A phosphorylation of RIPK3 kinase initiates an intracellular apoptotic pathway that promotes prostaglandin2α-induced corpus luteum regression
Figures
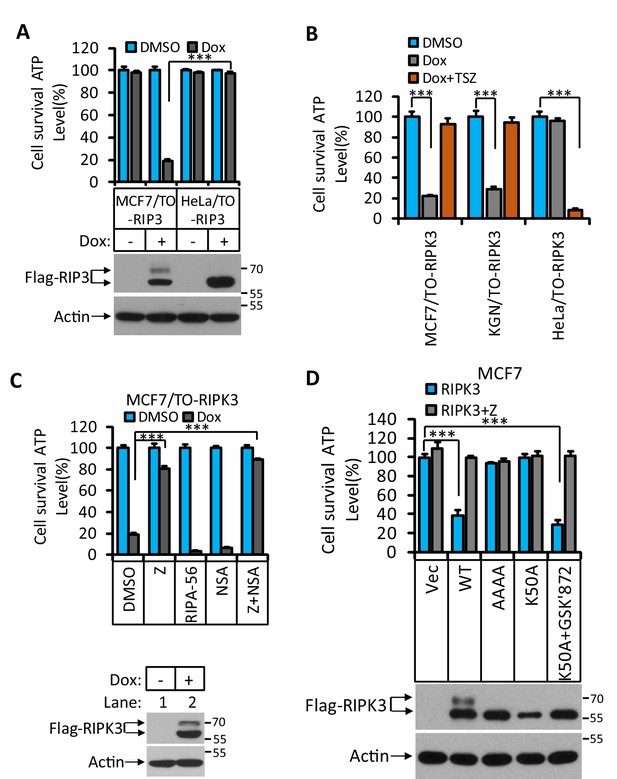
RIPK3-induced apoptosis in MCF7 and KGN cells.
(A) Cultured MCF7/TO-RIPK3 and HeLa/TO-RIPK3 cells were treated with DMSO or Dox (1 μg/ml) induction for 36 hr. Cell viability was determined by measuring cellular ATP levels (upper panel). The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. The cell lysates were analyzed by western blotting using antibodies against RIPK3 or β-actin (lower panel). (B) Cultured MCF7/TO-RIPK3, KGN/TO-RIPK3, and HeLa/TO-RIPK3 cells were treated with DMSO, Dox, or Dox plus TSZ for 36 hr. Cell viability was determined by measuring cellular ATP levels. The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. (C) Cultured MCF7/TO-RIPK3 cells were treated with DMSO or Dox, plus the indicated agents for 36 hr. Cell viability was determined by measuring cellular ATP levels (upper panel). The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. The cell lysates were analyzed by western blotting using antibodies against RIPK3 or β-actin (lower panel). 20 μM Z, pan-caspase inhibitor z-VAD; 2 μM RIPA-56, RIPK1 inhibitor; 2 μM NSA, MLKL inhibitor. (D) Cultured MCF7 cells were infected with lentiviruses encoding RIPK3(WT), RIPK3(AAAA), RIPK3(K50A), and RIPK3(K50A)+GSK’872 plus Z for 36 hr. Cell viability was determined by measuring cellular ATP levels (upper panel). The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. The lysates were measured by western blotting using antibodies against RIPK3 or β-actin as indicated (lower panel). GSK’872, RIPK3 inhibitor.
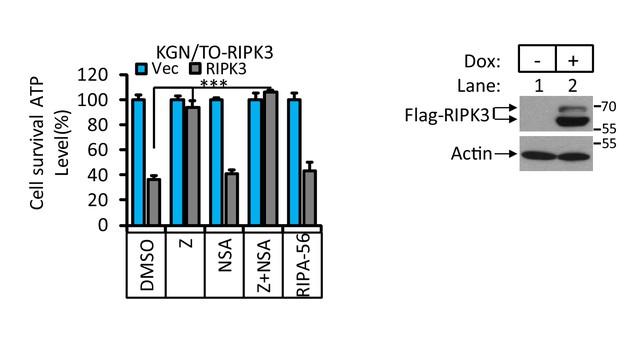
RIPK3-induced apoptosis in human granulosa lutein cells (KGN).
Cultured KGN/TO-RIPK3 cells were treated with DMSO or Dox (1 μg/ml) induction for 36 hr. Cell viability was determined by measuring cellular ATP levels (upper panel). The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. The cell lysates were analyzed by western blotting using antibodies against RIPK3 or β-actin (lower panel).
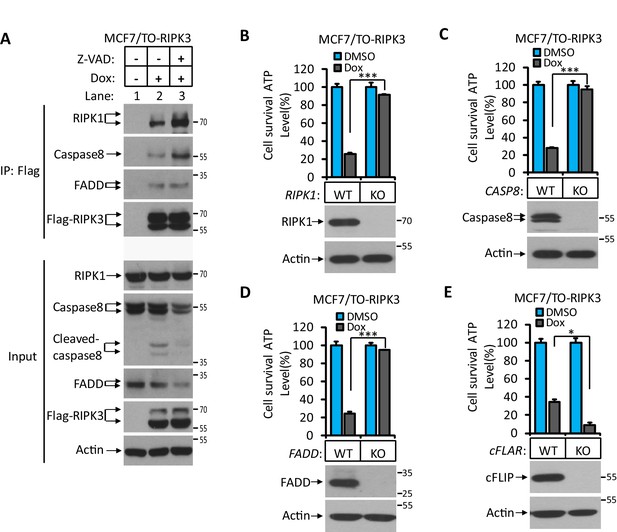
RIPK3-induced apoptosis was dependent on RIPK1, FADD, and caspase-8.
(A) Cultured MCF7/TO-RIPK3 cells were treated with DMSO or Dox plus the indicated agent for 24 hr. The cells were then harvested, and RIPK3 was immunoprecipitated from the cell lysates using anti-Flag resin. The cell lysates and immunocomplexes were analyzed by western blotting using antibodies as indicated. (B–E) Cultured MCF7/TO-RIPK3 (wild type [WT], RIPK1-/-, Caspase8-/-, FADD-/-, and cFLIF-/-) cells were treated with DMSO or Dox induction for 36 hr. Cell viability was determined by measuring cellular ATP levels (upper panel). The data are represented as the mean ± SD of triplicate wells. *p<0.05, ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. The cell lysates were analyzed by western blotting using antibodies against RIPK1, caspase-8, FADD, cFLIP, or β-actin (lower panel). Five independent knockout clones were test in each gene.
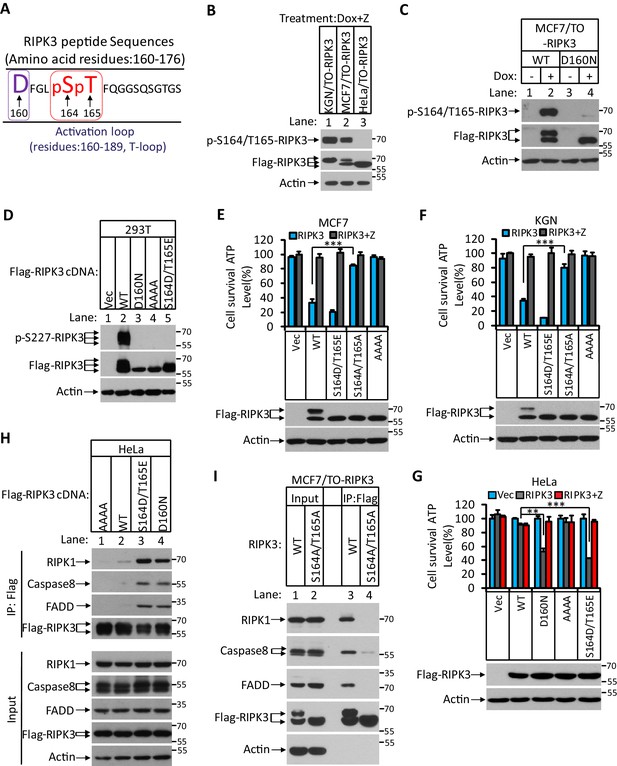
RIPK3-mediated apoptosis is dependent on the auto-phosphorylation of the S164/T165 sites.
(A) Cultured MCF7/TO-RIPK3 and HeLa/TO-RIPK3 cells were treated with Dox plus z-VAD for 24 hr. RIPK3 was immunoprecipitated from the cell lysates using anti-Flag resin. The RIPK3 bands were excised and subjected to mass spectrometry analysis. RIPK3-specific phosphorylation site in MCF7/TO-RIPK3 cells is highlighted in red. (B) Cultured KGN/TO-RIPK3, MCF7/TO-RIPK3, and HeLa/TO-RIPK3 cells were treated with Dox plus z-VAD for 24 hr. The lysates were analyzed by western blotting using antibodies against the phospho-serine 164/threonine 165 of RIPK3, Flag (RIPK3), and β-actin as indicated. (C) Cultured MCF7 stably transfected with either wild-type RIPK3 (WT) or kinase-dead mutant (D160N) cells under the control of Dox-inducible promoter were treated with DMSO(-) or Dox plus z-VAD for 24 hr. The lysates were analyzed by western blotting using antibodies against the phospho-serine 164/threonine 165 RIPK3, Flag (RIPK3), and β-actin as indicated. (D) Cultured 293T cells were transfected with Vector (Vec), RIPK3(WT), RIPK3(D160N), RIPK3(AAAA) (RIPK3-AAAA, residues 459–462 mutated to AAAA), and RIPK3(S164D/T165E) for 24 hr. The level of phospho-S227-RIPK3 and RIPK3 was measured by western blotting. (E, F) Cultured MCF7 (E) and KGN (F) cells were infected with lentiviruses encoding RIPK3(WT), RIPK3(S164D/T165E), RIPK3(S164A/T165A), and RIPK3(AAAA) plus z-VAD for 36 hr. Cell viability was determined by measuring cellular ATP levels (upper panel). The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. The lysates were measured by western blotting using antibodies against RIPK3 or β-actin as indicated (lower panel). (G) Cultured HeLa cells were infected with lentiviruses encoding RIPK3(WT), RIPK3(D160N), RIPK3(AAAA), and RIPK3(S164D/T165E) plus z-VAD for 36 hr. Cell viability was determined by measuring cellular ATP levels (upper panel). The data are represented as the mean ± SD of triplicate wells. **p<0.01, ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. The expressed RIPK3 in the cell lysates were measured by western blotting using antibodies against RIPK3 or β-actin as indicated (lower panel). Vector (Vec, control viruses) (H) Cultured HeLa cells were transfected with Flag-tagged RIPK3(WT), RIPK3(D160N), and RIPK3(S164D/T165E) for 24 hr. RIPK3 was immunoprecipitated using anti-Flag resin. The lysates and immunocomplexes were analyzed by western blotting using antibodies against RIPK1, caspase-8, FADD, and RIPK3 as indicated. (I) Cultured MCF7/TO-RIPK3 and MCF7/TO-RIPK3(S164A/T165A) cells were treated with Dox plus Z for 24 hr. The cells were then harvested, and RIPK3 was immunoprecipitated from the cell lysates using anti-Flag resin. The cell lysates and immunocomplexes were analyzed by western blotting using antibodies as indicated.
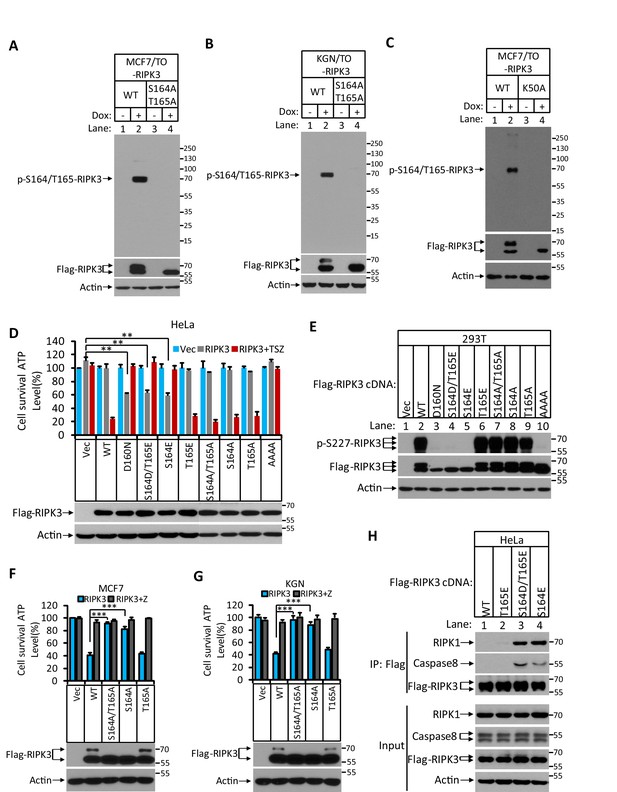
Characterization of RIPK3 auto-phosphorylation sites.
(A) Cultured MCF7/TO-RIPK3 and MCF7/TO-RIPK3(S164A/T165A) cells were treated with DMSO(-) or Dox plus z-VAD for 24 hr. The lysates were analyzed by western blotting using antibodies against the phospho-serine 164/threonine 165 of RIPK3, Flag (RIPK3), and β-actin as indicated. (B) Cultured KGN7/TO-RIPK3 and KGN/TO-RIPK3(S164A/T165A) cells were treated with DMSO or Dox plus z-VAD for 24 hr. The lysates were analyzed by western blotting using antibodies against the phospho-serine 164/threonine 165 of RIPK3, Flag (RIPK3), and β-actin as indicated. (C) Cultured MCF7/TO-RIPK3 and MCF7/TO-RIPK3(K50A) cells were treated with DMSO(-) or Dox plus z-VAD for 24 hr. The lysates were analyzed by western blotting using antibodies against the phospho-serine 164/threonine 165 of RIPK3, Flag (RIPK3), and β-actin as indicated. (D) Cultured HeLa cells were infected with lentiviruses encoding wild-type RIPK3(WT), and mutant RIPK3 including RIPK3(D160N), RIPK3(S164D/T165E), RIPK3(S164E), RIPK3(T165E), RIPK3(S164A/T165A), RIPK3(S164A), RIPK3(T165A), and RIPK3(AAAA) and treated with TSZ for 36 hr. Cell viability was determined by measuring cellular ATP levels (upper panel). The data are represented as the mean ± SD of triplicate wells. **p<0.01. p-values were determined by two-sided unpaired Student’s t-tests. The levels of expressed RIPK3 in the cell lysates were measured by western blotting (lower panel). (E) RIPK3 single site (S164E) mutation blocks auto-phosphorylation. 293T cells were transfected with Flag-tagged RIPK3(WT), RIPK3(D160N), RIPK3(S164D/T165E), RIPK3(S164E), RIPK3(T165E), RIPK3(S164A/T165A), RIPK3(S164A), RIPK3(T165A), and RIPK3(AAAA) for 24 hr. The level of p-S227-RIPK3 and RIPK3 was measured by western blotting. (F, G) Cultured MCF7 (E) and KGN (F) cells were infected with lentiviruses encoding wild-type RIPK3(WT), and mutant forms of RIPK3(S164A/T165A), RIPK3(S164A), and RIPK3(T165A) and treated with z-VAD as indicated for 36 hr. Cell viability was determined by measuring cellular ATP levels (upper panel). The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. The levels of RIPK3 in the cell lysates were measured by western blotting (lower panel). (H) Cultured HeLa cells were transfected with Flag-tagged wild-type RIPK3(WT), and mutant forms of RIPK3(T165E), RIPK3(S164D/T165E), and RIPK3(S164E) for 24 hr. RIPK3 was immunoprecipitated from the cell lysates using anti-Flag resin. The lysates and immunocomplexes were analyzed by western blotting using antibodies against RIPK1, caspase-8, RIPK3, and β-actin as indicated.
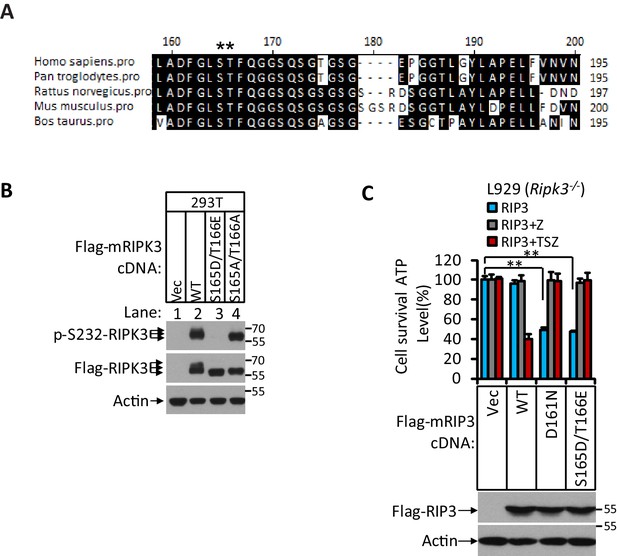
The phosphorylation site of RIPK3 is conserved among different mammalian species.
(A) Alignment of amino acid sequences of RIPK3 orthologs in five mammalian species. Amino acid residues conserved in 80% or more of the sequences are shaded in black. The putative phosphorylation residues are denoted by asterisks (*). (B) Cultured 293T cells were transfected with Vector (Vec), mouse RIPK3(WT), RIPK3(S165D/T166E), and RIPK3(S165A/T165A) for 24 hr. The level of phospho-S232-RIPK3 and RIPK3 was measured by western blotting. (C) Cultured mouse sarcoma cells L929(Ripk3-/-) were transfected with Vector, wild-type mouse RIPK3, and mutant forms of mRIPK3(D161N), and mRIPK3(S165D/T166E) and treated with z-VAD or TSZ as indicated for 36 hr. Cell viability was determined by measuring cellular ATP levels (upper panel). The data are represented as the mean ± SD of triplicate wells. **p<0.01. p-values were determined by two-sided unpaired Student’s t-tests. The lysates were analyzed by western blotting using antibodies as indicated (lower panel).
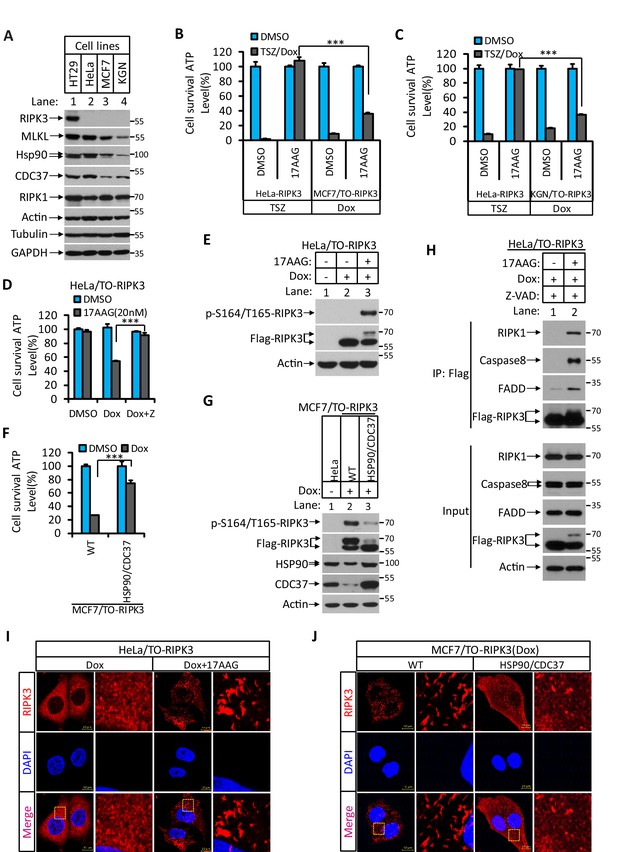
Hsp90/CDC37 chaperone determined the apoptotic and necroptotic function of RIPK3 kinase.
(A) The cell lysates from cultured HT29, HeLa, MCF7, and KGN cells were analyzed by western blotting using antibodies as indicated. (B, C) Cultured HeLa-RIPK3, MCF7/TO-RIPK3, and KGN/TO-RIPK3 cells were treated with the indicated stimuli for 36 hr. Cell viability was determined by measuring cellular ATP levels. The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. 17AAG, Hsp90 inhibitor. (D, E) HeLa/TO-RIPK3 cells were treated with the indicated stimuli for 36 hr. Cell viability was determined by measuring cellular ATP levels in (D). The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. 24 hr after treatment, the cell lysates were analyzed by western blotting using antibodies against phospho-serine 164/threonine 165 of RIPK3, RIPK3, and β-actin as indicated in (E). (F, G) Cultured MCF7/TO-RIPK3 cells co-transfected with HSP90 and CDC37 as indicated were treated with DMSO or Dox for 36 hr. Cell viability was determined by measuring cellular ATP levels in (F). The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. 24 hr after treatment, the cell lysates were analyzed by western blotting using antibodies against phospho-serine 164/threonine 165 of RIPK3, RIPK3, Hsp90, CDC37, and β-actin as indicated in (G). (H) Cultured HeLa/TO-RIPK3 cells were treated with Dox or Dox plus 17AAG for 24 hr. The cells were then harvested, and RIPK3 was immunoprecipitated from the cell lysates using anti-Flag resin. The cell lysates and immunocomplexes were analyzed by western blotting using antibodies as indicated. (I) Cultured HeLa/TO-RIPK3 cells were treated with Dox or Dox plus 17AAG for 24 hr. Immunofluorescence of the cells with Flag-RIPK3 (red) antibody. Counterstaining with DAPI (blue). Scale bar, 10 μm. Higher-power views (right panels) were acquired from the selected boxed areas from the left panel. (J) Cultured MCF7/TO-RIPK3 cells co-transfected with HSP90 and CDC37 as indicated were treated with Dox for 24 hr. Immunofluorescence of the cells with Flag-RIPK3 (red) antibody. Counterstaining with DAPI (blue). Scale bar, 10 μm. Higher-power views (right panels) were acquired from the selected boxed areas from the left panel.
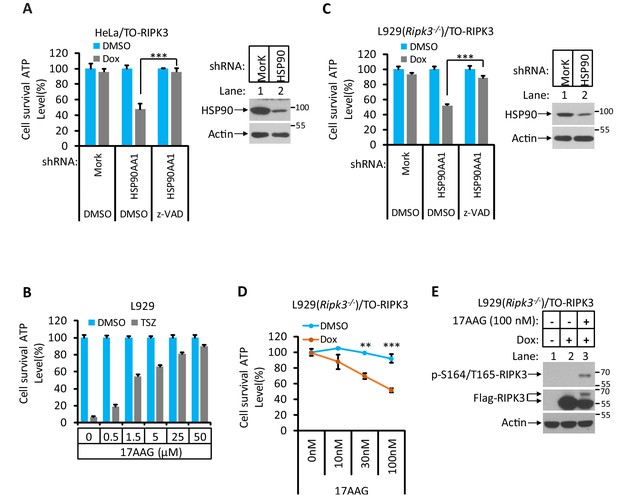
Hsp90/CDC37 chaperone determines the necroptotic or apoptotic function of RIPK3 kinase.
(A) HeLa/TO-RIPK3 and HeLa/TO-RIPK3-shRNA-HSP90 cells were treated with the indicated stimuli for 36 hr. Cell viability was determined by measuring cellular ATP levels. The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. The cell lysates were analyzed by western blotting using antibodies as indicated (right panel). (B) L929 cells were treated with the indicated stimuli for 5 hr. Cell viability was determined by measuring cellular ATP levels. The data are represented as the mean ± SD of triplicate wells. (C) L929(Ripk3-/-)/TO-RIPK3 and L929(Ripk3-/-)/TO-RIPK3-shRNA-HSP90 cells were treated with the indicated stimuli for 36 hr. Cell viability was determined by measuring cellular ATP levels. The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. The cell lysates were analyzed by western blotting using antibodies as indicated (right panel). (D, E) L929(Ripk3-/-)/TO-RIPK3 cells were treated with the indicated stimuli for 36 hr. Cell viability was determined by measuring cellular ATP levels in (D). The data are represented as the mean ± SD of triplicate wells. **p<0.01, ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. 24 hr after treatment, the cell lysates were analyzed by western blotting using antibodies against phospho-serine 165/threonine 166 of RIPK3, RIPK3, and β-actin as indicated in (E).
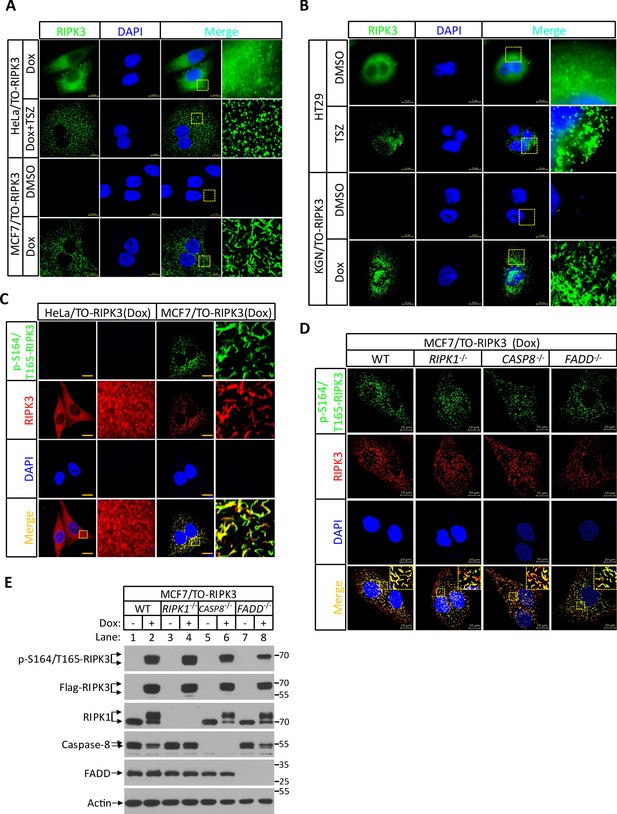
RIPK3 form amyloid-like structure in MCF7 and KGN cells.
(A) Cultured HeLa/TO-RIPK3 and MCF7/TO-RIPK3 cells were treated with DMSO or Dox plus TSZ for 24 hr. Immunofluorescence of the cells with Flag-RIPK3 (green) antibody. Counterstaining with DAPI (blue). Scale bar, 10 μm. Higher-power views (right panels) were acquired from the selected boxed areas from the left panel. (B) Cultured HT29 and KGN/TO-RIPK3 cells were treated with DMSO, TSZ, or Dox for 24 hr. Immunofluorescence of the cells with Flag-RIPK3 (green) antibody. Counterstaining with DAPI (blue). Scale bar, 10 μm. Higher-power views (right panels) were acquired from the selected boxed areas from the left panel. (C) Cultured HeLa/TO-RIPK3 and MCF7/TO-RIPK3 cells were treated with Dox plus Z for 24 hr. Immunofluorescence of the cells with Flag-RIPK3 (red) and p-S164/T165-RIPK3 (green) antibody. Counterstaining with DAPI (blue). Scale bar, 10 μm. Higher-power views (right panels) were acquired from the selected boxed areas from the left panel. (D) Cultured MCF7/TO-RIPK3(WT, RIPK1-/-, caspase-8-/- and FADD-/-) cells were treated with Dox plus Z for 24 hr. Immunofluorescence of the cells with Flag-RIPK3 (red) and p-S164/T165-RIPK3 (green) antibody. Counterstaining with DAPI (blue). Scale bar, 10 μm. Higher-power views (right panels) were acquired from the selected boxed areas from the left panel. (E) Cultured MCF7/TO-RIPK3(WT, RIPK1-/-, caspase-8-/- and FADD-/-) cells were treated with DMSO or Dox plus Z for 24 hr. The lysates were analyzed by western blotting using antibodies as indicated.
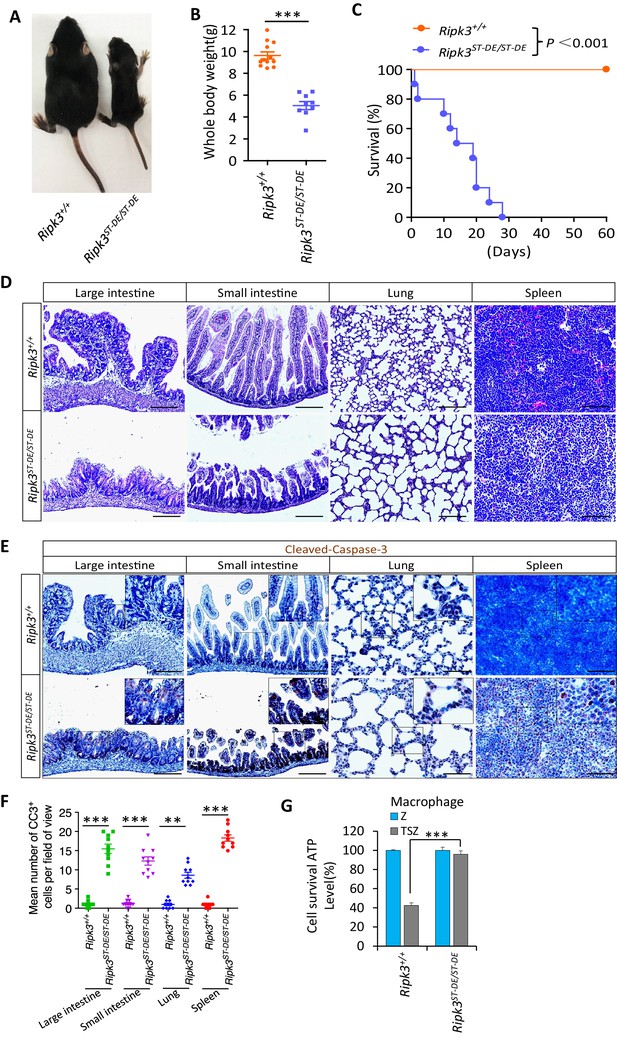
Ripk3S165D-T166D/S165D-T166D (Ripk3ST-DE/ST-DE) mice die within 1 month after birth.
(A, B) Macroscopic features (A) and body weights (B) of Ripk3+/+ and Ripk3ST-DE/ST-DE littermate mice at 14 days of age (n ≥ 9). The result from each individual animal is presented as an indicated dot. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. (C) Kaplan–Meier plot of survival of Ripk3+/+ and Ripk3ST-DE/ST-DE littermate mice (n = 10 for each genotype) after birth within 2 months. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. (D) Histological analysis of large intestine, small intestine, lung, and spleen of Ripk3+/+ and Ripk3ST-DE/ST-DE littermate mice (n = 5) at 14 days of age. Scale bar, 20 μm. (E, F) Representative immunohistochemistry (IHC) images of the large intestine, small intestine, lung, and spleen of Ripk3+/+ and Ripk3ST-DE/ST-DE littermate mice (n = 5, 14 days) stained with a cleaved-caspase-3 (C–C3) antibody in (E). C-C3-positive cells were counted in two fields per organ and quantified in (F). Scale bar, 10 μm. Data represent the mean ± s.e.m. **p<0.01, ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. (G) Cell viability measurement of bone marrow-derived macrophages from the Ripk3+/+ and Ripk3ST-DE/ST-DE littermate mice (n = 3, 14 days) after treatment with the indicated Z-VAD or necroptosis stimuli for 24 hr. Cell viability was determined by measuring cellular ATP levels. The data are represented as the mean ± SD of triplicate wells. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. .
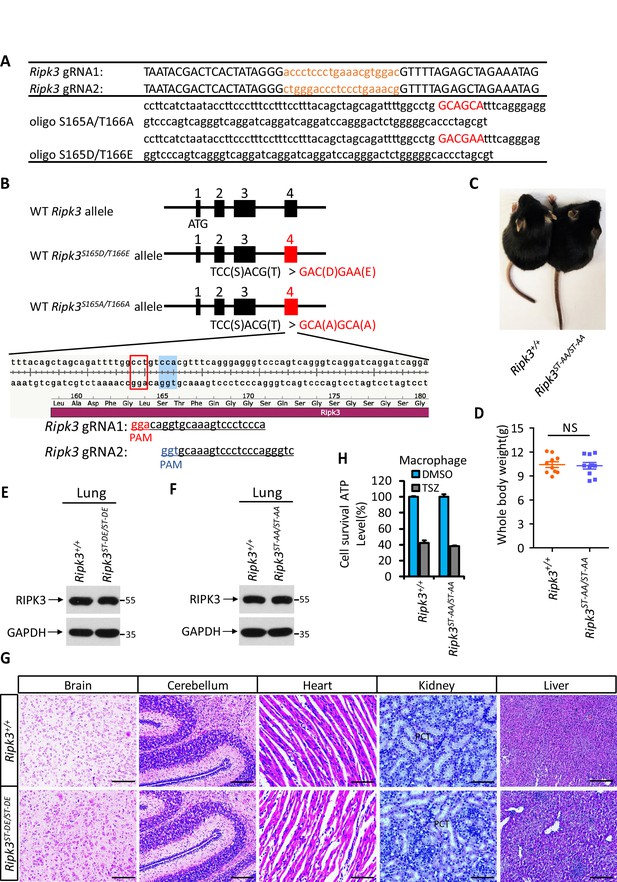
Generation of Ripk3ST-DE/ST-DE and Ripk3ST-AA/ST-AA mice.
(A) Two guide RNA and donate oligo sequences of Ripk3(S165D/T166E) and Ripk3(S165A/T166A) knock-in mice. (B) Schematic of CRISPER-Cas9 strategy for the generation for Ripk3(S165D/T166E) and Ripk3(S165A/T166A) knock-in mice. The gene structure of RIPK3 and two guide RNA sequences targeting the exon 4 of RIPK3 is shown with the PAM sequences highlighted in red and blue. (C, D) Macroscopic features (C) and body weights (D) of Ripk3+/+ and Ripk3ST-AA/ST-AA littermate mice at 14 days of age (n = 10). The result from each individual animal is presented as an indicated dot. NS: not significant. p-values were determined by two-sided unpaired Student’s t-tests. (E, F) Immunoblot of RIPK3 from lung extracts of 14 days old Ripk3+/+, Ripk3ST-DE/ST-DE, and Ripk3ST-AA/ST-AA littermates using antibodies against RIPK3 and GAPDH as indicated (n = 3). (G) Histological analysis of brain, cerebellum, heart, kidney, and liver of Ripk3+/+ and Ripk3ST-DE/ST-DE littermate mice (n = 5) at 14 days of age. Scale bar, 20 μm. PTC: proximal tubular cell. (H) Cell viability measurement of bone marrow-derived macrophages from the Ripk3+/+ and Ripk3ST-AA/ST-AA littermate mice (n = 3, 14 days) after treatment with the indicated necroptosis stimuli for 24 hr. Cell viability was determined by measuring cellular ATP levels. The data are represented as the mean ± SD of triplicate wells.
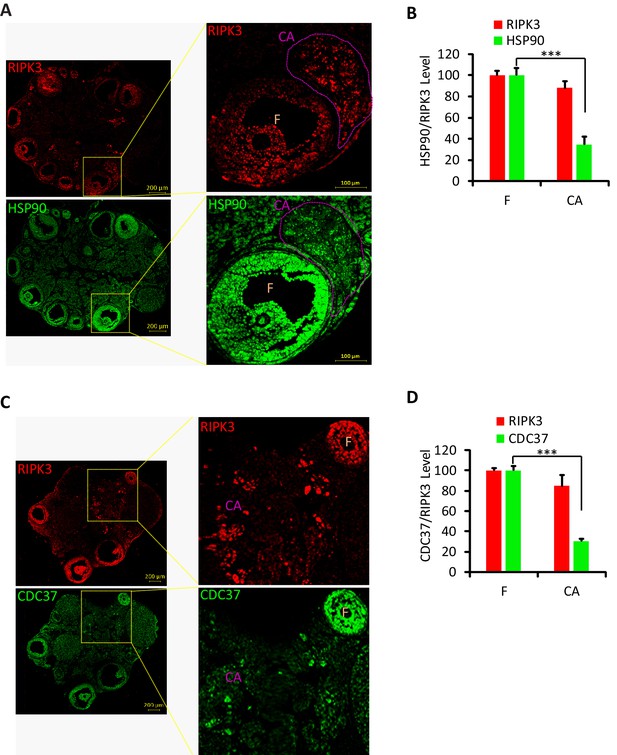
Hsp90/CDC37 chaperone protein levels were low in corpus luteum and corpus albicans.
(A, B) Immunofluorescence of ovary from wild-type mice (8 months; n = 3) with RIPK3 (red) and HSP90 (green) antibody in (A). Higher-power views of selected areas were acquired in right panel. The HSP90/RIPK3 levels were quantified in (B). F: follicle; CA: corpus albicans. Scale bar, 100/200 μm. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. (C, D) Immunofluorescence of ovary from wild-type mice (8 months; n = 3) with RIPK3 (red) and CDC37 (green) antibody in (C). Higher-power views of selected areas were acquired in right panel. The CDC37/RIPK3 levels were quantified in (D). F: follicle; CA: corpus albicans. Scale bar, 200 μm. ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests.
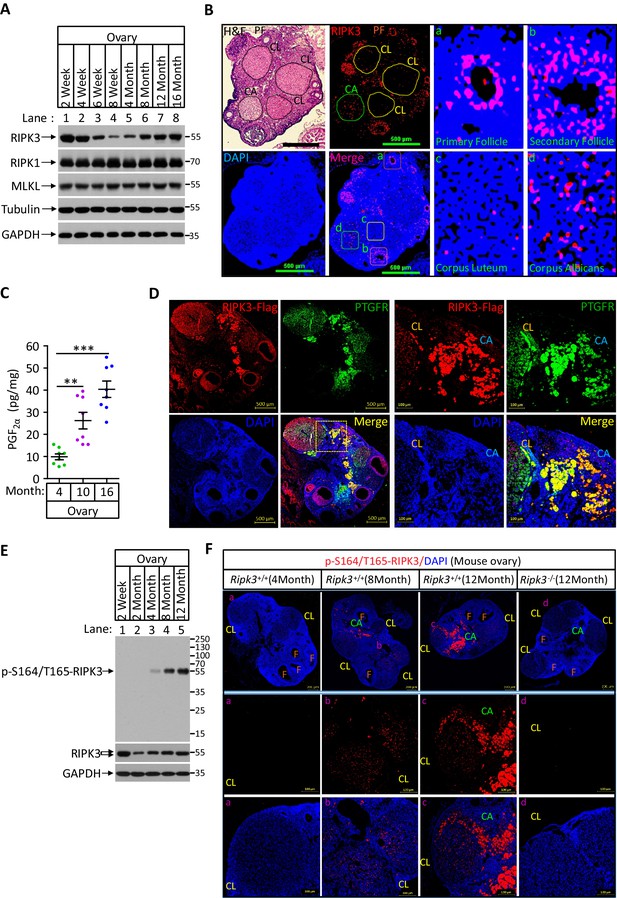
Phospho-serine 164/threonine 165-RIPK3 signals were present in aged ovary corpus albican.
(A) Western blot analysis of RIPK1, RIPK3, and MLKL levels in perfused mouse ovary extracts of different ages. Each group is representative of at least three mice. (B) H&E and immunofluorescence (IF) imaging of an 8-month-old ovary. Two adjacent sections were analyzed. One section was stained with H&E, and the other was IF stained with a RIPK3 antibody (red) and DAPI (blue). Scale bar, 500 μm. Higher-power views of selected areas were acquired in a (primordia follicle), b (secondary follicle), c (corpus luteum), and d (corpus albicans) as indicated. PF: primary follicle; CL: corpus luteum; CA: corpus albicans. (C) Ovarian PGF2α levels of wild-type mice (n = 8) at the indicated age assayed by ELISA. Data represent the mean ± s.e.m. **p<0.01, ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. (D) Immunofluorescence images of a RIPK3 C-terminus HA-3xFlag knock-in mouse ovary (n = 5; 12 months) stained with antibodies against prostaglandin F receptor (PTGFR, green) and Flag (red). Counterstaining with DAPI (blue). Scale bar, 500 μm. Higher-power views (right panels) were acquired from the indicated boxed area in the second lower left panel. CL: corpus luteum; CA: corpus albicans. Scale bar, 100 μm. (E) Western blot analysis of p-S164/T165-RIPK3 and RIPK3 levels in extracts from perfused ovaries prepared from mice at the indicated age. Each group is representative of at least three mice. (F) Immunofluorescence images of ovaries from Ripk3+/+ and Ripk3-/- mice (4 months, 8 months and 12 months; n = 3) at the indicated ages stained with the p-S164/T165-RIPK3 antibody (red). Counterstaining with DAPI (blue). Scale bar, 200 μm. Higher-power views (lower two panels) were acquired from the selected boxed areas from the upper panel. a (CL), b (CL), c (CL, CA), and d (CL). F: follicle; CL: corpus luteum; CA: corpus albicans. Scale bar, 100 μm.
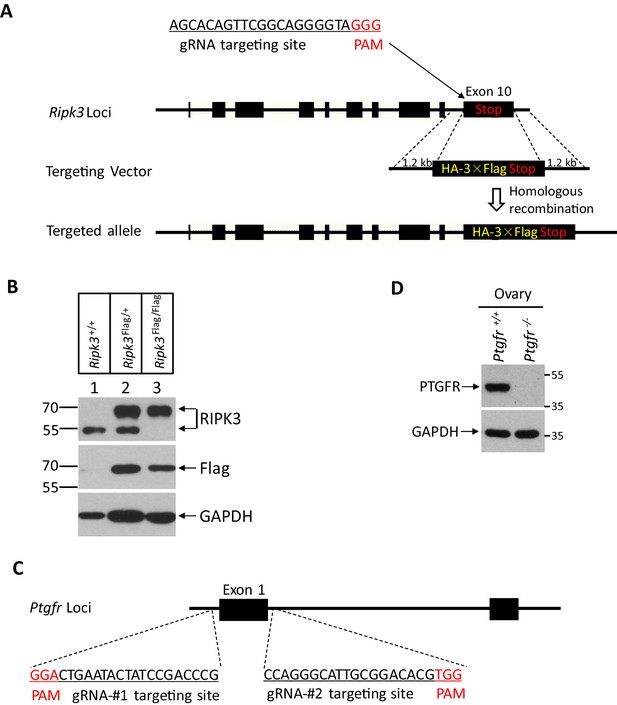
Generation of RIPK3 C-terminus HA-3xFlag knock-in and Ptgfr-/- mice.
(A) Schematic of CRISPER-Cas9 strategy for RIPK3 C-terminus HA-3xFlag knock-in mice. The gene structure of RIPK3 and guide RNA sequences targeting the Ripk3 is shown with the PAM sequences highlighted in red. (B) Western blotting analysis using protein extracts from the ovary of wild-type, heterozygous knock-in, and homozygous knock-in mice generated as illustrated in (A). (C) Schematic of CRISPER-Cas9 strategy for the generation for Ptgfr-/- mice. The gene structure of prostaglandin F receptor (PTGFR) and two guide RNA sequences targeting the Ptgfr is shown with the PAM sequences highlighted in red. (D) Immunoblot of PTGFR from ovary extracts of 2-month-old Ptgfr+/+ and Ptgfr-/- littermates using antibodies against PTGFR and GAPDH as indicated (n = 3).
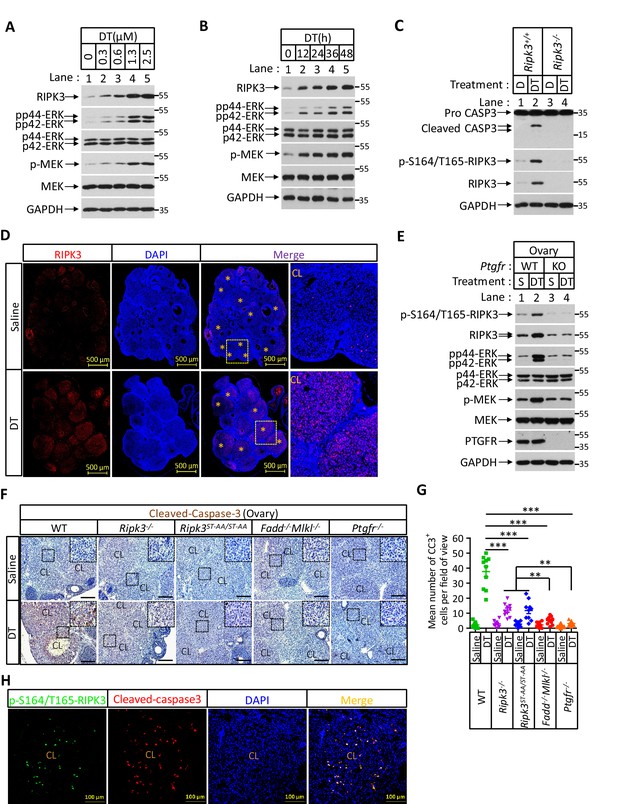
Prostaglandin F2alpha (PGF2α) induces ovarian RIPK3 expression for corpus luteum involution.
(A–C) Primary granulosal lutein cells (WT, Ripk3-/-) were isolated from 3-month-old mice ovaries. The cells were treated with dinoprost tromethamine (DT) at the indicated concentration for 36 hr in (A); with 1.5 μM DT at the indicated time in (B); or with 1.5 μM DT for 36 hr in (C). The cell lysates from the DT-treated cells were analyzed by western blotting using antibodies as indicated. (D, E) Ptgfr+/+ and Ptgfr-/- littermate female mice (n = 16; 25–26 days) were given 7.5 IU pregnant mare serum gonadotropin (PMSG) intraperitoneally (IP) followed by 7.5 IU serum gonadotropin and chorionic gonadotropin (SCG) 46 hr later to synchronize ovulation. The animals were then injected with DT (10 μg, IP) or saline 24 hr post-ovulation. Ovaries were then collected 12 hr later and stained with anti-RIPK3 antibody (red) in (D). The ovary lysates were analyzed by western blotting using antibodies as indicated in (E). * indicates corpus luteum. Counterstaining with DAPI (blue). Scale bar, 500 μm. (F, G) wild-type (WT), Ripk3-/-, Ripk3S165A-T166A/S165A-T166A, Fadd-/-Mlkl-/- and Ptgfr-/- female mice (each group, n = 16; 25–26 days) were treated as in (D, F). Ovaries from each group were then collected 24 hr after injecting with DT and stained with anti-cleaved-caspase-3 antibody in (F). The Cleaved-Caspase3+ cells were counted in five fields per ovary CL and quantified in (G). Scale bar, 20 μm. Data represent the mean ± s.e.m. **p<0.01, ***p<0.001. p-values were determined by two-sided unpaired Student’s t-tests. (H) WT female mice (n = 3; 25–26 days) were treated as in (D, F). Ovaries were then collected 12 hr after injecting with DT and stained with anti-cleaved-caspase-3 (red) and p-S164/T165-RIPK3 (green) antibody. Counterstaining with DAPI (blue). Scale bar, 100 μm.
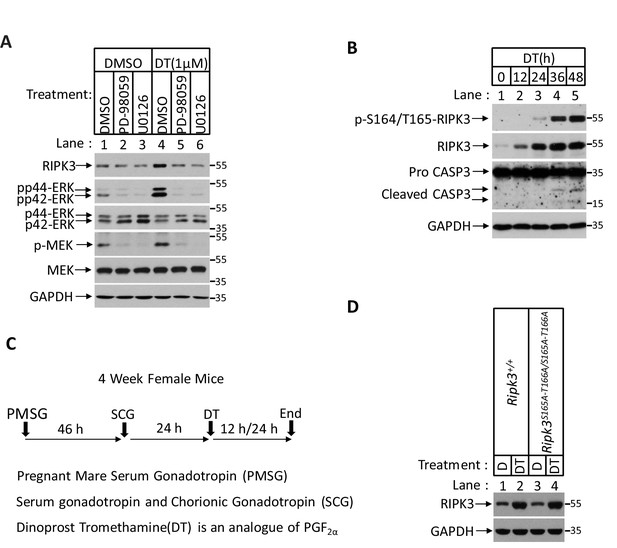
Prostaglandin F2alpha (PGF2α) stimulates RIPK3 expression through the MAPK pathway.
(A) Primary granulosal lutein cells were isolated from the 3-month-old mice ovary. The cells were then treated with 1 μM dinoprost tromethamine (DT) or plus MAPK inhibitors PD-98059 (5 μM) and U0126 (5 μM) as indicated for 36 hr. The lysates were analyzed by western blotting using antibodies as indicated. (B) Primary granulosal lutein cells were isolated from 3-month-old mice ovaries. The cells were treated with 1 μM DT at the indicated time. The cell lysates from the DT-treated cells were analyzed by western blotting using antibodies as indicated. (C) Diagram of induction of corpus luteum regression in vivo. (D) Ripk3+/+ and Ripk3S165A-T166A/S165A-T166A and littermate female mice (n = 3; 25–26 days) were given 7.5 IU pregnant mare serum gonadotropin (PMSG) intraperitoneally (IP) followed by 7.5 IU serum gonadotropin and chorionic gonadotropin (SCG) 46 hr later to synchronize ovulation. The animals were then injected with DT (10 μg, IP) or saline 24 hr post-ovulation. The ovary lysates were analyzed by western blotting using antibodies as indicated.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Homo sapiens) | HEK293T | ATCC | CRL-11268 | Female |
| Cell line (Homo sapiens) | HeLa | ATCC | CCL-2 | Female |
| Cell line (Homo sapiens) | HT-29 | ATCC | HTB-38 | Female |
| Cell line (Homo sapiens) | MCF7 | ATCC | HTB-22 | Female |
| Cell line (Homo sapiens) | KGN | Nishi et al., 2001 | N/A | Female |
| Cell line (Homo sapiens) | L929(Ripk3-/-) | Dr. Xiaodong Wang lab at National Institute of Biological Sciences, Beijing | N/A | |
| Cell line (Homo sapiens) | KGN/TO-RIPK3 | Dr. Xiaodong Wang lab at National Institute of Biological Sciences, Beijing | N/A | |
| Cell line (Homo sapiens) | KGN/TO- RIPK3(S164A/T165A) | Dr. Xiaodong Wang lab at National Institute of Biological Sciences, Beijing | N/A | |
| Cell line (Homo sapiens) | MCF7/TO-RIPK3 | Dr. Xiaodong Wang lab at National Institute of Biological Sciences, Beijing | N/A | |
| Cell line (Homo sapiens) | MCF7/TO- RIPK3(D160N) | Dr. Xiaodong Wang lab at National Institute of Biological Sciences, Beijing | N/A | |
| Cell line (Homo sapiens) | MCF7/TO- RIPK3(K50A) | Dr. Xiaodong Wang lab at National Institute of Biological Sciences, Beijing | N/A | |
| Cell line (Homo sapiens) | MCF7/TO- RIPK3(S164A/T165A) | Dr. Xiaodong Wang lab at National Institute of Biological Sciences, Beijing | N/A | |
| Cell line (Homo sapiens) | MCF7/TO-RIPK3 (RIPK1-/-/Caspase-8-/-/FADD-/-/cFLIP-/-) | Dr. Xiaodong Wang lab at National Institute of Biological Sciences, Beijing | N/A | |
| Antibody | Anti-RIPK3 (Rabbit polyclonal) | ProSci | Cat# 2283; RRID:AB_203256 | WB (1:1000) |
| Antibody | Anti-p-S164/T165-RIPK3 (Rabbit monoclonal) | Abcam | Cat# Ab255705; | Firstly described in this paper; WB (1:1000) IF (1:100) |
| Antibody | Anti-RIPK3 (Mouse monoclonal) | LSBio | Cat# LS-C336804 | WB (1:1000) |
| Antibody | Anti-GAPDH-HRP (Mouse monoclonal) | MBL | Cat# M171-1; RRID:AB_10699462 | WB (1:20,000) |
| Antibody | Anti-β-actin-HRP (Rabbit polyclonal) | MBL | Cat# PM053-7; RRID:AB_10697035 | WB (1:20,000) |
| Antibody | Anti-Tubulin-HRP (Rabbit polyclonal) | MBL | Cat# PM054-7; RRID:AB_10695326 | WB (1:20,000) |
| Antibody | Anti-Flag-HRP (Mouse monoclonal) | Sigma-Aldrich | Cat# A8592; RRID:AB_439702 | WB (1:10,000) |
| Antibody | Anti-RIPK1 (Rabbit polyclonal) | Cell Signaling | Cat# 3493S; RRID:AB_2305314 | WB (1:1000) |
| Antibody | Anti-cleaved-caspase3 (Rabbit polyclonal) | Cell Signaling | Cat# 9661; RRID:AB_2341188 | WB (1:1000) IF(1:100) |
| Antibody | Anti-Mouse-MLKL (Rabbit polyclonal) | ABGENT | Cat# AP14272b; RRID:AB_11134649 | WB (1:1000) |
| Antibody | Anti-Human-MLKL (Rabbit monoclonal) | Abcam | Cat# ab184718; RRID:AB_2755030 | WB (1:1000) |
| Antibody | Anti-caspase-8 (Mouse monoclonal) | Cell Signaling | Cat# 9746; RRID:AB_2275120 | WB (1:1000) |
| Antibody | anti-FADD (Rabbit polyclonal) | Cell Signaling | Cat# 2782; RRID:AB_2100484 | WB (1:1000) |
| Antibody | Anti-p-S227-RIP3 (Rabbit monoclonal) | Abcam | Cat# ab209384; RRID:AB_2714035 | WB (1:1000) |
| Antibody | Anti-p-S232-RIP3 (Rabbit monoclonal) | Abcam | Cat# ab222302 | WB (1:1000) |
| Antibody | Anti-cFLIP (Rabbit polyclonal) | Abcam | Cat# ab6144; RRID:AB_305314 | WB (1:1000) |
| Antibody | Anti-cleaved-caspase-3 (Mouse monoclonal) | St John's Laboratory | Cat# STJ97448 | IHC (1:100) |
| Antibody | Anti-prostaglandin F2 alpha (PTGFR) (Rabbit polyclonal) | Abcam | Cat# ab203342 | WB (1:1000) IF (1:100) |
| Antibody | Anti-HSP90 (Rabbit polyclonal) | Proteintech | Cat# 13171-1-AP;RRID:AB_2120924 | WB (1:1000) IF (1:100) |
| Antibody | Anti-CDC37 (Rabbit monoclonal) | Abcam | Cat# ab108305; RRID:AB_10861724 | WB (1:1000) IF (1:100) |
| Antibody | anti-FLAG M2 (Mouse monoclonal) | Sigma-Aldrich | Cat# F1840 | IHC (1:100) |
| Antibody | Anti-phospho-p44/42 MAPK (Rabbit monoclonal) | Cell Signaling | Cat# 4370S; RRID:AB_2315112 | WB (1:1000) |
| Antibody | Anti-p44/42 MAPK (Rabbit monoclonal) | Cell Signaling | Cat# 4965S | WB (1:1000) |
| Antibody | Anti-phospho-MEK1/2 (Rabbit monoclonal) | Cell Signaling | Cat# 9154S; RRID:AB_2138017 | WB (1:1000) |
| Antibody | Anti-MEK1/2 (Rabbit monoclonal) | Abcam | Cat# ab178876 | WB (1:1000) |
| Antibody | Donkey anti-Mouse, Alexa Fluor 488 (Mouse polyclonal) | Thermo Fisher | Cat# A-21202; RRID:AB_141607 | IF (1:500) |
| Antibody | Donkey anti-Mouse, Alexa Fluor 555 (Mouse polyclonal) | Thermo Fisher | Cat# A-31570; RRID:AB_2536180 | IF (1:500) |
| Antibody | Donkey anti-Rabbit, Alexa Fluor 488 (Rabbit polyclonal) | Thermo Fisher | Cat# A-21206; RRID:AB_141708 | IF (1:500) |
| Antibody | Donkey anti-Rabbit, Alexa Fluor 555 (Rabbit polyclonal) | Thermo Fisher | Cat# A-31572; RRID:AB_162543 | IF (1:500) |
| Antibody | Anti-Flag M2 affinity gel | Sigma-Aldrich | A2220 | |
| Recombinant DNA reagent | pWPI-HA-3xFlag-RIPK3 | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag- RIPK3(D160N) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag- RIPK3(K50A) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag- RIPK3(AAAA) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag-mRIPK3 | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag-RIPK3(S164D/T165E) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag- RIPK3(S164E) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag- RIPK3(T165E) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag- RIPK3(S164A) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag- RIPK3(T165A) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag- RIPK3(S164A/T165A) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag-mRIPK3(D161N) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pWPI-HA-3xFlag- mRIPK3(S165D/T166E) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pLVX-Tight-HA-3xFlag-RIPK3 | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pLVX-Tight-HA-3xFlag- RIPK3(D160N) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pLVX-Tight-HA-3xFlag- RIPK3(K50A) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pLVX-Tight-HA-3xFlag- RIPK3(S164A/T165A) | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pX458-GFP-RIPK1 | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pX458-GFP-caspase-8 | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pX458-GFP-FADD | This paper | N/A | Described in Materials and methods; available upon request |
| Recombinant DNA reagent | pX458-GFP-cFLIP | This paper | N/A | Described in Materials and methods; available upon request |
| Peptide, recombinant protein | 3xFlag peptide | ChinaPeptides | DYKDHDGDYKDHDIDYKDDDDK | 1 mg/ml |
| Software, algorithm | ImageJ | NIH | N/A | |
| Software, algorithm | Photoshop | Adobe | N/A | |
| Software, algorithm | Lasergene | DNASTAR | N/A | |
| Software, algorithm | GraphPad | GraphPad Software | N/A | |
| Software, algorithm | Prism | GraphPad Software | N/A | |
| Software, algorithm | Nikon A1-R | Nikon | https://www.nikoninstruments.com/Products/Confocal-Microscopes/A1R-HD |





