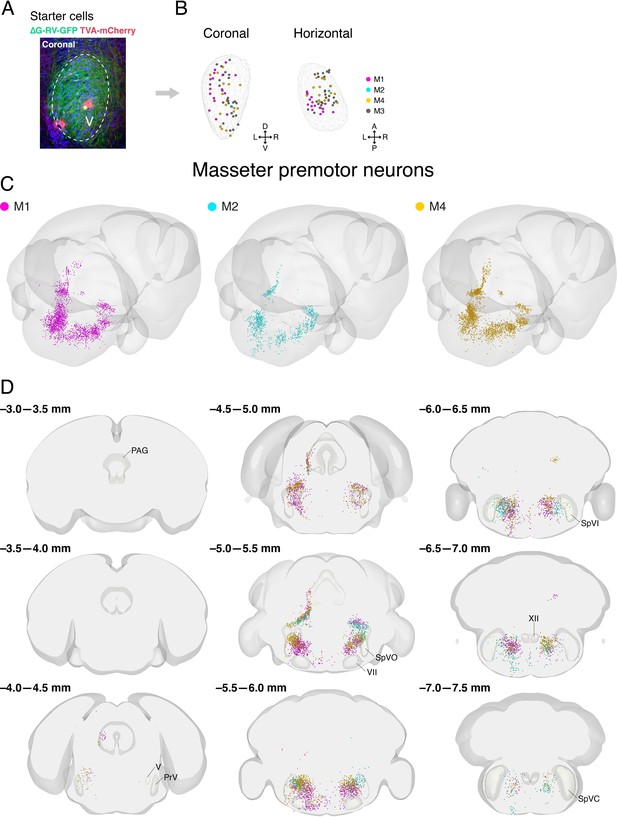
Distribution of starter cells in the trigeminal motor nucleus and labeled masseter premotor neurons from individual animals in Allen mouse brain common coordinate framework.
(A) A representative image of starter cells in the trigeminal motor nucleus (V) that co-express TVA-mCherry (red) and GFP (green) from ∆G-RV-GFP. (B) Reconstructed locations of starter cells in V projected onto coronal (left) and horizontal (right) planes. The contour of V is shown by the wireframe. (C) Three-dimensional reconstruction of labeled masseter premotor neuron with the posterior oblique view from three different mice (M1, magenta; M2, cyan; M3, gold). (D) Coronal views of reconstructed masseter premotor neurons from all three mice in the same coordinates. Anterior-posterior levels (referenced to Bregma) are shown on the top left of each panel.
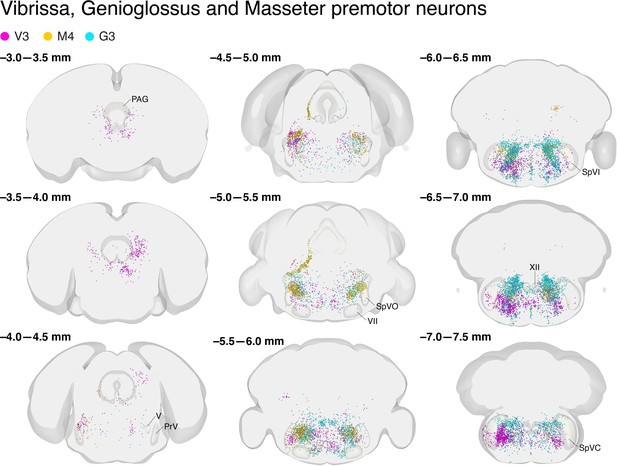
Vibrissa, genioglossus, and masseter premotor neurons overlapped with respect to the Allen mouse brain common coordinate framework.
Coronal views of reconstructed vibrissa (magenta), genioglossus (cyan), and masseter (gold) premotor neurons. Anterior-posterior levels (referenced to Bregma) are shown on the top left of each panel. The identification numbers of animals are shown on top.
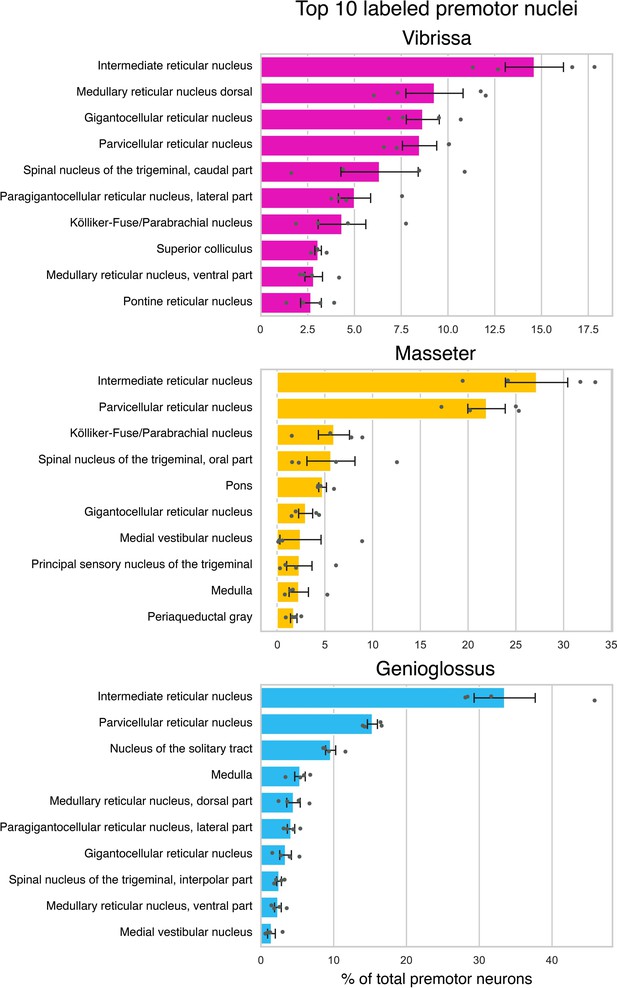
Quantification of transsynaptically labeled neurons in top 10 labeled brain areas for each motor group based on Allen mouse brain common coordinate framework (CCF) nomenclature.
Summary of the distributions of vibrissa (top, magenta, four mice), masseter (middle, gold, four mice), and genioglossus (bottom, cyan, four mice) premotor neurons. Brain areas were automatically annotated based on Allen CCF coordinates. The value is normalized against the total numbers of labeled neurons and averaged across animals. Data are mean ± SEM (n = 4).
Three-dimensional reconstructed vibrissa (magenta), genioglossus (cyan), and masseter (gold) premotor circuits.
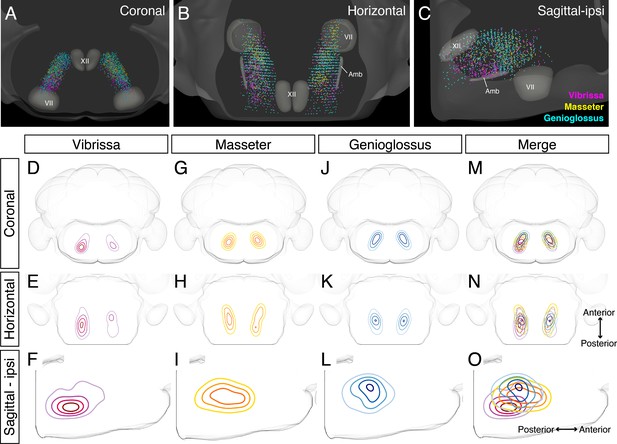
Detailed comparison of spatial organizations of orofacial premotor circuits within intermediatereticular nucleus (IRt).
(A–C) Distribution of vibrissa (magenta), masseter (gold), and genioglossus (cyan) premotor neurons within IRt from representative animals in coronal (A), horizontal (B), and sagittal (C) planes. (D–O) Density analysis of vibrissa (D–F, magenta, an average of four mice), masseter (G–I, yellow, an average of four mice), genioglossus (J–L, blue, an average of four mice) premotor neuron distributions. Merged images (M–O).
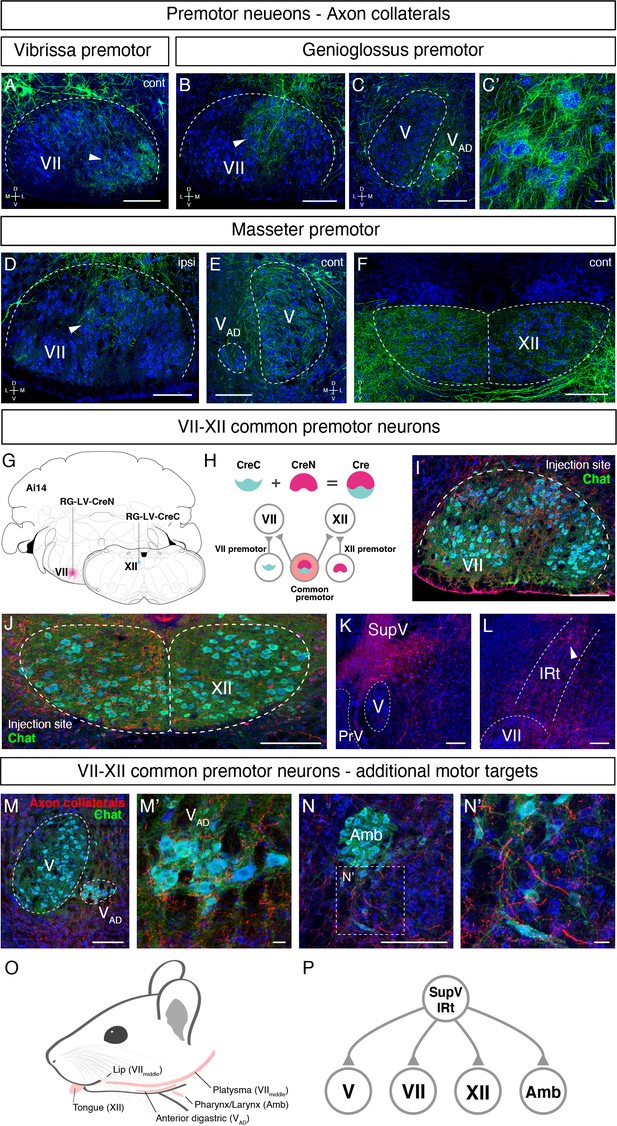
Common premotor neurons innervate multiple distinct orofacial motor nuclei.
(A–F) Representative images of axon collaterals from rabies labeled premotor neurons traced from one muscle innervating other orofacial motor nuclei. Sections were counterstained with fluorescent Nissl (blue). (A) Axon collaterals from ipsilateral vibrissa premotor neurons innervate the contralateral vibrissa motoneurons in the lateral part of VII (arrowhead). (B–C') Axon collaterals of some genioglossus premotor neurons also innervate the middle part of VIImiddle (arrowhead, B) and innervate the anterior digastric part of V (VAD) (C, magnified view is shown in C'). (D–F) Axon collaterals from masseter premotor neurons also innervate the middle part of VIImiddle (D), the contralateral V (E), and the dorsal part of XII (F). (G–P) Identifying VIImiddle-XII common premotor neurons. (G, H) Schematic of split-Cre tracing strategy. (G) RG-LV-CreN and RG-LV-CreC were injected into the left side of VIImiddle and XII of Ai 14 mice, respectively. (H) Cre is reconstituted only in neurons innervating both VIImiddle and XII, and which induces tdTomato reporter expression. (I, J) Representative images of axons/axon collaterals in the injection sites. Sections were counterstained with fluorescent Nissl (blue). Motoneurons were stained with anti-chat antibody (green). VII (I). XII (J). (K, L) Representative images of VIImiddle-XII common premotor neurons in supratrigeminal region (SupV) (K) and the dorsal intermediatereticular nucleus (IRt) (L). (M–N') Representative images of axon collaterals from VIImiddle-XII common premotor neurons in VAD (M, magnified view is shown in M') and Amb (N, magnified view is shown in N'). Scale bars, 200 µm (A–F, I–N); 20 µm (C', M', N'). (O) Schematic showing orofacial muscle targets of motor nuclei. (P) Schematic of all motor nuclei innervated by VIImiddle-XII common premotor neuron in SupV and IRt.
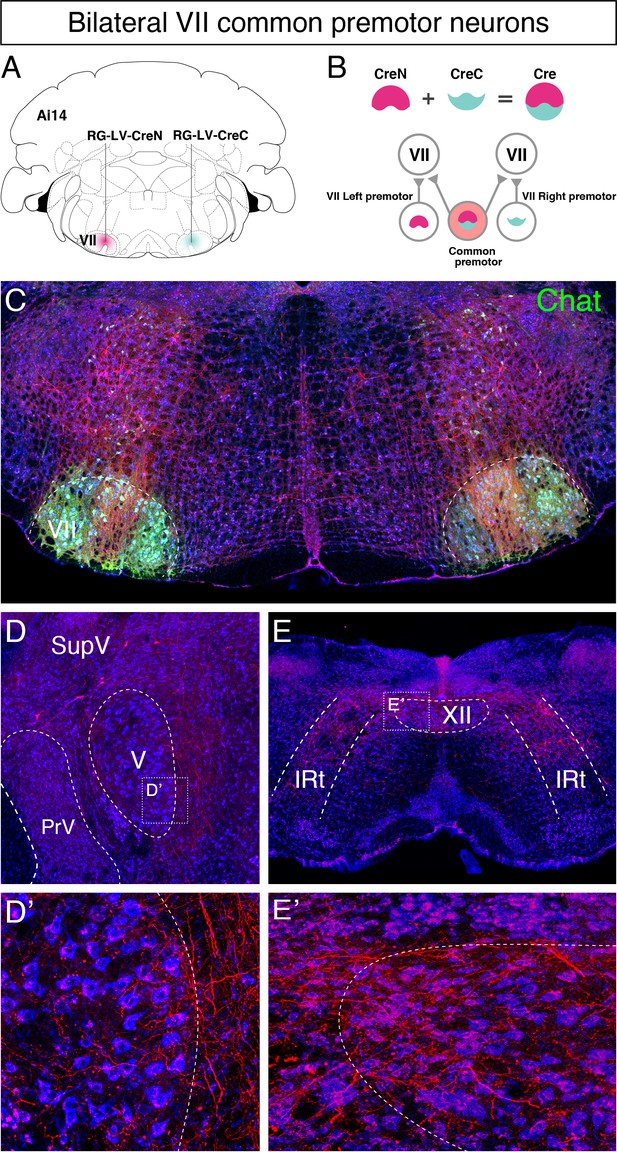
Identifying common premotor neurons with bilateral collateral projections to VIImiddle.
(A, B) Schematic of split-Cre tracing strategy. (B) RG-LV-CreN and RG-LV-CreC were injected into the left and right VIImiddle of Ai 14 mice, respectively. Cre is reconstituted only in neurons innervating both left and right VIImiddle, which induces tdTomato reporter expression. (C) Representative images of axons/axon collaterals in the injection sites. Note the dense tdTomato signal in VIImiddle and bilateral VIImiddle common premotor neurons in the intermediatereticular nucleus (IRt) dorsal to VII. Motoneurons were stained with anti-chat antibody (green). (D–E') bilateral VIImiddle common premotor neurons are observed in supratrigeminal region (D) and the dorsal IRt (E). Axon collaterals of bilateral VIImiddle premotor neurons also innervate V (D'; the boxed area in D) and XII (E'; the boxed area in E) motor neurons. Sections were counterstained with fluorescent Nissl (blue).
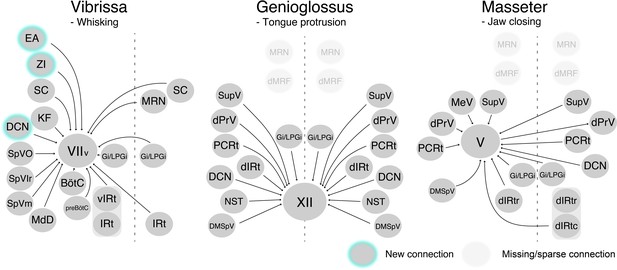
Schematic of vibrissa, tongue-protruding genioglossus, and jaw-closing premotor circuits in the adult mice.
Newly emerged connections in adults that were not observed in neonates are outlined in turquoise. Neonatal connections that appear lost or becoming sparse are shown as translucent spheres.





